1. History of Coffee Leaf Rust Discovery and Dissemination in Brazil
Arabica coffee was introduced in Brazil in 1727 and soon became an important agricultural product intimately linked to the country’s history and economic development. However, this agribusiness was shaken in January 1970 by the first observation of coffee leaf rust (CLR) in Brazil, in the south of Bahia, identified by the Brazilian researcher Arnaldo Gomes Medeiros [
1]. The etiologic agent of CLR is the biotrophic fungus
Hemileia vastatrix, described in 1869 by Berkeley and Broome [
2,
3].
There are two hypotheses to explain the origin of this disease. The first is that it came from cocoa seedlings brought from Africa; the second suggests that it arrived via spores, also from Africa, carried by high-altitude air currents across the Atlantic Ocean [
4]. Until then, Brazilian technicians knew of the disease only through literature references. Therefore, there was substantial fear that CLR would destroy coffee crops in the country, which led the Brazilian government, through the Brazilian Coffee Institute and the Ministry of Agriculture, to promote expert visits from abroad to assess the problem; in particular, from the scientist Branquinho d’Oliveira from the Coffee Rusts Research Center (CIFC, Centro de Investigação das Ferrugens do Cafeeiro), located in Oeiras, Portugal.
Considering the gravity of the problem, the Brazilian government soon established a series of control measures and implemented a coffee leaf rust control program. The first action was to diagnose affected areas for the eradication of disease outbreaks. The survey began in Bahia by establishing an approximately 50-km-wide safety strip to separate infected areas from the major producing regions located further to the south. All coffee plants found in the infected areas were eradicated. Simultaneously, information campaigns on CLR were conducted to identify new disease outbreaks that were initially eradicated by cutting and burning the infected trees. Subsequently, a defoliating agent (Paraquat) or eradicating fungicide (Pyracarbolid) was applied depending on the case [
4].
Despite efforts to eradicate and isolate CLR foci and prevent its expansion, the disease spread rapidly throughout the country. In a short time, CLR was reported in the most important Brazilian coffee regions, such as the south of Minas Gerais in June 1970 and São Paulo in January 1971. At that time, adapted traps in airplanes detected the presence of H. vastatrix spores up to 1000 m above ground level, revealing wind action as a spreading agent of CLR over long distances.
After verifying that the policy of eradicating and isolating rust outbreaks was unfeasible, a program of coexistence with CLR began, based on research, technical assistance, and targeted financial aid. Research institutions have focused on two main control strategies. The first is chemical control, which is an attractive short-term strategy. Most Brazilian coffee crops are planted at relatively high altitudes and have distinct dry seasons, making them more suitable for chemical control than coffee crops in Africa, Asia, and the Pacific. The second major control strategy is genetic breeding.
Soon after the initial control attempts, Monaco [
5] postulated that rust control is more an economic issue than a technical one because of the availability of efficient chemical control methods. Thus, a compromise between disease control and coffee production was established in Brazil, which led to the elimination of low-yield crops and the onset of coffee crop-integrated pest and disease control management routines. First, the importation of fungicides increased, and spraying equipment adapted to Brazilian coffee-growing conditions was developed. The recommendation was four sprays of 5 kg of copper oxychloride per hectare, which required approximately 50,000 tons of fungicide to protect the entire coffee crop at that time. This resulted in an additional cost of more than 200 million USD in capital investment and labor costs.
Thus, regional research centers were installed, technical assistance networks, building infrastructure, and personnel training were expanded, a coffee-growing agroclimatic zoning was established, and technical and economic studies with new management practices for CLR control were developed. In 1969, the “Coffee Crop Renewal and Reinvigorating Plan” was created to promote new coffee plantations, which ran until 1980. The renovation was financed and technically assisted for almost two billion coffee trees.
The new crops incorporated major technological improvements, developed and adapted by research, and were made available to producers through regional technical assistance, with emphasis on the adoption of zoned areas for new planting, new planting spacing, new cultivars, more rational and technical management, and the introduction of systematic control of coffee pests and diseases within an integrated management program. The aim was to improve productivity and reduce production costs.
Although CLR is the main disease influencing the coffee culture in Brazil, dissemination of the disease had positive impacts on Brazilian coffee crops because it boosted modernization through the generation and adoption of new technologies, as well as more efficient management practices, resulting in more competitive coffee production and expansion into new production areas. At the beginning of the 1980s, Brazil had a coffee tree stand comprising 3.4 billion individuals, which was 55% larger than that in 1970, with an average harvest of 25 million 60-kg green coffee bags, representing an annual increase of approximately five million bags from the 1970 harvest [
6]. This increase in production occurred despite the serious damage caused by the severe frost of 1975, which reduced the 1976 coffee harvest to just six million bags. In the following decades, Brazilian coffee-growing farms continued to expand and increase their productivity owing to new technologies. Currently, Brazil produces approximately 45–65 million 60-kg bags of green coffee per year, in an area of approximately 2.2 million hectares, with an average yield of 32.18 bags of green coffee/ha for Arabica and 38.78 bags/ha for Robusta [
7]. The country is internationally recognized for its ability to produce coffee in large volumes and at competitive prices, but, as of recently, it also stands out as a prestigious origin of specialty coffees. Brazil is also the world’s second largest consumer of the beverage, with an estimated consumption for 2021 of 23.53 million bags (14.4% of world consumption), surpassed only by the United States of America (16%) [
8,
9].
In Brazil, CLR can causes losses of up 50% in coffee production, depending on the level of resistance of the cultivar, favorable climatic conditions to the disease, and management measures [
1], and in other coffee producing countries, such as Mexico, Colombia, Costa Rica, and El Salvador, the coffee yield losses by CLR are also quite significant [
10,
11,
12,
13].
2. Characterization of Rust in Coffee
The genus Hemileia is a member of the phylum Basidiomycota, class Pucciniomycetes, and order Pucciniales. The name Hemileia reflects the characteristic half-smooth (half-rough) morphology of urediniospores, which helps dispersal and infection.
H. vastatrix urediniospores are reniform (28–36 × 18–28 µm), covered with a hyaline wall that is warted on the convex face and smooth on the straight or concave face, and is 1 µm thick [
3,
14,
15].
The first CLR symptoms are small, pale yellow spots, which gradually increase in diameter, preceding the differentiation of orange-colored uredinia on the lower leaf surface (
Figure 1). During severe rust infection, the leaves become covered with pustules, inducing them to fall prematurely, which reduces plant photosynthetic area. Repeated infections debilitate the plant and can cause branch dieback [
16,
17].
More than 50 physiological races of
H. vastatrix have been identified worldwide [
1,
3,
18,
19], 15 of which have been identified in Brazil, where the predominant race is II [
20,
21,
22,
23,
24].
H. vastatrix physiological races are identified according to their spectra of virulence on a set of 23 coffee differentials [
3,
25,
26]. A differential clone is classified as susceptible if uredospores are formed in a pustule of an inoculated coffee leaf or leaf discs [
27]. These differential clones were identified by CIFC and sent to several research institutions in other countries [
1,
3,
28].
The epidemic of CRL is mainly influenced by temperature and humidity. Urediniospores of
H. vastatrix germinate only when there is leaf wetness for 6 to 24 h [
29]. The temperature range of 21–25 °C and absence of light favors germination [
30,
31,
32], while germination is inhibited in temperatures above 32.5 °C and below 12.5 °C [
33,
34].
The temperature also influences the latency period. The common period of latency lasts 20 to 55 days (commonly lasting 25 to 35 days), but is significantly extended when temperatures are higher than 28 °C or lower than 18 °C [
2,
35]. The crop side exposed to the sun in the morning exhibits less CLR infection because the leaf wetness period is reduced. On the other hand, leaves located in the lower and middle third of the coffee trees have higher infection levels than the outer leaves and those in the upper third because of a more humid microclimate. This moistened environment with greater disease intensity also occurs in denser planting systems, shaded or wooded areas, plants with excess stems, and more closed crops [
36]. Another aspect that influences CLR incidence and severity is the plant nutritional status. The incidence involves estimating only the proportion of diseased leaves in a plant; in contrast, severity refers to the area of plant tissue affected by diseases based on lesion counts or descriptive scales. Unbalanced nutrition plants are more affected by CLR and become more susceptible because of greater physiological wear [
17].
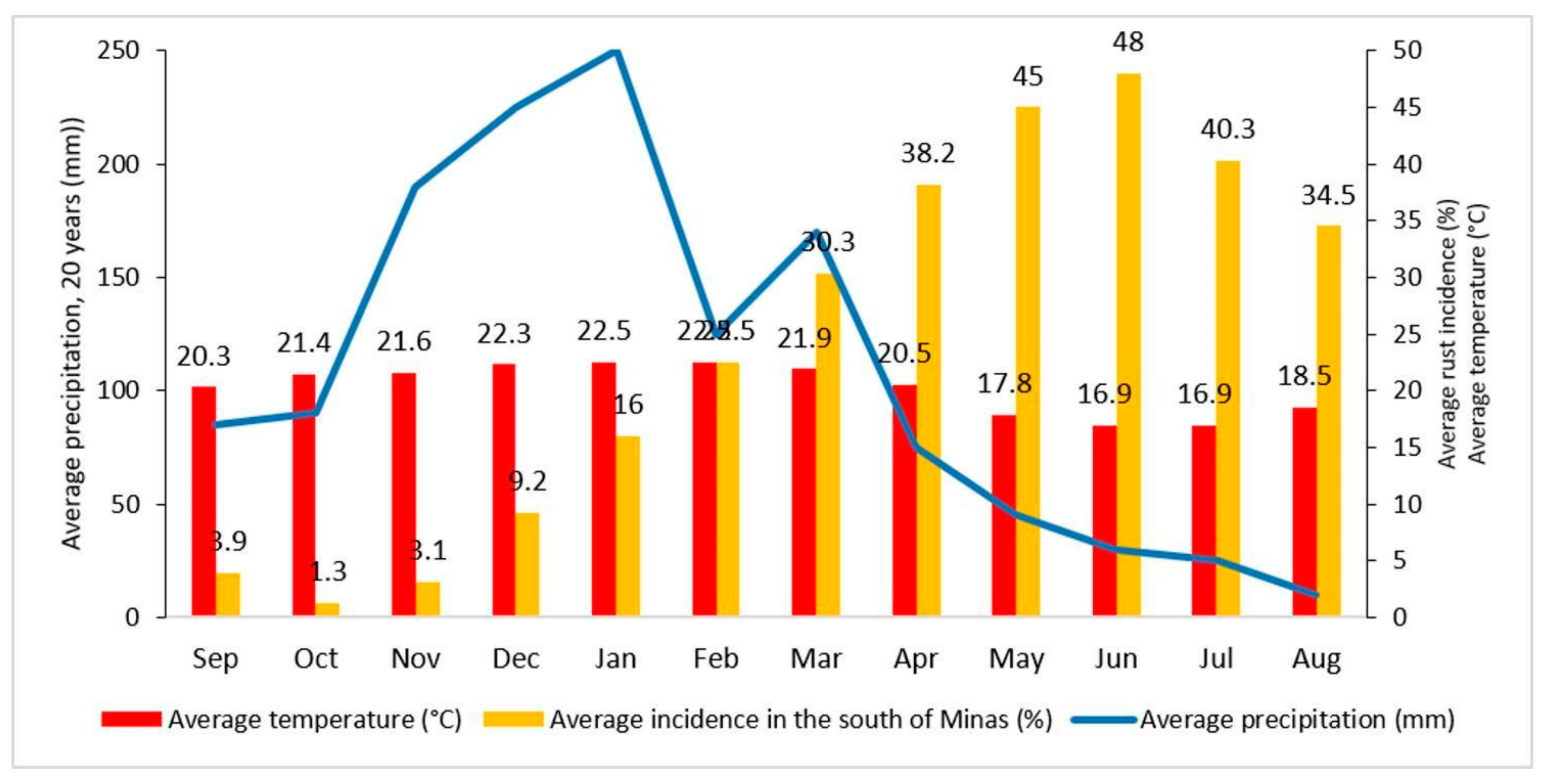
In most Arabica Brazilian coffee-growing regions, the main rainy season begins in spring in September–October, reaches a peak in December–January, then starts to decrease to minimum precipitation values in winter between June and August [
37]. Temperature fluctuations follow a similar pattern to precipitation (
Figure 2). The main flowering stage typically occurs during the months of September and October, when the greatest vegetative growth of coffee trees also begins. Bean filling takes place from January to March, and the main harvest season is between May and July. CLR incidence starts to increase after the rainy season in November, gradually escalating until it reaches a maximum in late autumn in June. Substantial leaf fall occurs because of damage caused by CLR and harvesting operations, leaving the coffee trees with few leaves until the beginning of the next rainy season. From September to November, the plant infection level decreases and remains low until the residual inoculum restarts a new cycle in November–December.
According to the growing conditions of Brazilian coffee culture, coffee trees grow in full sun and exhibit biennial bearing cycles, with alternating years of high and low harvest. Regardless of whether the year has a high or low crop load, the CLR incidence curve shows the same behavior, albeit with higher infection levels in years of high load [
1].
3. Genetic Breeding of Arabica Coffee for CLR Resistance in Brazil
Genetic breeding of coffee to obtain rust-resistant cultivars began in Brazil in 1954, in a partnership between the Agronomic Institute (IAC) and the CIFC [
38]. Pioneering studies conducted at the CIFC on the genetics of CLR resistance identified physiological races of the pathogen and helped national programs develop resistant cultivars. After the arrival of rust in the 1970s, other institutions responsible for technological development and coffee research in Brazil initiated genetic breeding programs aimed at developing new cultivars with resistance to the disease, namely Epamig (Agricultural Research Company of Minas Gerais)/UFV (Federal University of Viçosa)/UFLA (Federal University of Lavras), the Procafé Foundation (ex-IBC), IDR-Paraná (Institute of Rural Development of Paraná, ex-Iapar), and Embrapa Café, all of which were supported by the Research Café Consortium, coordinated by the Brazilian Agricultural Research Corporation (Embrapa Café).
3.1. Resistance Levels of Coffee Genotypes
CLR resistance can be qualitative/vertical or quantitative/horizontal. Qualitative resistance occurs because of the action of genes with a major effect on resistance (major genes), called
SH genes, which promote a high level of resistance in their homozygous state [
18,
39,
40]. Quantitative-type resistance occurs because of secondary-effect genes (minor genes), which promote intermediate levels of resistance [
40,
41]. In the interaction between CLR and coffee, the physiological races of
H. vastatrix attempt to overcome coffee-resistance genes. If the race does not have the virulence gene (
v) that breaks the resistance of the respective
SH gene, a high resistance reaction occurs without the appearance of sporulation symptoms. When a v gene (new race) emerges that can break the resistance of the respective
SH gene, the plants still manage to defend against rust, probably through minor genes that promote intermediate resistance levels
Coffee resistance levels to CLR can be classified as highly resistant (HR), moderately resistant (MR), slightly resistant (SR), susceptible (S), or highly susceptible (HS) depending on the plant reaction type to the pathogen, symptoms, and disease intensity (
Table 1). Plants with HR and SR levels are commonly termed immune and moderately susceptible, respectively, by other authors. The HR level is governed by qualitative-type resistance genes; however, cultivars classified at this level generally also have quantitative resistance genes. MR, SR, S, and HS levels are related to quantitative resistance. The HR level is also referred to as complete resistance, and the MR and SR levels are generically termed incomplete, intermediate, or partial resistance.
3.2. Sources of Qualitative Resistance
Known qualitative genes of CLR resistance include
SH1,
SH2,
SH3,
SH4,
SH5,
SH6,
SH7,
SH8,
SH9, and
SH? [
18,
25,
42]. The main gene sources used in Brazil to promote high qualitative resistance are coffee trees from the Híbrido de Timor (HdT) and its derivatives (e.g., Villa Sarchi × HdT, Caturra × HdT, Catuaí × HdT), Icatu and its derivatives (e.g., Icatu × Catuaí, Icatu × Catimor, Icatu × Sarchimor), coffee trees from the BA series carrying the
SH3 gene and its derivatives (e.g., Catuaí × BA-10), and wild Arabica coffee trees from Ethiopia, including landraces such as Geisha. Recently, Barka et al. [
43] and Almeida et al. [
44] found two different genes in HdT 832/1 and HdT 832/2 using molecular biology techniques, which were named
SH10 and
SH11, respectively.
Two hundred and fifty-five
H. vastatrix isolates were collected in Brazil, Honduras, Venezuela, and Costa Rica from 2018 to 2020 from HdT derivatives that had lost their resistance [
1]. In this study, the term pathotype was proposed to refer to
H. vastatrix isolates that could not be differentiated into races according to the methodologies and the set of coffee differentials used at CIFC. Ten
H. vastatrix isolates (Hv01 to Hv10) were identified in Brazil, three of which, Hv01 (
v1,
5,
6,
7,
8,
9, and
?), Hv02 (
v1,
5,
6,
8,
9, and
?), and Hv08 (
v1,
2,
5,
6,
7,
8,
9, and
?), did not infect HdT 832/1, HdT 832/2, or the hybrid Kawisari 644/18, respectively, suggesting that these genotypes have more resistance genes in their genomes. Twenty differentiating hosts were used to distinguish the 10 Brazilian pathotypes; however, they could not differentiate the isolates into races. Nevertheless, these hosts were particularly important for distinguishing the 10 pathotypes as pathogen genome recognition depends on the interaction between pathogen isolates and the differentiating hosts.
Coffee trees derived from HdT crossings were important sources of CLR qualitative resistance in Brazil because they contain the genes
SH5,
SH6,
SH7,
SH8,
SH9, and
SH?, either individually or in association [
42]. The main Brazilian HdT coffee trees are HdT CIFC 832/1, HdT CIFC 832/2, and HdT CIFC 2570, which are crossed with Caturra Vermelho CIFC 19/1 (giving rise to the Catimor group), Villa Sarchi CIFC 971/10 (originating from the Sarchimor group), and cultivars from the Catuaí group (
Table 2 and
Table 3). It is noteworthy that Catimor and Sarchimor are cultivars developed directly from progeny selections from the original crossings, as well as from crossings with descendants of these original crossings. However, the resistance of some of these cultivars has subsequently been overcome and attacked by isolates and pathotypes from the American continent.
Icatu originated from an artificial cross, developed at the IAC, between the doubled-chromosome species
Coffea canephora var. Robusta, and
Coffea arabica var. Bourbon Vermelho, with two more backcrosses of this hybrid with the
C. arabica cultivar Mundo Novo [
45]. In addition to Icatu, the IAC developed a coffee tree called “Dwarf Icatu” which was crossed with Mundo Novo and then with Catuaí Amarelo. Most cultivars of the Icatu group commercially released in Brazil have already had their qualitative resistance broken by races of
H. vastatrix present in Brazilian crops and currently exhibit SR levels, with the exception of the cultivar Icatu Amarelo IAC 2944 (
Table 3).
The spontaneous hybridization between Icatu Vermelho and Catuaí, called Catucaí, has led to several Brazilian cultivars already having their CLR qualitative resistance broken, with most of these currently classified as SR (
Table 3). Dwarf Icatu was crossed with Catuaí at the IAC, which produced the cultivars IPR 102 and IPR 103, both with their qualitative resistance already broken. However, in some places, IPR 102 has an HR level, indicating that it carries
SH genes lacking in IPR 103. Although the qualitative resistance is already broken in these coffee trees derived from Icatu × Catuaí and Dwarf Icatu × Catuaí, these genotypes carry
SH genes that can be used in breeding programs.
The genes SH1, SH2, SH4, and SH5 have already been supplanted by CLR in several coffee regions, and cultivars from the Sarchimor group generally exhibit high rust resistance in most coffee regions. Therefore, because of the large diversity of H. vastatrix races, some cultivars exhibit HR levels in one region and SR in others. For example, the cultivar Obatã (Sarchimor × Catuaí) exhibits HR in several places in Minas Gerais, but is classed as SR in parts of the states of Paraná and São Paulo. Thus, it seems that SH? is one of the single resistance factors of HdT and Icatu coffee trees that has not yet been broken in Brazil. Although the resistance of SH1, SH2, SH4, and SH5 genes in wild Arabica coffee trees from Ethiopia has already been broken by CLR in Brazil, these genotypes are important in gene pyramiding for durable resistance.
The physiological races of
H. vastatrix that possess the
v3 virulence gene have not yet been detected in Brazil [
26,
46]; therefore, cultivars carrying
SH3 are classified as HR. Among several coffee trees from the series BA, which are
SH3 carriers, BA-10, also called IAC 1110, is the main tree used in Brazil. In addition to BA-10, other coffee trees from this series are also used, such as BA-2, BA-8, BA-13, BA-14, BA-16, and BA-21, designated IAC 1111, IAC 1109, IAC 1112, IAC 1106, IAC 1116, and IAC 1107, respectively.
3.3. Sources of Quantitative/Horizontal Resistance
The manifestation of coffee tree quantitative/horizontal resistance becomes evident when the
H. vastatrix races succeed in breaking the qualitative/vertical resistance of
SH genes. Different levels of quantitative resistance were observed because of the minor genes. Incomplete or intermediate resistance caused by minor genes has been identified in HdT and Icatu plants [
40], as well as in
C. arabica and
C. canephora varieties [
47,
48]. The most common resistance levels in coffee trees, promoted by these minor genes, are SR and MR. However, even coffee trees known to be susceptible (S) worldwide, such as cultivars from the Catuaí group, seem to have minor genes that promote quantitative resistance because they are less susceptible than cultivars from the Bourbon group. Even HS cultivars, such as those from the Bourbon group, probably have quantitative resistance genes, but with no significant reduction in disease intensity. The term “intensity”, which involves attributes of incidence and severity, was used here as a general characterization measure of disease in a specific area.
Ethiopian wild coffee trees, HdT, Icatu, and their derivatives are also important sources of quantitative resistance at the SR and MR levels, and the Brazilian BA series coffee trees seem to have few quantitative resistance genes, because when their progenies lose the SH3 gene, they are then classified as susceptible. Intermediate resistance caused by the action of minor genes in wild Ethiopian accessions and their derivatives is more common in plants carrying SH1 and SH4. Plants carrying SH2 and SH5 seem to have fewer minor genes because they are generally susceptible when their qualitative resistance is broken.
3.4. Breeding Methods and Strategies Used in Brazil for CLR Resistance
In Brazil, the breeding methods normally used for transferring CLR resistance genes are pedigree, bulk, and backcrosses. In the first two methods, initial crossings occur between parents with different qualitative or quantitative resistance genes, whereas backcrossing aims to transfer one or two major genes. The pyramiding of qualitative and quantitative SH genes in a single genotype represents an improvement strategy aimed at developing cultivars with durable resistance to CLR. Pyramiding of qualitative genes can be performed through pedigree, bulk, and backcrosses, whereas quantitative genes use the first two methods.
The development of molecular markers associated with major SH genes assists breeding programs with pyramiding these genes. Marker-assisted selection associated with SH3 is already being used in breeding programs in Brazil. Further development of molecular markers associated with minor resistance genes will allow the pyramiding of these genes, even by the backcrossing method.
Immediately after CLR entered Brazil, great emphasis was placed on developing resistant cultivars from the Catimor, Sarchimor, and Icatu groups, as well as from the Icatu and Catuaí crossings, called Catucaí. The first rust-resistant cultivars were released for commercial cultivation during the 1980s and the 1990s [
49]. Shortly after commercial cultivation, a break in resistance began to appear in several cultivars, mainly those of the Catimor and Icatu groups and, to a lesser extent, in the Sarchimor group. In many cases, the resistance breakdown was incomplete and partial resistance remained, likely as a result of the action of minor genes. Therefore, genetic breeding programs have sought to overcome this resistance breakdown by adopting several strategies.
Considering that quantitative resistance is more durable than qualitative resistance, Brazilian breeders started to develop cultivars that combined different minor genes and were aimed at more durable resistance. Preferably, current breeding programs aim to develop HR and MR cultivars, originating from quantitative resistance genes, because they can provide high durability and eliminate or reduce fungicide applications to control CLR. HR and MR cultivars are being developed from minor genes by crossing SR and SR, SR and MR, and MR and MR coffee trees.
HdT coffee trees and their derivatives originate from a different C. canephora tree to that used to develop Icatu. For this reason, the combination of these two resistance sources has been used to increase the number of minor genes and generate trees with a higher resistance level, such as MR. Wild accessions from Ethiopia have likewise been crossed with Icatu, HdT, and their derivatives to combine different minor genes and increase the resistance level. In Brazil, the different crossing types made with this objective are Sarchimor × Icatu, Catimor × Icatu, Sarchimor × (Icatu × Catuaí), and Sarchimor × wild coffee trees from Ethiopia. Normally, when there is a qualitative resistance breakdown of Sarchimor, Icatu, Icatu × Catuaí coffee trees, and accessions of wild coffee trees from Ethiopia, the resistance level decreases from HR to SR and MR.
When a coffee tree is selected based only on its qualitative resistance, without considering the quantitative resistance covered by the former, genetic erosion of the minor genes may occur. A cultivar with few alleles of quantitative resistance that experiences qualitative resistance breakdown displays only partial resistance or susceptibility caused by a decrease in the rust resistance level. For example, the cultivar Oeiras, which belongs to the Catimor group, exhibited an HR level when released for commercial cultivation in the 1990s; however, after a few years, it lost its qualitative resistance and was reclassified as SR. A similar situation occurred with the cultivar IPR 100 carrying the SH2 and SH5 genes; upon the resistance breakdown of these two genes in the 1980s, the cultivar resistance dropped to S level, as did Catuaí.
To avoid this quantitative resistance loss caused by qualitative resistance selection, breeders should cross coffee that has major and minor genes, selecting HR coffee trees and intermediate resistance plants such as MR plants. Considering only the qualitative resistance, Brazilian breeders intensify crosses between SH3-carrying coffees with HdT and Sarchimor genotypes carrying the SH5, SH6, SH7, SH8, SH9, and SH? genes. Moreover, the latter genotypes have also been crossed with wild Arabica coffee trees from Ethiopia that carry the SH1, SH2, and SH4 genes, alone or in combination. The use of these genotypes allows the simultaneous transfer of minor genes aimed at intermediate resistance in case of qualitative resistance breakdown.
3.5. Cultivars with CLR Resistance Developed in Brazil
Of the 138 cultivars registered in Brazil, 23 are HR because they do not have their major genes supplanted by the physiological races present in Brazilian crops. Ten cultivars are HR in some coffee regions but MR or SR in others because their major genes have been supplanted; however, their minor genes act to promote different levels of intermediate resistance. In general, the Brazilian cultivars that are still HR in Brazil are those of the Sarchimor, Catuaí × HdT groups, and BA-10 derivatives carrying
SH3 (
Table 2). Of the cultivars derived from HdT, 12 have intermediate resistance levels (SR and MR) in all coffee regions of Brazil (
Table 3), and MGS Aranãs and IPR 108 exhibit major and minor resistance genes from HdT and Icatu.
Crossings between HdT CIFC 832/1 and Caturra Vermelho CIFC 19/1 have given rise to cultivars of the Catimor group named Oeiras MG 6851 and Katipó, which contain SH genes and were previously HR but currently MR and SR. Crossings between HdT CIFC 832/2 and Villa Sarchi CIFC 971/10 have resulted in the cultivars of the Sarchimor group named IAC 125 RN, IAPAR 59, IPR 97, IPR 98, IPR 104, Sarchimor MG 8840, IPR 99 and Tupi IAC 1669-33, which are HR throughout Brazil, except for the last two, which exhibit MR and SR levels in some locations. Subsequently, other cultivars of the Sarchimor group have been released from artificial or spontaneous hybridizations between Sarchimor and Catuaí or Mundo Novo; these exhibit greater productive potential and vegetative vigor than Sarchimor, and some still remain HR. Even if some Sarchimor cultivars, such as Tupi IAC 1669-33 and IPR 99, start to lose their high resistance in some places in Brazil, the chance of a qualitative resistance breakdown is much greater for cultivars of the Sarchimor group crossed with susceptible coffees such as Catuaí and Mundo Novo. The cultivar Obatã IAC 1669-20 is derived from spontaneous hybridization between Sarchimor and Catuaí, whereas Arara and IAC Obatã 4739 originate from spontaneous hybridization between Obatã IAC 1669-20 and Catuaí Amarelo; therefore, they exhibit two crossings with the susceptible coffee Catuaí. Like Tupi IAC 1669-33, these last three cultivars are HR in several regions of Brazil, but MR and SR in some locations with more virulent races of H. vastatrix. The cultivars Acauã, Acauãma, IPR Alvorada, Acauãnovo, Asabranca, Graúna, IPR 107, and IPR Pérola originate from Sarchimor × Mundo Novo; however, the first three are HR in several regions and MR and SR in other localities, whereas the last five are HR throughout Brazil. By crossing the Catuaí group with HdT CIFC 2570, EPAMIG and partners developed 10 cultivars named Araponga MG1, Catiguá MG1, Catiguá MG2, MGS Ametista, MGS Catiguá 3, MGS Paraíso 2, MGS Turmalina, Paraíso MG H 419-1, Pau Brasil MG1, and Sacramento MG1, all of which are HR throughout Brazil except Araponga MG 1, which is MR and SR in some locations, and MGS Paraíso 2 and MGS Turmalina, which are MR and SR in almost all locations.
In Brazil, 15 cultivars from the Icatu group have been released, 13 by the IAC, one by the Procafé Foundation, and one by the IDR-Paraná (
Table 3). In general, all 15 Icatu cultivars exhibit intermediate resistance to CLR at the SR level, except for Icatu Amarelo IAC 2944, which is MR. The MR reaction varies minimally according to the favorable environmental conditions for CLR, probably because this cultivar has more minor genes for resistance than SR cultivars.
From the spontaneous crossing between Icatu Vermelho and Catuaí, 19 cultivars emerged from the Procafé Foundation. These cultivars are typically SR and sometimes S. The exception is the Azulão cultivar, which is MR. The cultivars released by IDR-Paraná, called IPR 102 and IPR 103, are derived from the crossing between Dwarf Icatu and Catuaí and exhibit MR and SR levels, respectively. Depending on the races present at the site, IPR 102 may behave as an HR cultivar because some major genes have not yet been supplanted by CLR (
Table 3).
The three HR Brazilian cultivars derived from
SH3-carrying coffee genotypes are IAC Catuaí SH3, IPR 105, and Saíra, which were registered by the IAC, IDR-Paraná, and Procafé Foundation, respectively. The first two possess
SH3 originating from BA-10 (IAC 1110-8), whereas the last one originates from Catindu UFV 374 cv 643. The cultivar IPR 100 is derived from BA-10 and is rust-susceptible because of the lack of
SH3 (
Table 2). Cultivars from the Mundo Novo and Catuaí, and six other cultivars from Catuaí × Mundo Novo groups (e.g., IAC Ouro Verde, MGS Epamig 1194, Maracatiá, MGS Travessia, Rubi MG 1192, Topázio MG 1190) are S, whereas those from Bourbon are HS.
3.6. Distribution of Resistant Cultivars in Brazil
Currently, approximately 80% of Brazilian coffee plantations consist of CLR-susceptible cultivars from the Catuaí and Mundo Novo groups. With the introduction of new CLR-resistant cultivars in the 1990s, rural producers slowly began planting them. Between the 1990s and the 2000s, there was little compliance with HR cultivars from the Sarchimor group, which, despite having high productive potential, typically exhibit weaker vegetative vigor if inadequately managed in terms of nutrient and water supplies. From the 2000s onwards, new CLR-resistant HR cultivars with better vegetative vigor originating from Sarchimor × Catuaí, Sarchimor × Mundo Novo, derivatives from BA-10, and Catuaí × HdT were released. At the same time, SR and MR cultivars originating from Icatu × Catuaí, Dwarf Icatu × Catuaí, and Icatu × Catimor were released. All of these new cultivars are dwarf cultivars that were increasingly adopted because of other desirable traits in addition to CLR resistance, such as high productivity combined with stronger vegetative vigor, excellent cup quality, larger grain size, resistance to diseases such as Phoma spp. and bacterial halo blight, resistance to the nematodes Meloidogyne exigua, M. paranaensis, and M. incognita, and higher resistance to drought.
Despite the low percentage of CLR-resistant cultivars in the Brazilian coffee tree sector, the adoption of resistant cultivars has intensified in the last 10 years. Nevertheless, new cultivars are normally chosen for their additional interesting characteristics to the producer.
4. Chemical Management for Rust Control
The chemical control of CLR in Brazil has been simplified and rationalized in over 50 years of living with the disease, exhibiting both efficiency and a good cost–benefit ratio. Therefore, selection of the correct fungicides, dosage, time, and spraying technology must be observed. Typically, Brazilian coffee farmers that use advanced technology to obtain higher yields, above the moving average of 30 60-kg bags of green coffee/ha in areas without irrigation, manage to keep rust levels below at 5% incidence. Thus, suitable management techniques are required [
50]. Among these techniques, chemical control is currently the best way to guarantee food security in addition to social, financial, and environmental sustainability, where control is typically based on crop monitoring, decision support, or forecasting systems that help farmers apply the correct fungicide dosage at the ideal time, interrupt the host-pathogen relationship cycle, and reduce the disease progress rate.
As the different Brazilian biomes possess varied edaphoclimatic conditions and relief forms, chemical control must be adjusted to these characteristics. For example, in hilly regions with steep slopes, mechanization and spraying are more difficult to apply, making chemical control less efficient. For these reasons and others, CLR is still a problem for the Brazilian coffee-growing sector, causing significant losses for producers who do not adopt the available and efficient technologies for rust control. It is noteworthy that the application technology is as important as the choice of fungicide. Currently, contact, mesostemic, and systemic fungicides are available for the chemical control of CLR in Brazil [
17].
4.1. Contact Fungicides
Contact fungicides are protective fungicides from the cupric and dithiocarbamate chemical groups. The cupric chemical group includes several molecules, such as copper hydroxide, copper oxychloride, copper sulfate, and cuprous oxide, which are still available in different formulations [
51]. The first two are the most commonly used molecules in the Brazilian coffee-growing sector. These fungicides can help control CLR, especially in years with low crop load or productivity, in addition to contributing to rotation of the active principles of fungicides used in coffee growing, thereby avoiding the selection of resistant pathogens to systemic and mesostemic fungicides [
2,
17]. Among the various products and formulations offered to producers, one of the most important is the amount of metallic copper or Cu
2+ present in the formulation [
52]. In other words, it is necessary to have a charge, in this case, a positive one, to bind sites capable of making metabolism unfeasible and thus killing the pathogen. This information is usually found in the fungicide package insert. The Cu
2+ concentration in the tank mix must be greater than 1200 ppm to efficiently kill the fungus. In other words, a suspension with 1000 L of water must contain 1.2 kg of the active ingredient Cu
2+.
Concerning contact or protective fungicides, it is extremely important to maintain good spraying equipment and invest in application technology so that spraying can provide good coverage on both sides of the coffee leaves. Adhesion caused by adjuvants or the product formulation is essential to estimate the interval between the sprays of protectors, especially under Brazilian conditions, whereby rainfall is distributed throughout the summer months [
53].
Moreover, copper solubilization can be slow depending on the solubility of the applied product. This is important for ensuring that plants do not absorb high amounts of metallic copper, thereby avoiding phytotoxicity. Although fungicides have copper concentrations of more than 1200 ppm, the copper is not absorbed by the plant in these amounts. Thus, copper is frequently found in Brazilian crops, with sequential copper sprays and concentrations of more than 150 ppm (mg/kg) in the leaves. This concentration can cause phytotoxicity symptoms; therefore, Cu2+ concentrations should ideally remain below 70 ppm (mg/kg) in the leaves, as in most cultivars planted in Brazil, with a moving average of production between 30 and 35 60-kg bags of green coffee/ha in non-irrigated areas.
The particle size of copper products is another variable to consider because it is inversely proportional to the contact area. However, an effective spray on both leaf sides using volume at an ideal pH and without precipitates, reaching the greatest possible extension of the tree’s canopy or its leaf area, may compensate for the particle size. Most existing commercial products have different particle sizes and are distributed in different amounts.
Beyond copper molecules of conventional sizes, nanoparticles can contribute to reducing the amount of metallic copper applied per hectare because of their increased contact area with the leaf, which provides better coverage. This reduction is in line with new global laws implemented to minimize the impact of metallic compounds in agriculture in several countries. Nanoparticle concentrations currently vary from 250 to 500 ppm (mg/kg) with 100% Ag and Cu nanoparticles in the size range of 20–30 nm.
Copper chelates associated with ethylenediaminetetraacetic acid, amino acids, and nitrates, among others, are available on the market in various formulations. In most cases, as the concentrations of metallic copper are below 300 ppm (mg/kg), its effect is both nutritional and resistance inducing. Therefore, these chelates must be applied more than once to plants in good nutritional status and conditions to produce compounds such as adenosinetriphosphate and adenosinediphosphate (energy), amino acids, and micronutrients, as well as products for the shikimic acid and jasmonic acid routes, which in turn produce resistance products such as lignin and phenols. Chelates rarely show direct toxicity to the pathogen, depending on the formulation. Some should be sprayed during the flowering season, whereas other formulations mixed with fungicides should be applied during the rainy season in Brazil, i.e., the tropical summer. These chelates can exhibit high absorption, making Cu2+ readily available; therefore, they should not provide large amounts of the element to avoid phytotoxicity.
Mancozeb is a protective fungicide from the dithiocarbamate chemical group. It is also a multisite fungicide used in fungal resistance management when combined with triazoles, carboxamides, and strobilurin fungicides. Its formulation also contains Zn and Mn, which are capable of contributing to coffee tree nutrition. Moreover, triple mixtures with triazoles and strobilurins are already available on the market.
The spraying of contact fungicides should be performed effectively by seeking to cover both the abaxial (lower) and adaxial (upper) surfaces of the leaves. H. vastatrix penetrates through the stomata on the abaxial side of the leaf; therefore, it should be thoroughly covered by the product to prevent pathogen penetration. The volume spraying for adult coffee trees must be at least 200 L ha−1 (for plants up to 1.5 m high) and up to 500 L ha−1 (for plants heights of 3.5–4 m), depending on the plant size, foliage, and planting and cultivation density. As the cultivars developed in Brazil are grown in different biomes, they possess different canopy architectures, internode lengths, tree heights, leaf sizes, and intensity of plagiotropic branching, which can interfere with spraying efficiency. Therefore, it is particularly important to consider the syrup volume. Taller plants with larger canopy diameters, closed-canopy architectures, and a higher ramification intensity of plagiotropic branches require a higher syrup volume. Additionally, spray nozzles must be in good condition so that the correct syrup volume can be applied according to the recommendation so that the product can reach the leaves uniformly on both surfaces.
4.2. Mesostemic Fungicides
The mesostemic fungicide group includes strobilurins with the following molecules, in alphabetical order: azoxystrobin, metominostrobin, picoxystrobin, pyraclostrobin, and trifloxystrobin. They are usually mixed with triazoles or carboxamides, or both, creating mixtures of systemic and mesostemic fungicides (MixSM). For better effects, it is recommended to combine their application with adjuvants, such as mineral and vegetable oils, which are capable of diminishing the wax layer, breaking the surface tension of the superficial cuticle of leaves, flowers, branches, and fruits, and providing absorption in lethal doses to pathogens.
4.3. Systemic Fungicides
Systemic fungicides include the chemical groups of triazoles, strobirulin, and carboxamides for the control of CLR and other coffee pathogens in Brazil [
17]. In alphabetical order, the triazole molecules currently used include cyproconazole, epoxiconazole, flutriafol, tebuconazole, and triadimenol, whereas the carboxamide molecules are benzovindiflupir, fluxapyroxade, and thifluzamide, among others. These fungicides can be applied via foliar application and are also recommended to be combined with strobilurins; triple mixtures with contact products also exist in this formulation.
In the soil, triazoles are applied via drenching or squirting under the tree canopies, either in isolation or mixed with insecticides, at the beginning of the rainy season between October and November, following technical recommendations. It is important to monitor the climate in different Brazilian edaphoclimatic conditions and biomes because the ideal moment for application is when the soil is moistened and more able to absorb these products via roots. Currently, this soil application method must be combined with foliar sprays to control CLR in high-productivity systems in Brazil. Furthermore, producers and technicians should always be aware of the tree size to avoid under- or overdoses and prevent inefficient treatment or phytotoxicity, depending on the number of branches and leaves in the tree canopy of the different cultivars in different cultivation systems.
The efficiency of triazoles mixed with strobilurins is indisputably higher than that of contact fungicides in production systems with moving averages greater than 30 60-kg bags in dryland and 40 60-kg bags of green coffee/ha in irrigated crops. However, contact fungicides are essential as multisite fungicides in managing pathogen resistance to systemic fungicides with a specific mode of action, such as triazoles and carboxamides. Moreover, contact fungicides exhibit more efficient systemic curative effects in the pathogen colonization phase and eradicative effects in the sporulation or reproduction phase.
Although systemic fungicides lead to very efficient distribution within the leaf, spraying must be as careful as for contact fungicides. If there is a possibility of low relative humidity (lower than 40%) or high temperature (higher than 30 °C), the spraying efficiency will be compromised and must be stopped until the environmental conditions improve. That is, when possible, spraying should be performed in the early hours of the morning because of dew, or in the late afternoon and early evening, avoiding the hottest hours of the day.
Two other important factors influencing systemic fungicides are the water quality of the spraying syrup and the state of the application equipment, such as turbo atomizers and knapsack sprayers. The water must be clean and clay-free at a pH between 6 and 7.5, similar to the spraying syrup. Triazoles and carboxamides are polar charged compounds that influence their absorption and systemic action; therefore, tank mixtures with products capable of impairing these characteristics should be avoided. Furthermore, control efficiency is greater when MixSM foliar applications are interspersed with micronutrient and copper foliar applications rather than mixed together.
4.4. Frequency of Fungicide Spraying in Brazil
Some authors propose models to predict the development of the disease, aiming to plan the sprays based on the variables of the disease triangle, that is, the pathogen, the host, and the environment [
54]. These models are mainly based on CLR severity values, occurrence of favorable leaf temperature and humidity, and meteorological data. Since the 1960s, more than 20 models have been developed to predict different indicators of the disease’s development and can help manage it [
55,
56,
57,
58,
59]. However, these models are not yet used to control rust in coffee farms. In several Brazilian coffee regions, there are warning systems developed by research institutes that release data about climate, such as temperature and precipitation, and the occurence of CLR in the coffee plantations, in order to support producers regarding the best time to start chemical control.
Considering the climatic, planting, and management conditions of Brazilian coffee crops, if control methods apply only cupric products, they should be applied preventively from the beginning to the end of the rainy season and repeated every 20 to 30 days, depending on the rain volume. However, in Brazil, the unique application of cupric fungicides is rare and can lead to phytotoxicity. Normally, these fungicides are used for resistance management because they are multisite products that can be used in mixtures or interspersed with triazoles or carboxamides with strobilurin spraying.
The most common fungicides applied to Brazilian coffee crops to control CLR are systemic and mesostemic, or even MixSM, with the number of sprays ranging from two to four, in intervals of 45 to 60 days. These intervals and numbers of applications depend on the weather conditions, especially rain and favorable temperatures, plant nutrition, planting spacing, productivity, resistance levels of cultivars, and other cultural practices such as pruning or removing suckers [
60]. The beginning of chemical control is not based on the percentage of leaves exhibiting symptoms of CLR, because previous research found that, in Brazilian crops with a high productivity depending on susceptible cultivars, control is less efficient when the fungicide is applied after the onset of symptoms. This is because the CLR will have already infected other leaves, but without manifesting symptoms, leading to late control, which is less efficient. Therefore, the start of foliar spraying to control CLR in Brazil usually occurs in December, when symptoms typically begin to appear. This period coincides with an increase in rainfall and temperature (
Figure 1), which favors the spread of CLR. Therefore, applications are commonly performed from December to April, with three sprays of MixSM or five sprays of exclusively copper fungicides. In most cases, MixSM is combined with protective fungicides; however, control is more efficient when MixSM is interspersed with two more sprays of protective fungicides with foliar micronutrients.
Many Brazilian crops have exceedingly high productivity, with moving averages reaching more than 50- to 60-kg bags of green coffee per hectare in cultivars susceptible to CLR, such as those of the Catuaí and Mundo Novo groups. With these yields, up to four sprays of systemic, mesostemic, or MixSM may be necessary for susceptible cultivars, particularly if the environmental conditions are favorable for fungal multiplication, as in the case of summers with above normal rainfall coinciding with high rainfall in the autumn and winter, that is, during the harvest period in Brazil.
When the fungicide is applied to the soil as a single application of triazoles, it is necessary to complement it with two to three foliar sprays of MixSM for effective CLR control. Application via the soil is performed prior to foliar spraying. In addition to contributing to the management of CLR, soil application can also control pests, as the fungicides are also mixed with insecticides and increase the vigor of both the root system and the coffee tree crown.
For cultivars with intermediate resistance to CLR at SR and MR levels, such as those of the Icatu, Icatu × Catuaí group, and some Sarchimors derivatives, it is typically possible to control CLR with one or two fewer sprays than required for susceptible cultivars. These reductions in the number of sprayings depend on yield goals, soil fertility, and weather conditions. In some situations, such as in years of low yield, chemical control of CLR is not required for moderately resistant cultivars.
However, even for growers who use HR cultivars to control CLR, the use of fungicides is still necessary for controlling other diseases, such as brown eye spot caused by Cercospora coffeicola. The latter occurs at approximately the same time as CLR, and the fungicides employed to control these diseases are almost always the same; thus, even crops with HR cultivars are sprayed in this case. As brown eye spot is sometimes a less aggressive disease than CLR, fewer sprays will be necessary. Therefore, even if the use of fungicides is necessary for crops with HR cultivars, the number of applications required is generally less than that in S and HS cultivars.
In crops containing HR cultivars to control CLR, with productivity higher than 40 60-kg bags of green coffee per hectare, producers often continue with two to three sprays during the summer. Depending on the management, climate, and cultural practices adopted for crops containing HR cultivars to control CLR, the intensity of brown eye spot may be reduced, which may reduce the number of applications of MixSM.
The Brazilian coffee-growing conditions demand high productivity per area to preserve forests and the Cerrado, which leads to the use of chemical control in cultivars susceptible to diseases. For example, in Minas Gerais, the largest Brazilian coffee-producing state, properties have an average area of 34% designated for permanent protection of legal forests and reserves. Regarding chemical control, qualified technicians trained in technical and agronomic schools recommend that most properties apply the appropriate fungicides according to the correct dosage, time, and frequency of sprays. Therefore, fungicides only represent a poison for pathogens and a medicine for the plants, leading to food security with coffee production free of agrochemical residues.
5. Cultural Management
In the implementation and management of coffee cultivation, the adoption of correct cultural practices can help control CLR by conditioning disease reduction and facilitating chemical control. Abiotic environmental factors such as precipitation, temperature, the sunlight position on coffee branches, and plant morphology play an important role in the dynamics of CLR and its natural enemy population [
2,
12,
61,
62,
63]. In coffee plantation formation, the choice of spacing, cultivar, nutrition, and weed management are important [
64]. The crop characteristics must also be considered to facilitate management of the disease, with emphasis on the crop load, topography of the land, size and type of cultivation system, technological level of the producer, equipment and labor force available for work, water availability, climatic conditions in each region, simultaneous occurrence of other pests and diseases, and costs/benefits of the system.
The use of denser spacing creates a moistened and shaded environment, which facilitates CLR infection [
65]. Planting in mechanized rows, which is typical in Brazilian coffee-growing regions, and characterized by more openness between rows and less openness within rows, offers good aeration and results in a shorter wetting duration within the crop. It also facilitates the passage of the tractor and the sprayer, and leads to less waste of spray solution over a continuous row of plants. With the current equipment available in the Brazilian market, preference should be given to small cultivars, which are more easily reached by sprayed drops. An excess of stems, caused by a lack of pruning new sprouts post-planting, also creates a moistened and shaded environment. Therefore, whenever necessary, adequate thinning and pruning of coffee trees should be performed to reduce plant height and improve aeration of the coffee canopy. Removing suckers and excess branches is also important for ensuring a less moist microclimate and reducing the period of leaf wetness. Additionally, very tall weeds accumulate moisture in the crop, especially in young crops, in addition to shading and favoring infection in the lower third of the canopy of adult plants. Weeds can also absorb fungicides applied to the soil, reducing their availability to the coffee tree. Adequate fertilization that provides effective and balanced levels of nutrients makes plants less susceptible to diseases and favors their recovery after infection with
H. vastatrix. In summary, all of these factors must be considered when proposing integrated coffee rust management.
6. Final Considerations
The arrival of CLR in Brazil was initially seen as a serious threat to coffee production in Brazil. Nevertheless, the actions taken to solve this problem have led to renewal and modernization of the coffee-growing sector, further establishing the coffee industry and increasing coffee production in Brazil.
In recent decades, genetic coffee breeding programs in Brazil have developed a lot of cultivars that are resistant to CLR. Nevertheless, the adoption of these new cultivars by coffee growers has been slow. This is because traditional and susceptible cultivars have a good performance, and chemical rust control methods have low cost and high efficiency. There is also a need for strategies to disseminate new cultivars among coffee growers and agents responsible for technical assistance.
Resistance to CLR is not the only characteristic that determines the choice of a cultivar by Brazilian coffee growers. Characteristics including high yield potential, bean size, vegetative vigor, response to pruning, maturation cycle, easy mechanized harvesting, resistance to other diseases, and nematodes are also highly considered when choosing cultivars. In this way, the most coffee plantations in Brazil, such as Catuaí and Mundo Novo, are still susceptible to CLR.
Although the use of chemical control is commonly used for the management of CLR, the utilization of cultural management, the use of safety practices for pesticides, and forecast models to minimize the number of sprayings can result in rational use of fungicides. Therefore, environmental sustainability could be balanced with the economic and social needs of agricultural production.
It stands out that a considerable part of Brazilian coffee production is located in mountainous areas, where mechanization is not possible, making chemical control of rust difficult and resulting in substantial production losses. In these locations, resistant cultivars are the most efficient method of controlling CLR, and are slowly but surely replacing traditional cultivars.
Despite CLR being the most important coffee disease in this country with the potential to cause substantial economic setbacks, Brazilian coffee growers have learned to live with the disease.