Evaluation of ‘Lorca’ Cultivar Aptitude for Minimally Processed Artichoke
Abstract
:1. Introduction
2. Materials and Methods
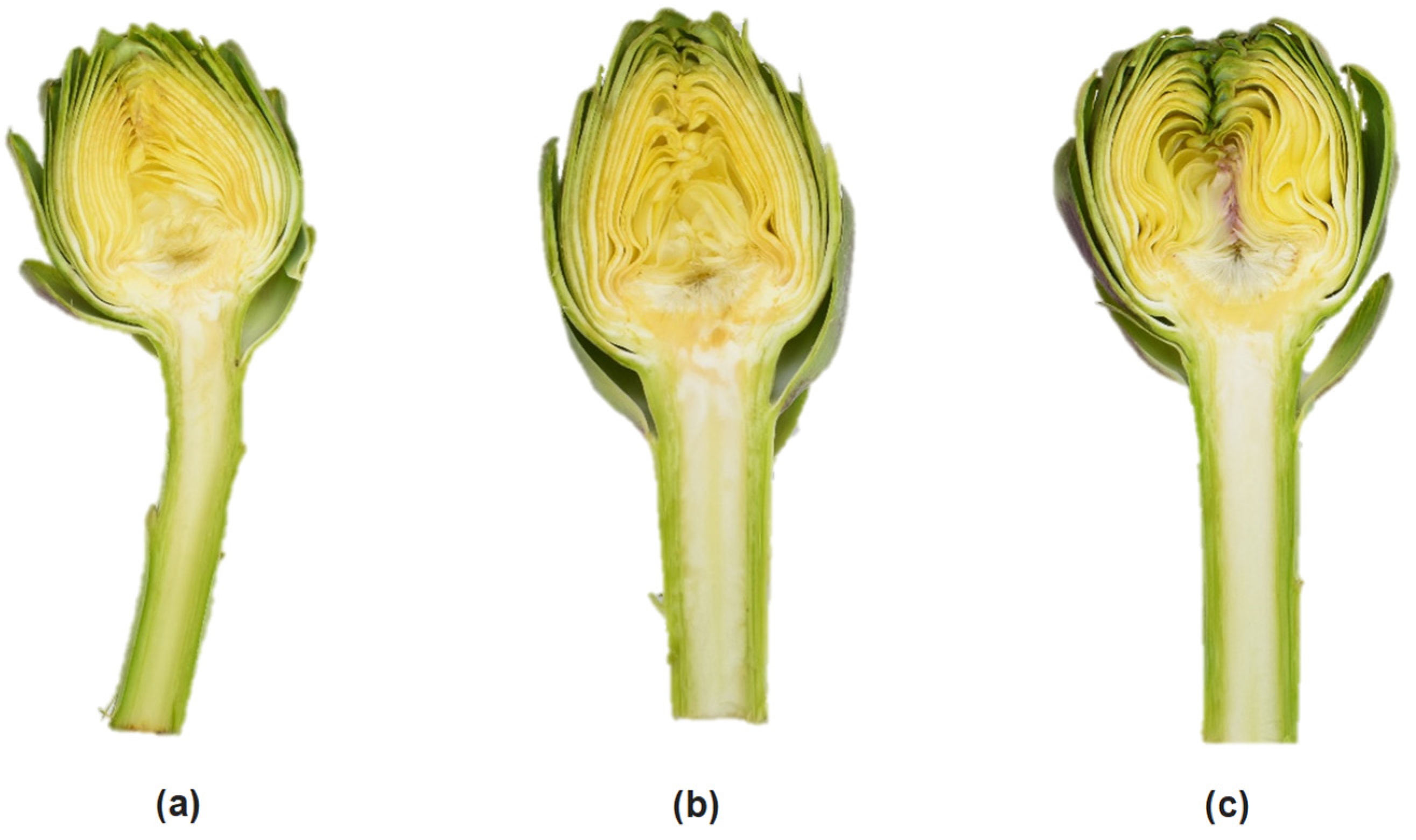
2.1. Plant Material and Experimental Design
2.2. Extraction and Quantification of Total Phenolic Compounds
2.3. Browning Evaluation by Digital Image Analysis
2.4. PPO Enzyme Activity Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Influence of Variance Components Analysis on Phenolic Content
3.2. Influence of Artichoke Head Order and Internal Development Stage on Total Phenolic Content
3.3. PPO Activity for Different Artichoke Head Orders
3.4. Evaluation of Artichoke Browning for Different Artichoke Head Orders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Muratore, G.; Restuccia, C.; Licciardello, F.; Lombardo, S.; Pandino, G.; Mauromicale, G. Effect of packaging film and antibrowning solution on quality maintenance of minimally processed globe artichoke heads. Innov. Food Sci. Emerg. Technol. 2015, 31, 97–104. [Google Scholar] [CrossRef]
- Cabezas-Serrano, A.B.; Amodio, M.L.; Cornacchia, R.; Rinaldi, R.; Colelli, G. Screening quality and browning susceptibility of five artichoke cultivars for fresh-cut processing. J. Sci. Food Agric. 2009, 89, 2588–2594. [Google Scholar] [CrossRef]
- Brat, P.; Georgé, S.; Bellamy, A.; du Chaffaut, L.; Scalbert, A.; Mennen, L.; Arnault, N.; Amiot, M.J. Daily Polyphenol Intake in France from Fruit and Vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar] [CrossRef] [Green Version]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Characterization of phenolic acids and flavonoids in leaves, stems, bracts and edible parts of globe artichokes. Acta Hortic. 2012, 942, 413–417. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Giménez, M.; Giménez-Berenguer, M.; García-Pastor, M.; Parra, J.; Zapata, P.; Castillo, S. The Influence of Flower Head Order and Gibberellic Acid Treatment on the Hydroxycinnamic Acid and Luteolin Derivatives Content in Globe Artichoke Cultivars. Foods 2021, 10, 1813. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, N.; Curadi, M.; Picciarelli, P.; Martelloni, L.; Sbrana, C.; Giovannetti, M. Globe artichoke as a functional food. Mediterr. J. Nutr. Metab. 2010, 3, 197–201. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauro, R.; Mauromicale, G. Variation of Phenolic Content in Globe Artichoke in Relation to Biological, Technical and Environmental Factors. Ital. J. Agron. 2009, 4, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Phenolic acids and flavonoids in leaf and floral stem of cultivated and wild Cynara cardunculus L. genotypes. Food Chem. 2011, 126, 417–422. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Mauromicale, G. Choice of time of harvest influences the polyphenol profile of globe artichoke. J. Funct. Foods 2013, 5, 1822–1828. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Monaco, A.L.; Mauromicale, G. Variation of polyphenols profile in globe artichoke capitulum during harvest time. Acta Hortic. 2013, 407–413. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knödler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Di Venere, D.; Linsalata, V.; Calabrese, N.; Pieralice, M.; Bianco, V. Morphological and biochemical changes during growth and development of artichoke buds. Acta Hortic. 2005, 437–444. [Google Scholar] [CrossRef]
- Ricci, I.; Amodio, M.L.; Colelli, G. Influence of pre-cutting operations on quality of fresh-cut artichokes (Cynara scolymus L.): Effect of harvest dates. Postharvest Biol. Technol. 2013, 83, 90–96. [Google Scholar] [CrossRef]
- Massignan, L.; Lovino, R.; De Cillis, M.; Di Venere, D.; Linsalata, V.; Bianco, V. Effect of harvest time, processing and packaging on the quality of ‘ready to use’ artichokes. Acta Hortic. 2005, 681, 629–636. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- García-Martínez, N.; Andreo-Martínez, P.; Almela, L. Characterization of six artichoke cultivars and their suitability for agro-industrial processing. J. Food Nutr. Res. 2017, 5, 234–242. [Google Scholar] [CrossRef]
- Cefola, M.; D’Antuono, I.; Pace, B.; Calabrese, N.; Carito, A.; Linsalata, V.; Cardinali, A. Biochemical relationships and browning index for assessing the storage suitability of artichoke genotypes. Food Res. Int. 2012, 48, 397–403. [Google Scholar] [CrossRef]
- Todaro, A.; Peluso, O.; Catalano, A.E.; Mauromicale, G.; Spagna, G. Polyphenol Oxidase Activity from Three Sicilian Artichoke [Cynara cardunculus L. Var. scolymus L. (Fiori)] Cultivars: Studies and Technological Application on Minimally Processed Production. J. Agric. Food Chem. 2010, 58, 1714–1718. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Ierna, A.; Mauromicale, G. Variation of polyphenols in a germplasm collection of globe artichoke. Food Res. Int. 2012, 46, 544–551. [Google Scholar] [CrossRef]
- Pandino, G.; Courts, F.L.; Lombardo, S.; Mauromicale, G.; Williamson, G. Caffeoylquinic Acids and Flavonoids in the Immature Inflorescence of Globe Artichoke, Wild Cardoon, and Cultivated Cardoon. J. Agric. Food Chem. 2010, 58, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Gambacorta, G.; Elia, A. Morphological and qualitative characterisation of globe artichoke head from new seed-propagated cultivars. J. Sci. Food Agric. 2010, 90, 2689–2693. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esplá, A.; García-Pastor, M.E.; Zapata, P.J.; Guillén, F.; Serrano, M.; Valero, D.; Gironés-Vilaplana, A. Preharvest application of oxalic acid improves quality and phytochemical content of artichoke (Cynara scolymus L.) at harvest and during storage. Food Chem. 2017, 230, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The influence of pre-harvest factors on the quality of globe artichoke. Sci. Hortic. 2018, 233, 479–490. [Google Scholar] [CrossRef]
- Giménez, M.J.; Giménez-Berenguer, M.; García-Pastor, M.E.; Castillo, S.; Valverde, J.M.; Serrano, M.; Valero, D.; Zapata, P.J. Influence of flower head order on phenolic content and quality of globe artichoke at harvest and during twenty-one days of cold storage. Sci. Hortic. 2021, 295, 110846. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliani, M.M.; Carucci, F.; Francavilla, M.; Gatta, G. Effects of the Irrigation with Treated Wastewaters on the Proximate Composition, Mineral, and Polyphenolic Profile of the Globe Artichoke Heads [Cynara cardunculus (L.)]. Agronomy 2019, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Lattanzio, V.; Cardinali, A.; Di Venere, D.; Linsalata, V.; Palmieri, S. Browning phenomena in stored artichoke (Cynara scolymus L.) heads: Enzymic or chemical reactions? Food Chem. 1994, 50, 1–7. [Google Scholar] [CrossRef]
- Amodio, M.; Cabezas-Serrano, A.; Peri, G.; Colelli, G. Post-cutting quality changes of fresh-cut artichokes treated with different anti-browning agents as evaluated by image analysis. Postharvest Biol. Technol. 2011, 62, 213–220. [Google Scholar] [CrossRef]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lamuela-Raventos, R.; Buxaderas, S.; Codina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, Agronomical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Vazquez-Olivo, G.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Osuna-Enciso, T.; León-Félix, J.; Heredia, J.B. Cellular antioxidant activity and in vitro intestinal permeability of phenolic compounds from four varieties of mango bark (Mangifera Findica L.). J. Sci. Food Agric. 2019, 99, 3481–3489. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.-Y.; Tadmor, Y. Analysis of Antioxidative Phenolic Compounds in Artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Serrano, A.; Amodio, M.; Colelli, G. Effect of solution pH of cysteine-based pre-treatments to prevent browning of fresh-cut artichokes. Postharvest Biol. Technol. 2013, 75, 17–23. [Google Scholar] [CrossRef]
- Hashim, N.; Daniel, O.; Rahaman, E. A Preliminary Study: Kinetic Model of Drying Process of Pumpkins (Cucurbita Moschata) in a Convective Hot Air Dryer. Agric. Agric. Sci. Procedia 2014, 2, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, S. Computer vision technology for food quality assurance. Trends Food Sci. Technol. 1996, 7, 245–256. [Google Scholar] [CrossRef]
- Sapers, G.M.; Douglas, F.W. Measurement of Enzymatic Browning at Cut Surfaces and in Juice of Raw Apple and Pear Fruits. J. Food Sci. 1987, 52, 1258–1285. [Google Scholar] [CrossRef]
- Gorny, J.R.; Hess-Pierce, B.; Cifuentes, R.A.; Kader, A.A. Quality changes in fresh-cut pear slices as affected by controlled atmospheres and chemical preservatives. Postharvest Biol. Technol. 2002, 24, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Heimdal, H.; Kühn, B.F.; Poll, L.; Larsen, L.M. Biochemical Changes and Sensory Quality of Shredded and MA-Packaged Iceberg Lettuce. J. Food Sci. 1995, 60, 1265–1268. [Google Scholar] [CrossRef]
- Peiser, G.; López-Gálvez, G.; Cantwell, M.; Saltveit, M.E. Phenylalanine ammonia lyase inhibitors control browning of cut lettuce. Postharvest Biol. Technol. 1998, 14, 171–177. [Google Scholar] [CrossRef]
- Brecht, J.K.; Saltveit, M.E.; Talcott, S.T.; Schneider, K.R.; Felkey, K.; Bartz, J.A. Fresh-Cut Vegetables and Fruits. Hortic. Rev. 2003, 30, 185–251. [Google Scholar] [CrossRef]
- Lattanzio, V. Bioactive polyphenols: Their role in quality and storability of fruit and vegetables. J. Appl. Bot. Food Qual. 2003, 77, 128–146. [Google Scholar]
- Saltveit, M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Rizzo, V.; Lombardo, S.; Pandino, G.; Barbagallo, R.; Mazzaglia, A.; Restuccia, C.; Mauromicale, G.; Muratore, G. Active Packaging-Releasing System with Foeniculum vulgare Essential Oil for the Quality Preservation of Ready-to-Cook (RTC) Globe Artichoke Slices. Foods 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Del Nobile, M.; Conte, A.; Scrocco, C.; Laverse, J.; Brescia, I.; Conversa, G.; Elia, A. New packaging strategies to preserve fresh-cut artichoke quality during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2009, 10, 128–133. [Google Scholar] [CrossRef]
- La Zazzera, M.; Amodio, M.L.; Colelli, G. Designing a modified atmosphere packaging (MAP) for fresh-cut artichokes. Adv. Hortic. Sci. 2015, 29, 24–29. [Google Scholar] [CrossRef]
- Ghidelli, C.; Mateos, M.; Rojas-Argudo, C.; Pérez-Gago, M.B. Novel approaches to control browning of fresh-cut artichoke: Effect of a soy protein-based coating and modified atmosphere packaging. Postharvest Biol. Technol. 2015, 99, 105–113. [Google Scholar] [CrossRef]
- Rizzo, V.; Lombardo, S.; Pandino, G.; Barbagallo, R.N.; Mazzaglia, A.; Restuccia, C.; Mauromicale, G.; Muratore, G. Shelf-life study of ready-to-cook slices of globe artichoke ‘Spinoso sardo’: Effects of anti-browning solutions and edible coating enriched with Foeniculum vulgare essential oil. J. Sci. Food Agric. 2019, 99, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.; Garcia, E. Preservative Treatments for Fresh-cut Fruits and Vegetables. Fresh-Cut Fruits Veg. 2002, 267–304. [Google Scholar] [CrossRef]


| Flower Head Order | Number of Artichokes |
|---|---|
| Main | 40 |
| Secondary | 278 |
| Tertiary | 127 |
| Total | 445 |
| Source | Percentage (%) |
|---|---|
| Plant | 4.55 |
| Artichoke head order | 22.17 |
| Internal development stage | 15.55 |
| Error | 57.74 |
| Artichoke Head Order | Total Phenolic Content |
|---|---|
| Main heads | 2.155 ± 0.065 c |
| Secondary heads | 2.782 ± 0.023 b |
| Tertiary heads | 3.221 ± 0.019 a |
| Internal Development Stage | Total Phenolic Content |
|---|---|
| 1 (initial) | 2.889 ± 0.036 a |
| 2 (intermediate) | 2.827 ± 0.032 a |
| 3 (advanced) | 2.411 ± 0.023 b |
| Artichoke Head Order | PPO Activity (U g−1 FW) |
|---|---|
| Main heads | 311.30 ± 19.05 b |
| Secondary heads | 411.90 ± 19.71 a |
| Tertiary heads | 451.31 ± 20.17 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez-Berenguer, M.; García-Pastor, M.E.; García-Martínez, S.; Giménez, M.J.; Zapata, P.J. Evaluation of ‘Lorca’ Cultivar Aptitude for Minimally Processed Artichoke. Agronomy 2022, 12, 515. https://doi.org/10.3390/agronomy12020515
Giménez-Berenguer M, García-Pastor ME, García-Martínez S, Giménez MJ, Zapata PJ. Evaluation of ‘Lorca’ Cultivar Aptitude for Minimally Processed Artichoke. Agronomy. 2022; 12(2):515. https://doi.org/10.3390/agronomy12020515
Chicago/Turabian StyleGiménez-Berenguer, Marina, María E. García-Pastor, Santiago García-Martínez, María J. Giménez, and Pedro J. Zapata. 2022. "Evaluation of ‘Lorca’ Cultivar Aptitude for Minimally Processed Artichoke" Agronomy 12, no. 2: 515. https://doi.org/10.3390/agronomy12020515
APA StyleGiménez-Berenguer, M., García-Pastor, M. E., García-Martínez, S., Giménez, M. J., & Zapata, P. J. (2022). Evaluation of ‘Lorca’ Cultivar Aptitude for Minimally Processed Artichoke. Agronomy, 12(2), 515. https://doi.org/10.3390/agronomy12020515











