Abstract
Grapevine is highly susceptible to fungal diseases, whose incidence and severity increase due to climate change. The present work focuses on the assessment of eight combinations of natural products with chitosan oligomers with fungicidal capacity that may be effective in the integrated control of powdery mildew, in compliance with Article 14 of the European Directive 2009/128/EC. Their efficacy was evaluated in field conditions against natural infections, in a plot with high disease pressure during a growing season (assaying both foliar or root application), and against overwintering inoculums (chasmothecia) through in vitro tests. In addition, their possible biostimulant capacities were evaluated based on harvest yields. Treatments based on chitosan oligomers in combination with secondary metabolites of Streptomyces spp. and chitosan oligomers combined with hydrolyzed gluten showed the best results in terms of disease control. Given the high efficacy of these formulations, comparable to that of conventional antifungals, they constitute an interesting alternative for the control of this disease whose treatment can, in some cases, represent almost half of the production costs.
Keywords:
antifungals; chasmothecia; chitosan; Erysiphe necator; gluten; Streptomyces; Vitis vinifera 1. Introduction
Grapevine (Vitis vinifera L.) is highly susceptible to numerous diseases caused by aerial pathogens, such as powdery mildew (Erysiphe necator Schwein., synonym Uncinula necator (Schwein.) Burrill), downy mildew (Plasmopara viticola (Berk. & M.A. Curtis) Berl. & De Toni) and grey mold (Botrytis cinerea Pers.) [1]. The incidence and severity of these diseases are increasing as a consequence of climate change [2,3], and, in France, it has been estimated that their treatment accounts for about half of the production cost.
Under favorable environmental conditions, the pressure of these diseases forces the use of enormous quantities of phytosanitary products, which entails high economic and environmental costs and, in many cases, quickly generates resistance [4]. According to Eurostat data, the application of phytosanitary products per hectare per year in viticulture is the highest of all crops [5]. In some cases, the number of applications per growing season is higher than 12 [6], reaching up to 16 applications in times of high disease pressure. In a study on pecuniary and nonpecuniary costs of managing powdery mildew in California grape production, Sambucci et al. [7] estimated that powdery mildew control accounted for 89% of crop protection applications in this sector.
This disease is caused by a biotrophic fungus that survives during the winter in the form of ascospores contained in chasmothecia (sexual fruiting bodies) or in the form of mycelium in dormant buds [8]. These structures can survive for long periods under adverse conditions. Their epidemiological importance lies in the fact that they constitute one of the main sources of primary inoculum for grapevine powdery mildew [9] provided that, in spring, under optimal conditions, the chasmothecia open, dispersing the ascospores. When these ascospores reach green tissues (mainly the most basal leaves closest to the bark of the grapevine’s trunk), they germinate, giving rise to infections and onset of the disease. These first infections will give rise to multiple secondary infections [10]. Hence, effective control of this initial source of inoculum is vital to control the development of this fungus in the vineyard, thus breaking the disease cycle.
Currently, the search for alternative solutions for pathogen control has become a key objective to comply with the guidelines of the European Directive 2009/128/EC, which establishes the basis for the sustainable use of pesticides, highlighting the reduction of their use (e.g., that of copper in viticulture [6]) as a fundamental aspect. In addition, recently, in 2020, we have witnessed the signing of the European Green Pact, by which EU member states have agreed to reduce the use of chemically synthesized plant protection products by 50% by 2030. Breeding for resistant genotypes [11], flashes of UV-C light [12], out-of-season fungicide applications [13], use of spore traps [14], and application of non-synthetic chemicals and organic control measures have been put forward as promising alternative approaches for powdery mildew management.
The study presented herein explores the antifungal activity against powdery mildew on the grapevine of eight formulations based on natural products that have previously shown promise against other phytopathogenic fungi [15,16,17]. Their efficacy has been evaluated in field conditions, in a plot of Viñas del Vero winery (D.O. Somontano, Huesca, Spain), and in vitro against one of the primary sources of initial inoculum, chasmothecia.
2. Material and Methods
2.1. Reagents and Actinobacteria Isolates
High molecular weight chitosan (CAS 9012-76-4; 310,000–375,000 Da) was purchased from Hangzhou Simit Chemical Technology Co. (Hangzhou, China). The ε-polylysine (CAS 25104-18-1), silver nanoparticles (40 nm particle size (TEM), 0.02 mg·mL−1 in aqueous solution, with sodium citrate as a stabilizer), fluorescein diacetate (CAS 596-09-8), phosphate buffer (for microbiology, APHA, pH 7.2), ethyl acetate (CAS 141-78-6; ≥99.5%), and citric acid (CAS 77-92-9; ≥99.5%) were supplied by Sigma-Aldrich Química S.A. (Madrid, Spain). Neutrase® 0.8 L enzyme was supplied by Novozymes (Bagsvaerd, Denmark). Potato dextrose agar (PDA), yeast extract, and BactoTM Peptone were purchased from Becton, Dickinson & Company (Franklin Lakes, NJ, USA). Starch casein agar (SCA), Mueller Hinton agar (MHA), and malt extract agar (MEA) were purchased from Oxoid Ltd. (Hampshire, UK). Molasses were supplied by ACOR, Sociedad Cooperativa General Agropecuaria (Castilla y León, Spain).
The two Streptomyces spp. strains from which the secondary metabolites were produced, Streptomyces lavendofoliae (DSM 40217) and Streptomyces rochei (DSM 41729) were acquired from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ; Braunschweig, Germany).
2.2. Preparation of Chitosan Oligomers and Secondary Metabolites
Chitosan oligomers (COS) were prepared according to the procedure described in the work by Santos-Moriano et al. [18], with the modifications indicated in [17]. Commercial chitosan (MW = 310–375 kDa) was dissolved in aqueous 1% (w/w) acetic acid, and, after filtration, the filtrate was neutralized with aqueous 4% (w/w) NaOH. The precipitate was collected and washed thoroughly with hot distilled water, ethanol, and acetone. The purified chitosan was obtained by drying. The degree of deacetylation (DD) was determined to be 90% according to Sannan et al. [19]. 20 g of purified chitosan were dissolved in 1000 mL of Milli-Q water by adding 20 g de citric acid under constant stirring at 60 °C. Once dissolved, the commercial proteolytic preparation Neutrase® 0.8 L (a protease from Bacillus amyloliquefaciens) was added to obtain a product enriched in deacetylated chitooligosaccharides and to degrade the polymer chains. The mixture was sonicated for 3 min in 1 min of sonication/1 min without sonication cycles to keep the temperature in the 30–60 °C range. At the end of the process, a solution with a pH in the 4–6 interval with oligomers of molecular weight <2 kDa was obtained, with a polydispersity index of 1.6.
The two strains of the genus Streptomyces were grown on starch-casein agar medium at 28 °C for 10 days. The plates were stored at 4 °C. For long-term storage, lyophilizates of both strains were used.
The method described by Sadigh-Eteghad et al. [20] was followed to obtain the secondary metabolites. After completion of fermentation, each final solution of the cultures of both strains was treated with 50 mL of phosphate buffer (pH 6.4) and subjected to ultrasonication with a 1000 W probe-type ultrasonicator operated at 20 kHz (model UIP1000hdT, Hielscher Ultrasonics, Teltow, Germany) for 5 min. The solutions were then filtered twice through a sterile muslin cloth.
To determine the concentration of the bioactive compounds in the above solutions, the procedure described in Pazhanimurugan et al. [21] was followed: the filtrates were centrifuged, and the supernatant was extracted with 100 mL of ethyl acetate. The solvent with the crude bioactive compounds was concentrated under reduced pressure and then lyophilized. As a result, the culture filtrates had a concentration of approximately 2.0 mg∙mL−1 (1.96 mg∙mL−1 for secondary metabolites of S. lavendofoliae and 1.88 mg∙mL−1 for secondary metabolites of S. rochei).
2.3. Bioactive Products Tested
The treatments used are summarized in Table 1. The methods of preparation of each formulation are described in detail in previous work [15,16,17]. In short, the chitosan oligomers solution was mixed in a 1:1 (v/v) ratio with the silver nanoparticles (nAg), ε-polylysine (EPL), or hydrolyzed gluten solutions, or in a 3:1 (v/v) ratio with the secondary metabolites (either from S. lavendofoliae or from S. rochei) solutions. Mixtures were prepared by sonication at 20 kHz, in periods of 2 min each, and for a total time of 20 min, avoiding that the temperature exceeded 50 °C.

Table 1.
Summary of treatments used against powdery mildew in the field and in vitro against chasmothecia.
For root application and the in vitro tests on ascospores, no additives were used. For foliar application, 0.2% Tween® 20 (a sorbitan fatty acid ester ethoxylate) was added, because this highly effective nonionic surfactant effectively delivers multiple adjuvant functionalities such as retention, enhanced uptake, humectancy, wetting, and spreading properties.
2.4. Field Antifungal Activity Test
The field application trial was carried out from the end of April 2019 to the end of August 2019 at the Viñas del Vero winery estate called ‘Litonera’ (41°58′46.6″ N 0°08′05.6″ E), in the municipality of Barbastro (Huesca, Spain), included in the Somontano designation of origin. This particular vineyard plot was selected because it is subject to the area’s highest pressure of fungal diseases (attributable to a higher environmental humidity due to two nearby irrigation ponds fed from the Selgua irrigation canal).
The trials were carried out on grapevines of ‘Chardonnay’ variety, clone 151, on Richter 110 rootstock. For each treatment, two application types (foliar-spray and root) were tested, except for the freeze-dried Streptomyces strains, which were root-applied in a solid phase.
Each experimental unit consisted of four replicates and three plants per replicate, separated by a guard plant. The separation between two application modes (foliar and root) consisted of 3 guard plants. Each product was tested in different rows, leaving a guard row between treatments (Figure S1). The efficacy of the treatments was compared against untreated control grapevines and against grapevines treated with the usual treatment of the winery, based on conventional agrochemicals (Table S1).
The dose of the product tested was the same throughout the campaign: 40 mL of active product/plant. The application of the product was carried out with agricultural spray backpacks (one for each product to be tested) and with buckets (one per product to be tested), depending on whether the application was foliar or root-based, respectively.
The treatments were applied twice a month (25 April, 10 May, 27 May, 10 June, 24 June, 9 July, 22 July, and 5 August) until the harvest date (23 August).
The applications were accompanied by monitoring of disease pressure. Due to the season’s weather conditions (Figure S2), only powdery mildew attack was significant. A total of 5 counts of leaf attack and 4 counts of bunch attack were carried out, starting on 10 June and 24 June, respectively, and repeated every two weeks until 5 August in both cases. On each date, a sample of 40 randomly selected organs was observed in each block, and in due course, 40 bunches. The European and Mediterranean Plant Protection Organization (EPPO) procedure was followed to evaluate the parameters related to the presence and attack of powdery mildew. It establishes a scale based on the percentage of the area of the organ affected by the disease. The Townsend–Heuberger formula [22] was used to calculate the degree of damage by fungi attack:
where DS is the disease severity, n is the number of organs (leaves or bunches) in each class, v is the class value, N is the total number of assessed organs, and V is the highest class value.
On 23 August, coinciding with the harvest of the entire plot, the productivity of the different treatments was determined. To do this, the number of bunches and their weight were counted in one grapevine for each repetition (that is, in 4 grapevines per treatment and per mode of application of the active product). Once these measurements had been taken, 100 random grains were weighed, and the grape juice obtained was analyzed (pH, °Brix, potential alcohol, and acidity).
2.5. In Vitro Test of Antifungal Activity on Ascospores
2.5.1. Isolation of Chasmothecia
For chasmothecia isolation, the protocol described by Cortesi et al. [23] was used with slight modifications: 100 g of leaves from a powdery mildew susceptible grapevine variety (‘Godello’) with abundant production of chasmothecia on leaves (dark coloration, Figure 1) were collected. They were placed in a 2 L bottle with 1.5 L of distilled water (Figure 2A) and were vigorously shaken for 3 min. Chasmothecia were double-sieved through 0.2 and 0.1 mm mesh sieves (Figure 2B–D). The process was repeated three more times, shaking for only 1 min each time. Finally, the chasmothecia were collected with a brush, allowed to dry on a filter paper (Figure 2E), and stored in a cool, dry place until further use.
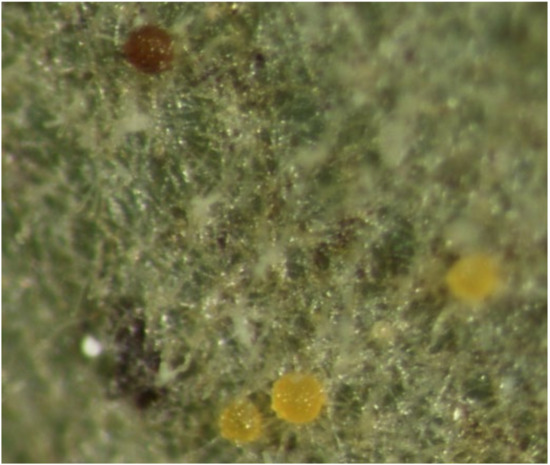
Figure 1.
Chasmothecia on ‘Godello’ variety grapevine leaves.
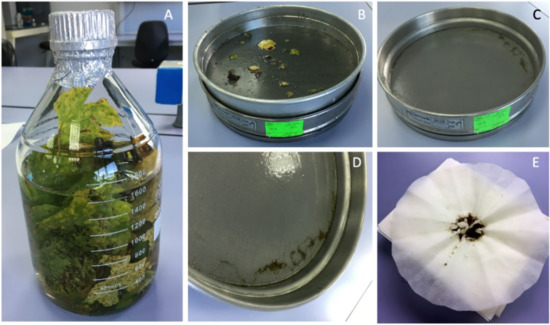
Figure 2.
Chasmothecia obtaining process from ‘Godello’ variety grapevine leaves: (A) 100 g of chopped grapevine leaves in a 2 L bottle with 1.5 L of distilled water; (B) 0.2 mm sieve; (C) 0.1 mm sieve; (D) clean chasmothecia on the 0.2 mm sieve; (E) recovered chasmothecia by drying on a filter paper for subsequent preservation.
2.5.2. Fluorescein Diacetate Staining of Ascospores
Three different protocols were evaluated, to which slight modifications were made regarding the staining time and the concentration of fluorescein diacetate (FDA). The tested protocols were: (i) the one proposed by Widholm [24]; (ii) that reported by Ingham and Klein [25]; and (iii) that of ibidi GmbH [26]. The selected protocol was the third one. Since this protocol contemplated a co-staining with propidium iodide, all volumes of propidium iodide were replaced by phosphate-buffered saline (PBS). Staining solution: FDA on PBS in a final concentration of 7.9 µg·mL−1 (stored at 4 °C in the dark for no more than 2 h).
Twenty-five chasmothecia were picked up with a brush and placed in a well of a multiwell plate where the compound to be evaluated was added, leaving it to act for 10 min. After this time, the compound was removed, rinsed with sterile distilled water, and the staining solution was added for 5 min. To facilitate the staining of the ascospores, the chasmothecia were broken with a micropistil.
For the same fields of observation, the number of viable ascospores (fluorescent under fluorescence microscopy; Figure 3) and the total number of ascospores in brightfield (Figure 3) were counted. For each treatment, the viability of 100 ascospores was assessed and expressed as a percentage of viable ascospores.
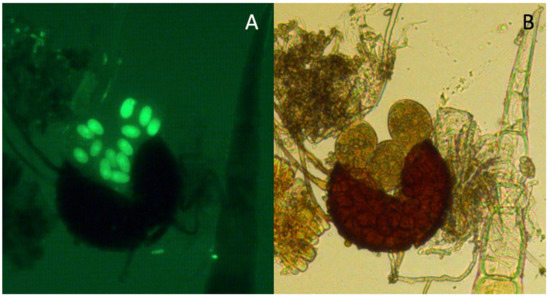
Figure 3.
Open chasmothecia showing asci with ascospores. Both images show the result of the same preparation stained with fluorescein diacetate under (A) fluorescence and (B) brightfield microscopy (40×).
The experiment was repeated once under the same conditions using chasmothecia obtained in the same isolation process.
2.6. Statistical Treatment
For the whole period, all the data of degree of damage by fungi attack (in leaves and bunches) were analyzed with Friedman’s test, non-parametric equivalent to the ANOVA test for repeated measures, with multiple comparisons by pairs employing the Nemenyi’s procedure/two-tailed test. Additionally, for the results of the degree of attack on each sampling date, a non-parametric analysis was carried out with the Kruskal–Wallis test, accompanied by the comparison of pairs with the Dunn and Conover–Iman methods, applying the Bonferroni correction. For the production measures (number of bunches, bunch weight, and weight of 100 grains), analysis of variance (ANOVA) was used with post hoc comparisons with Tukey’s test, when the requirements of normality and homoscedasticity of the data were fulfilled.
3. Results
3.1. Field Antifungal Activity Tests
3.1.1. Powdery Mildew Control on Leaves
Mean values of the degree of powdery mildew attack on leaves for each treatment and sampling date are presented in Table S2. Environmental conditions throughout the period of study are summarized in Figure S2.
From the results of Friedman’s test (Table S3), it was observed that the lowest degree of attack corresponded to root-applied COS + S. lavendofoliae metabolites treatment (T5R). Although there were no statistically significant differences, COS + hydrolyzed gluten treatments (foliar and root-applied, T6F and T6R, respectively) were the next most effective treatments.
The best treatments at each date, separately analyzed by the Kruskal–Wallis test, are summarized in Table 2. Again, root-applied COS + S. lavendofoliae metabolites treatment and COS + hydrolyzed gluten treatments (T5R, T6R, and T6F, respectively) were the most effective, comparable to the conventional treatment (Tconv).

Table 2.
Summary of the most effective treatments (lowest degree of attack) on leaves and grapevine clusters for each sampling date. Results are based on non-parametric analysis using the Kruskal–Wallis test.
3.1.2. Powdery Mildew Control on Grapevine Clusters
Mean values of the degree of powdery mildew attack on grapevine clusters for each treatment and sampling date are summarized in Table S4.
The results of Friedman’s test for the degree of attack on grapevine bunches (Table S5) were consistent with those obtained for the degree of attack on leaves, with a higher efficacy (statistically significant) of root-applied COS + S. lavendofoliae metabolites treatment, followed by COS + hydrolyzed gluten treatments (without statistically significant differences).
Based on the results of the degree of damage by fungi attack for each date, separately analyzed by the Kruskal–Wallis test, the best treatments in terms of grapevine bunch protection are summarized in the lower part of Table 2. If the 24 June result is excluded, the remaining results are consistent with those of Friedman’s test.
In the manual harvest, it was clearly seen that the berries of the grapevines treated with the natural treatments mentioned above showed a greater turgidity.
3.2. Effect on Production Quality and Yield
No statistically significant differences were observed in the number of bunches per sampling unit (average of 21 bunches/4 grapevines sampled) or in the weight of 100 grains (mean value of 153 g), except for the foliar-applied COS-only treatment, in which a presumable phytotoxicity reaction was found (with leaf chlorosis symptoms followed by foliage loss, Figure S3) and which led to almost no production (Table S6).
Nevertheless, significant differences were observed in terms of bunch weight/plant, with the highest weights associated with foliar-applied COS + hydrolyzed gluten treatment (T6F; 5.54 kg/plant), root-applied COS + S. lavendofoliae metabolites treatment (T5R; 4.55 kg/plant), and the conventional treatment (3.85 kg/plant), a result consistent with the lower degree of attack on bunches referred above. However, the low bunch weight obtained for the plants treated with root-applied COS + hydrolyzed gluten treatment was striking (T6R; 1.98 kg/plant).
Concerning the parameters measured in the samples of grape juice (Table S6), no statistically significant differences were detected between treatments in terms of pH (mean value of 3.36), acidity (mean value of 7.37 g tartaric acid∙L−1), sugar content (with a mean value of 22 °Brix), or potential alcohol (mean value of 15.1%), so it may be inferred that the quality of production was not affected.
3.3. In Vitro Effect of Antifungal Activity on Ascospores
All treatments showed efficacy in terms of loss of viability of ascospores compared to the control treatment (Tcontrol, 69% viability). Those that combined chitosan oligomers with secondary metabolites of the two Streptomyces species used (S. rochei and S. lavendofoliae) were the most effective (T4C and T5C, 0% viable ascospores), followed by COS + hydrolyzed gluten (T6C, 5%) and COS + nAg (T2C, 27%). Of all treatments, COS + ε-polylysine (T3C, 47%) and COS (T1C, 57%) showed the lowest fungicidal activity on ascospores (Figure 4).
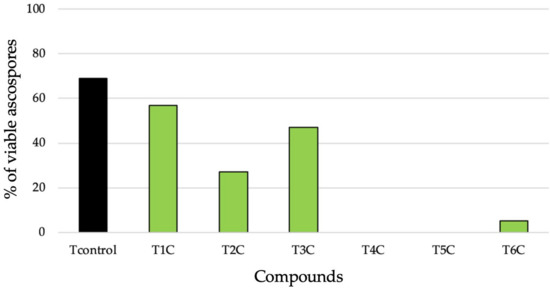
Figure 4.
Percentage of viable ascospores after subjecting chasmothecia to treatments with the following compounds: distilled H2O (Tcontrol), chitosan oligomers (T1C), COS + silver nanoparticles (T2C), COS + ε-polylysine (T3C), COS + Streptomyces rochei secondary metabolites (T4C), COS + S. lavendofoliae secondary metabolites (T5C), and COS + hydrolyzed gluten (T6C).
4. Discussion
4.1. Antifungal Behavior in Field Conditions and Effect on Yield
The results obtained should be considered preliminary, given that they correspond to trials carried out in a single location and during a single season. However, considering that disease pressure was high, the observations made throughout the season indicate that the treatment based on root-applied COS + S. lavendofoliae metabolites (T5R) was the most effective against powdery mildew among the treatments tested, both on leaves and bunches. Streptomyces species usually show a remarkable antimicrobial activity, making them potential biological control agents [27]. Numerous species have been described for controlling mildews in different crops, either by direct application or using substances produced by these bacteria [17,28,29,30], but powdery mildew in grapevine has been less widely addressed [31].
Treatments based on COS + hydrolyzed gluten (T6F and T6R) were the next most promising, which may be attributed to the high content of amino acids present in gluten. In this regard, there are previous reports on the high antifungal efficacy of COS−amino acid conjugate complexes [32,33], which try to mimic plant host defense peptides (HDPs). HDPs are generally cysteine-rich (nodule-specific cysteine-rich peptides, NCRs) and are considered one of the main barriers developed by plants to fight infective agents [34,35,36], including the Snakin class identified in the grapevine [37]. Further, in a study on the foliar application of Mn−amino acid complexes for powdery mildew control in cucumber (caused by Podosphaera fuliginea (Schltdl.) U.Braun & S.Takam.), Eskandari et al. [38] showed that the Mn−amino acid complexes resulted in disease suppression and that the spraying of free amino acids significantly decreased disease severity on the treated leaves, being more effective than sulfate.
The efficacy of the aforementioned products was superior to that of the conventional treatments on all dates in which the monitoring was carried out, and, therefore, they could be used as an alternative to synthetic phytochemicals. For example, on the sampling date prior to harvest (5 August), the grapevines treated with root-applied COS + S. lavendofoliae metabolites and with root and foliar-applied COS + hydrolyzed gluten (T5R, T6R, and T6F, respectively) showed degrees of attack on clusters of 33.1, 47.5 and 51.25%, compared to 75.6% for the conventional treatment. It should be emphasized that these attack results correspond to a plot with an unusually high fungal disease pressure, so in other plots it is foreseeable that the degree of attack would be considerably lower.
Regarding yield, the production per plant was significantly higher in the plants treated with root-applied COS + S. lavendofoliae metabolites (T5R) and foliar-applied COS + hydrolyzed gluten (T6F), surpassing the production of the strains treated with conventional products. This activity as biofertilizers, improving the yield, has previously been well documented for Streptomyces spp., making the species of this genus an interesting alternative to inorganic fertilizers [39]. However, little research works have combined COS with Streptomyces metabolites, and even less have been assayed in field conditions. On the other hand, the low production for the strains treated with root-applied COS + hydrolyzed gluten (T6R) discourages using this treatment.
4.2. In Vitro Efficacy against Chasmothecia
All the treatments used showed a fungicidal effect, evidenced by lower viability of the ascospores, highlighting the combinations of COS + Streptomyces spp. secondary metabolites. Chasmothecia isolated and preserved in suitable conditions (cool, dry place with a not very high temperature of around 15–18 °C) lose viability, with some authors describing up to 50% loss of viability after 16 weeks of preservation at 17 °C [40]. This could explain why the control treatment showed a percentage of viability in the ascospores used of 69%. However, works that study the viability of chasmothecia and/or ascospores of this pathogen are not very abundant and usually focus on controlling the formation and development of chasmothecia. An example is a recent work by Thiessen et al. [41] on the effect of using an organic oil to control chasmothecia production, or that by Legler et al. [42], in which a biofungicide based on Ampelomyces quisqualis Ces. (a hyperparasite) has shown good results in reducing the pathogen due to its activity in the later cycle stages. Although we do not know the effect that the assayed products have on the formation and maturation of chasmothecia, the fungicidal effect they have, not only on the viability of ascospores but also on the development of the disease, suggest that they could be an environmentally friendly alternative that could be combined with other treatments within an integrated powdery mildew management program. This makes the presented results interesting from a practical point of view for wine-growers, given that the presence of chasmothecia and the viability of their ascospores mark to a large extent the initial levels of disease, which is also closely related to the subsequent difficulty to adequately control the pathogen in the course of its cycle [10,43].
4.3. Justification of the Observed Antifungal Behavior
Several modes of action have been proposed regarding the mode of inhibition of chitosan oligomers [44]. The interaction of the positively charged chitosan with the negatively charged phospholipid components would result in increased permeability and leakage of cellular contents. Its chelating action would deprive fungi of trace elements essential for normal growth. In addition, their binding to fungal DNA would inhibit mRNA synthesis and affect protein and enzyme production.
The good antifungal behavior observed in vitro for treatments based on polyelectrolyte complexes (PECs) of COS and secondary metabolites of S. lavendofoliae and S. rochei has to be referred to the bioactive compounds present in the extracts of these actinobacteria (summarized in Table 3) and is consistent with that already observed against grapevine wood fungi [17].

Table 3.
Secondary metabolites with biological activity produced by S. lavendofoliae and S. rochei.
Regarding the differences in terms of activity between Streptomyces strains in field trials, it can be tentatively referred to solubility problems of some of the active principles of the secondary metabolites of S. rochei, despite the formation of PECs with COS (for example, regarding one of the active compounds present in the filtrates of S. rochei, lankacidine, Harada et al. [57] have referred that lankacidine group antibiotics are poorly soluble in water and that the dissolving parts rapidly decompose into compounds without antimicrobial activity). However, other options cannot be ruled out a priori, such as stability problems of the substances present in uncontrolled laboratory conditions, degradation due to the effect of temperature, inactivation due to leaf exudates, or even the activity of other microorganisms present in the plant.
Regarding the antifungal activity of the COS + hydrolyzed gluten complex, it can be attributed to the amino acids present in gluten, given that COS-amino acid conjugate complexes have shown antifungal activity in other works [32,33].
5. Conclusions
Among the treatments tested in the field, the highest antifungal activity against powdery mildew corresponded to the root-applied COS + S. lavendofoliae metabolites treatment, followed by the foliar-applied COS + hydrolyzed gluten treatment, in good agreement with the results from the in vitro studies of ascospore viability (in which the COS + S. rochei metabolites treatment was also very effective). The efficacy of the two aforementioned natural formulations was superior to that of the conventional fungicides used by the winery, resulting in higher yields per plant. Therefore, treatments such as the ones proposed herein, which in addition to being effective in controlling the development of the disease at different points in its cycle, are environmentally friendly, open a new avenue of action to reduce the use of chemically-synthesized phytosanitary products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12020495/s1, Figure S1: Distribution of the treatments on the vineyard plot; Figure S2. Environmental conditions (temperature and rainfall) in the vineyard plot throughout the study period; Figure S3. Phytotoxicity symptoms detected in grapevines upon foliar application of the COS-only treatment; Table S1: Phytosanitary applications carried out by Viñas del Vero winery; Table S2. Mean values of the degree of powdery mildew attack on leaves for each treatment and sampling date; Table S3. Results of Friedman’s test for multiple pairwise comparisons using the Nemenyi’s procedure/Two-tailed test for the treatments tested as a function of the degree of attack recorded on leaves; Table S4. Mean values of the degree of powdery mildew attack on grapevine clusters for each treatment and sampling date; Table S5. Results of Friedman’s test for multiple pairwise comparisons using the Nemenyi’s procedure/two-tailed test for the treatments tested as a function of the degree of attack recorded on grapevine bunches; Table S6. Average yield values and grape juice characteristics for the different treatments.
Author Contributions
Conceptualization, D.R.-R., P.M.-R. and J.C.-G.; methodology, D.R.-R., J.M.-G., S.T.-S. and J.C.-G.; validation, E.S.-H.; formal analysis, R.B.-F., P.M.-R. and J.C.-G.; investigation, D.R.-R., E.S.-H., R.B.-F., P.M.-R., J.M.-G., S.T.-S. and J.C.-G.; resources, P.M.-R., S.T.-S. and J.M.-G.; data curation, E.S.-H.; writing—original draft preparation, D.R.-R., E.S.-H., R.B.-F., P.M.-R., J.M.-G., S.T.-S. and J.C.-G.; writing—review and editing, D.R.-R., P.M.-R. and J.C.-G.; visualization, E.S.-H.; supervision, P.M.-R. and J.C.-G.; project administration, P.M.-R. and J.M.-G.; funding acquisition, P.M.-R. and J.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Junta de Castilla y León under project VA258P18, with FEDER co-funding; by Cátedra Agrobank under “IV Convocatoria de Ayudas de la Cátedra AgroBank para la transferencia del conocimiento al sector agroalimentario” program; and by Fundación Ibercaja-Universidad de Zaragoza under “Convocatoria Fundación Ibercaja-Universidad de Zaragoza de proyectos de investigación, desarrollo e innovación para jóvenes investigadores” program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to their relevance as part of an ongoing Ph.D. Thesis.
Acknowledgments
To José M. Ayuso-Rodríguez and Adrián Jarné-Casasús, from Viñas del Vero S.A. winery, and to Laura Buzón-Durán.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galet, P. Précis de Pathologie Viticole, 3rd ed.; Imprimerie JF Impression: Montpellier, France, 1999; p. 296. [Google Scholar]
- Bois, B.; Zito, S.; Calonnec, A. Climate vs grapevine pests and diseases worldwide: The first results of a global survey. OENO One 2017, 51, 133–139. [Google Scholar] [CrossRef]
- Caffarra, A.; Rinaldi, M.; Eccel, E.; Rossi, V.; Pertot, I. Modelling the impact of climate change on the interaction between grapevine and its pests and pathogens: European grapevine moth and powdery mildew. Agric. Ecosyst. Environ. 2012, 148, 89–101. [Google Scholar] [CrossRef]
- Kunova, A.; Pizzatti, C.; Saracchi, M.; Pasquali, M.; Cortesi, P. Grapevine powdery mildew: Fungicides for its management and advances in molecular detection of markers associated with resistance. Microorganisms 2021, 9, 1541. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. The Use of Plant Protection Products in the European Union; Office for Official Publications of the European Communities: Luxembourg, 2007; p. 215. [Google Scholar]
- Rousseau, J.; Chanfreau, S.; Bontemps, É. Les Cépages Résistants and Maladies Cryptogamiques; Groupe ICV: Bordeaux, France, 2013; p. 228. [Google Scholar]
- Sambucci, O.; Alston, J.M.; Fuller, K.B.; Lusk, J. The pecuniary and nonpecuniary costs of powdery mildew and the potential value of resistant grape varieties in California. Am. J. Enol. Vitic. 2019, 70, 177–187. [Google Scholar] [CrossRef]
- Pearson, R.C.; Gadoury, D.M. Cleistothecia, the source of primary inoculum for grape powdery mildew in New York. Phytopathology 1987, 77, 1509–1514. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Pearson, R.C. Germination of ascospores and infection of Vitis by Uncinula necator. Phytopathology 1990, 80, 1198–1203. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Cadle-Davidson, L.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine powdery mildew (Erysiphe necator): A fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef]
- Calonnec, A.; Jolivet, J.; Ramaroson, M.L.; Dufour, M.C.; Corio-Costet, M.F. Defence responses of grapevine cultivars to powdery mildew: Ontogenic resistance versus genetic resistance. Plant Pathol. 2021, 70, 1583–1600. [Google Scholar] [CrossRef]
- Ledermann, L.; Daouda, S.; Gouttesoulard, C.; Aarrouf, J.; Urban, L. Flashes of UV-C light stimulate defenses of Vitis vinifera L. ‘Chardonnay’ against Erysiphe necator in greenhouse and vineyard conditions. Plant Dis. 2021, 105, 2106–2113. [Google Scholar] [CrossRef]
- Redl, M.; Sitavanc, L.; Hanousek, F.; Steinkellner, S. A single out-of-season fungicide application reduces the grape powdery mildew inoculum. Crop Prot. 2021, 149, 105760. [Google Scholar] [CrossRef]
- Scott, E.S. 2019 Daniel McAlpine Memorial Lecture. Grapevine powdery mildew: From fundamental plant pathology to new and future technologies. Australas. Plant Pathol. 2021, 50, 1–6. [Google Scholar] [CrossRef]
- Matei, P.; Iacomi, B.; Martín-Gil, J.; Pérez-Lebeña, E.; Ramos-Sánchez, M.; Barrio-Arredondo, M.; Martín-Ramos, P. In vitro antifungal activity of composites of AgNPs and polyphenol inclusion compounds against Fusarium culmorum in different dispersion media. Agronomy 2018, 8, 239. [Google Scholar] [CrossRef]
- Matei, P.; Martín-Gil, J.; Michaela Iacomi, B.; Pérez-Lebeña, E.; Barrio-Arredondo, M.; Martín-Ramos, P. Silver nanoparticles and polyphenol inclusion compounds composites for Phytophthora cinnamomi mycelial growth inhibition. Antibiotics 2018, 7, 76. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Martín-Gil, J.; Pérez-Lebeña, E.; Ruano-Rosa, D.; Revuelta, J.L.; Casanova-Gascón, J.; Ramos-Sánchez, M.C.; Martín-Ramos, P. Antifungal agents based on chitosan oligomers, ε-polylysine and Streptomyces spp. secondary metabolites against three Botryosphaeriaceae species. Antibiotics 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2017, 36, 57–67. [Google Scholar] [CrossRef]
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on chitin, 2. Effect of deacetylation on solubility. Die Makromol. Chem. 1976, 177, 3589–3600. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Dehnad, A.; Shanebandi, D.; Khalili, I.; Razmarayii, N.; Namvaran, A.J.V.R.C. Identification and characterization of a Streptomyces sp. isolate exhibiting activity against multidrug-resistant coagulase-negative Staphylococci. Vet. Res. Commun. 2011, 35, 477–486. [Google Scholar] [CrossRef]
- Pazhanimurugan, R.; Radhakrishnan, M.; Shanmugasundaram, T.; Gopikrishnan, V.; Balagurunathan, R. Terpenoid bioactive compound from Streptomyces rochei (M32): Taxonomy, fermentation and biological activities. World J. Microbiol. Biotechnol. 2016, 32, 161. [Google Scholar] [CrossRef]
- Townsend, G.R.; Heuberger, J.W. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. Report. 1943, 27, 340–343. [Google Scholar]
- Cortesi, P.; Bisiach, M.; Ricciolini, M.; Gadoury, D.M. Cleistothecia of Uncinula necator—An additional source of inoculum in Italian vineyards. Plant Dis. 1997, 81, 922–926. [Google Scholar] [CrossRef]
- Widholm, J.M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 2009, 47, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Ingham, E.R.; Klein, D.A. Relationship between fluorescein diacetate-stained hyphae and oxygen utilization, glucose utilization, and biomass of submerged fungal batch cultures. Appl. Environ. Microbiol. 1982, 44, 363–370. [Google Scholar] [CrossRef]
- ibidi GmbH. Application Note 33: Live/Dead Staining with FDA and PI; ibidi GmbH: Munich, Germany, 2015. [Google Scholar]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef]
- Romanazzi, G.; Mancini, V.; Feliziani, E.; Servili, A.; Endeshaw, S.; Neri, D. Impact of alternative fungicides on grape downy mildew control and vine growth and development. Plant Dis. 2016, 100, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Islam, M. Bioactive natural products for managing downy mildew disease in grapevine. In Biocontrol of Major Grapevine Diseases: Leading Research; Compant, S., Mathieu, F., Eds.; CAB International: Wallingford, UK, 2016; pp. 125–149. [Google Scholar]
- Kurth, F.; Mailänder, S.; Bönn, M.; Feldhahn, L.; Herrmann, S.; Große, I.; Buscot, F.; Schrey, S.D.; Tarkka, M.T. Streptomyces-induced resistance against oak powdery mildew involves host plant responses in defense, photosynthesis, and secondary metabolism pathways. Mol. Plant Microbe Interact. 2014, 27, 891–900. [Google Scholar] [CrossRef]
- LongXian, R.; Du, S.H.; Li, H.P.; Zhen, Z.X.; Jesús, M.-B. Suppression of grape powdery mildew by Streptomyces and Pseudomonas spp. In Proceedings of the XVII Congreso de la Sociedad Española de Fitopatología, Lleida, Spain, 7–10 October 2014; p. 1. [Google Scholar]
- Buzón-Durán, L.; Martín-Gil, J.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Pérez-Lebeña, E.; Martín-Ramos, P. Antifungal activity of chitosan oligomers–amino acid conjugate complexes against Fusarium culmorum in spelt (Triticum spelta L.). Agronomy 2020, 10, 1427. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Pérez-Lebeña, E.; Martín-Ramos, P. On the applicability of chitosan oligomers-amino acid conjugate complexes as eco-friendly fungicides against grapevine trunk pathogens. Agronomy 2021, 11, 324. [Google Scholar] [CrossRef]
- Dos Santos-Silva, C.A.; Zupin, L.; Oliveira-Lima, M.; Vilela, L.M.B.; Bezerra-Neto, J.P.; Ferreira-Neto, J.R.; Ferreira, J.D.C.; de Oliveira-Silva, R.L.; Pires, C.d.J.; Aburjaile, F.F.; et al. Plant antimicrobial peptides: State of the art, in silico prediction and perspectives in the omics era. Bioinform. Biol. Insights 2020, 14, 1–22. [Google Scholar] [CrossRef]
- Sathoff, A.E.; Samac, D.A. Antibacterial activity of plant defensins. Mol. Plant Microbe Interact. 2019, 32, 507–514. [Google Scholar] [CrossRef]
- Su, T.; Han, M.; Cao, D.; Xu, M. Molecular and biological properties of snakins: The foremost cysteine-rich plant host defense peptides. J. Fungi 2020, 6, 220. [Google Scholar] [CrossRef]
- Ahmad, B.; Yao, J.; Zhang, S.; Li, X.; Zhang, X.; Yadav, V.; Wang, X. Genome-wide characterization and expression profiling of GASA genes during different stages of seed development in grapevine (Vitis vinifera L.) predict their involvement in seed development. Int. J. Mol. Sci. 2020, 21, 1088. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Khoshgoftarmanesh, A.H.; Sharifnabi, B. The effect of foliar-applied manganese in mineral and complex forms with amino acids on certain defense mechanisms of cucumber (Cucumis sativus L.) against powdery mildew. J. Plant Growth Regul. 2018, 37, 481–490. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Pérez-Lebeña, E.; Martín-Gil, J.; Sánchez-Báscones, M.; Martín-Ramos, P. Applications of Streptomyces spp. enhanced compost in sustainable agriculture. In Biology of Composts; Meghvansi, M.A.V., Ed.; Springer Nature: Cham, Switzerland, 2020; Volume 58, pp. 257–291. [Google Scholar]
- Redl, M.; Möth, S.; Koschier, E.; Spangl, B.; Steinkellner, S. Survival and viability of ascospores of Erysiphe necator in Austrian vineyards. Eur. J. Plant Pathol. 2021, 159, 615–626. [Google Scholar] [CrossRef]
- Thiessen, L.D.; Neill, T.M.; Mahaffee, W.F. Interruption and reduction of Erysiphe necator chasmothecia development utilizing fungicidal oil. Plant Health Prog. 2018, 19, 153–155. [Google Scholar] [CrossRef]
- Legler, S.E.; Caffi, T.; Rossi, V. Effect of Different Plant Protection Products on the Sexual Stage of Grapevine Powdery Mildew. In Proceedings of the IOBC/WPRS European Meeting of the Working Group “Integrated Protection and Production in Viticulture, Lacanau, France, 2–5 October 2011; p. 2. [Google Scholar]
- Magarey, P.; Moyer, M. Towards establishing slow input regimes in Australian viticulture 3: Use of ”epi-season” and ”lag phase control” in applying epidemiological knowledge of grapevine powdery mildew, to reduce the number of sprays and inoculum reservoirs for long-term control. In Proceedings of the 6th International Workshop on Grapevine Downy and Powdery Mildew, Bordeaux, France, 4–9 July 2010; pp. 114–116. [Google Scholar]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef]
- Al-Humiany, A.U.-R.A.A. Taificidin1 and Taificidin2, two anti-microbial agents isolated from the fermentation broth of Streptomyces roseodistaticus TA15 and Streptomyces lavendofoliae TA17. Res. J. Microbiol. 2011, 6, 328–342. [Google Scholar] [CrossRef][Green Version]
- Kim, W.S.; Youn, D.J.; Kim, H.R.; Rhee, S.K.; Choi, E.S. Metabolic conversion of aclacinomycins B and Y to A by pH shift during fermentation with Streptomyces lavendofoliae DKRS. Biotechnol. Tech. 1995, 9, 671–676. [Google Scholar] [CrossRef]
- Le Goff, G.; Ouazzani, J. Natural hydrazine-containing compounds: Biosynthesis, isolation, biological activities and synthesis. Biorg. Med. Chem. 2014, 22, 6529–6544. [Google Scholar] [CrossRef]
- Murakami, S.; Harada, S.; Yamazaki, T.; Takahashi, Y.; Hamada, M.; Takeuchi, T.; Aoyagi, T. Piperastatin A, a new selective serine carboxypeptidase inhibitor produced by Actinomycete. I. Taxonomy, production, isolation and biological activities. J. Enzym. Inhib. 2008, 10, 93–103. [Google Scholar] [CrossRef]
- Murakami, S.; Harada, S.; Takahashi, Y.; Naganawa, H.; Takeuchi, T.; Aoyagi, T. Piperastatin B: A new selective serine carboxypeptidase inhibitor from Streptomyces lavendofoliae MJ908-WF13. J. Enzym. Inhib. 2008, 11, 51–66. [Google Scholar] [CrossRef]
- Narayanaswamy, V.K.; Albericio, F.; Coovadia, Y.M.; Kruger, H.G.; Maguire, G.E.M.; Pillay, M.; Govender, T. Total synthesis of a depsidomycin analogue by convergent solid-phase peptide synthesis and macrolactonization strategy for antitubercular activity. J. Pept. Sci. 2011, 17, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, K.; Sugino, F.; Kodama, K.; Ishii, T.; Kinashi, H. Cyclization mechanism for the synthesis of macrocyclic antibiotic Lankacidin in Streptomyces rochei. Chem. Biol. 2005, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Anukool, U.; Gaze, W.H.; Wellington, E.M.H. In situ monitoring of Streptothricin production by Streptomyces rochei F20 in soil and rhizosphere. Appl. Environ. Microbiol. 2004, 70, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Shin-Ya, K.; Furihata, K.; Hayakawa, Y.; Seto, H. New Ravidomycin analogues, FE35A and FE35B, apoptosis inducers produced by Streptomyces rochei. J. Antibiot. 1998, 51, 1105–1108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanini, G.S.; Katsifas, E.A.; Savvides, A.L.; Karagouni, A.D. Streptomyces rochei ACTA1551, an indigenous Greek isolate studied as a potential biocontrol agent against Fusarium oxysporum f. sp. lycopersici. BioMed Res. Int. 2013, 2013, 387230. [Google Scholar] [CrossRef] [PubMed]
- Augustine, S.; Bhavsar, S.; Kapadnis, B. Production of a growth dependent metabolite active against dermatophytes by Streptomyces rochei AK 39. Indian J. Med. Res. 2005, 121, 164–170. [Google Scholar]
- Irdani, T.; Perito, B.; Mastromei, G. Characterization of a Streptomyces rochei endoglucanasea. Ann. N. Y. Acad. Sci. 1996, 782, 173–181. [Google Scholar] [CrossRef]
- Harada, S.; Okada, J.; Takeda, M.; Yamazaki, T. Inclusion compounds of lankacidin-group antibiotics with cyclodextrins. J. Antibiot. 1985, 38, 877–885. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).