The Sorption of Sulfamethoxazole by Aliphatic and Aromatic Carbons from Lignocellulose Pyrolysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Chemicals
2.2. Biochar Preparation
2.3. Characterization of Biochars
2.4. Sorption Experiment
2.5. Data Analysis
3. Results and Discussion
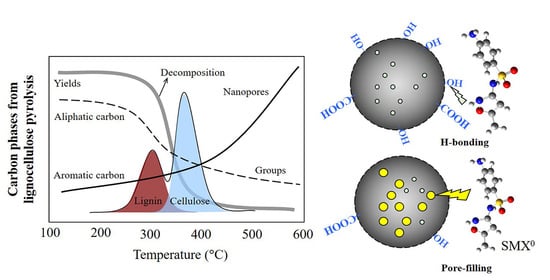
3.1. Pyrolysis Characteristics
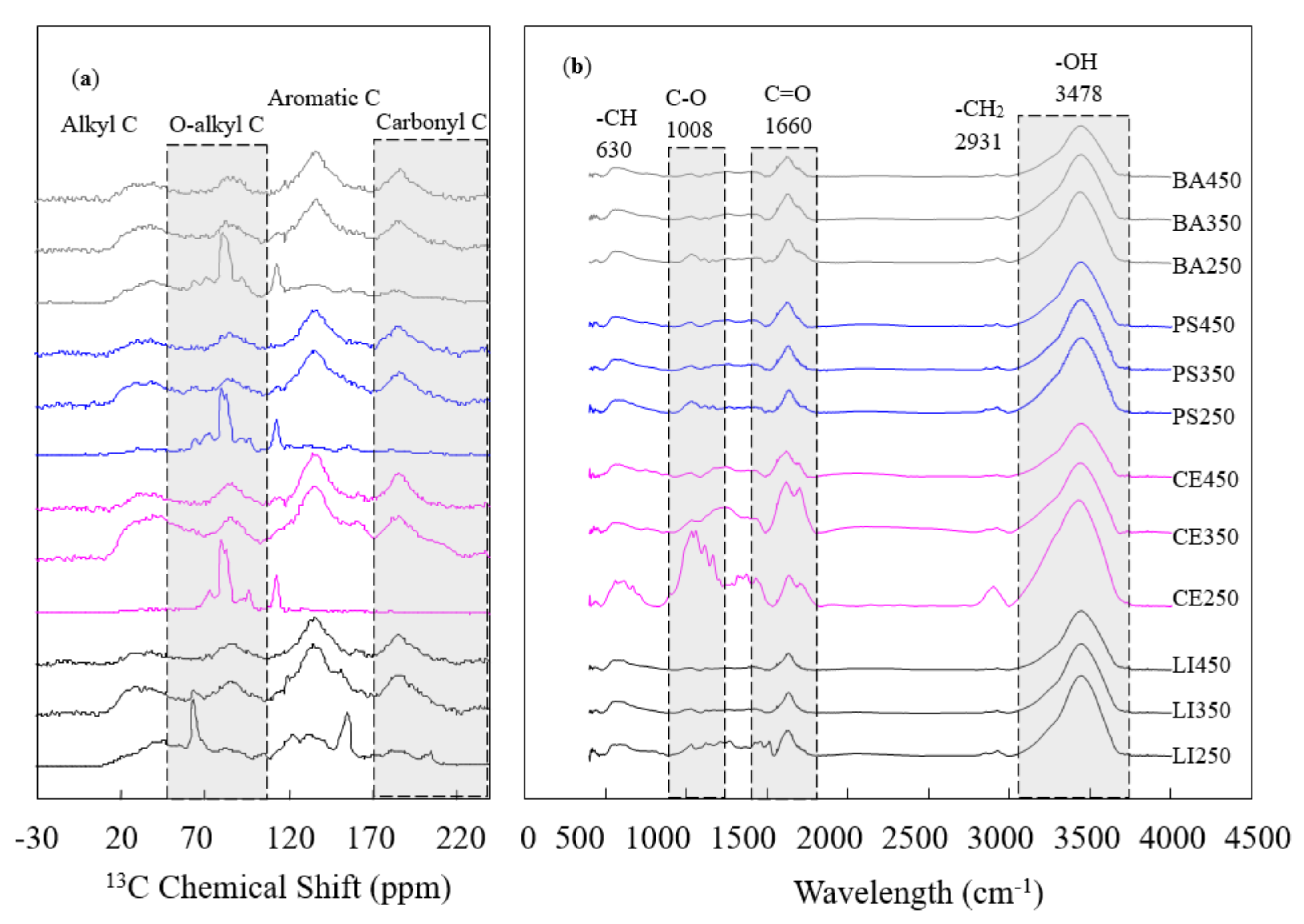
3.2. Evolution of Carbon Structure
3.3. Surface Functional Groups
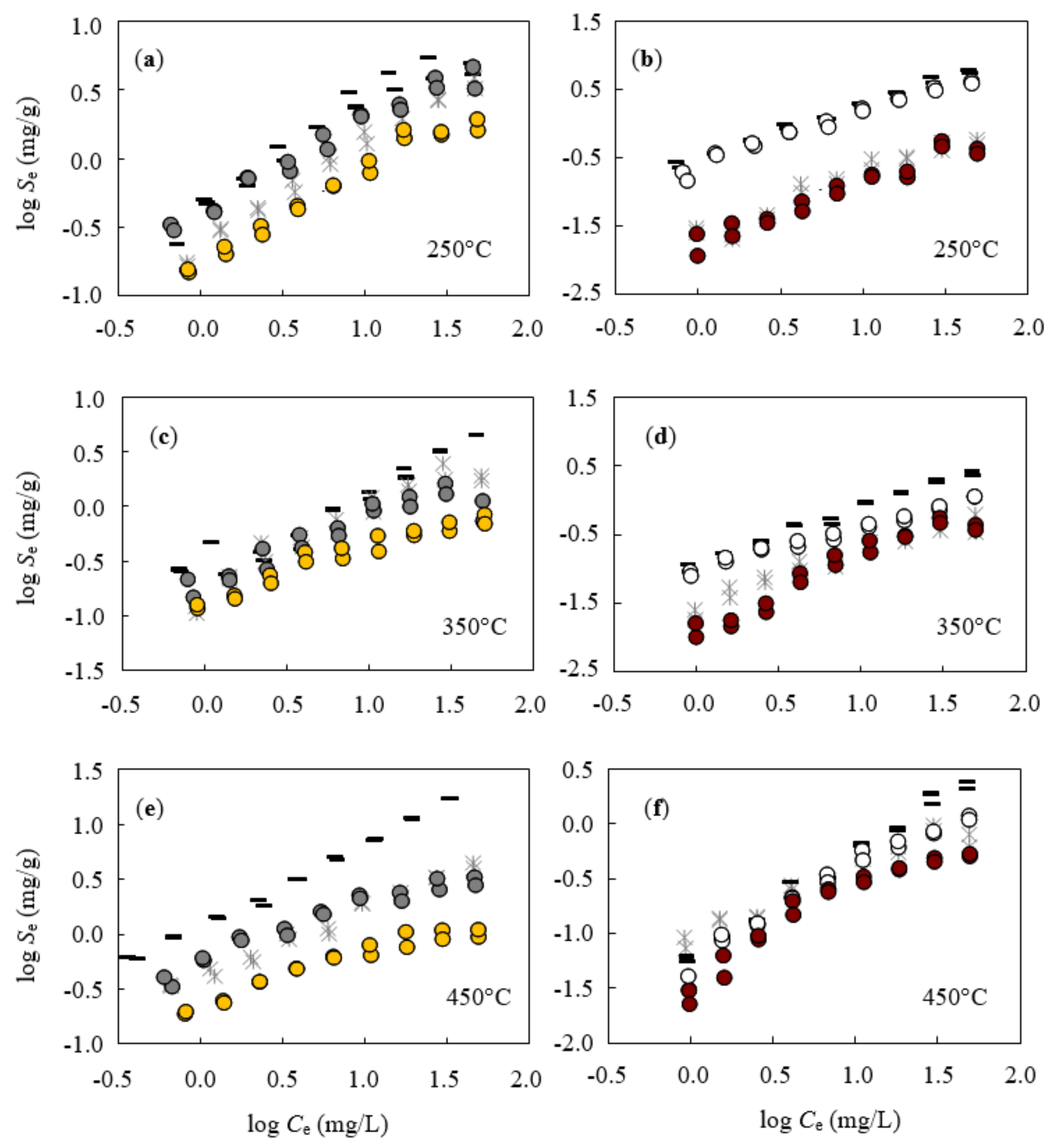
3.4. Isothermal Sorption
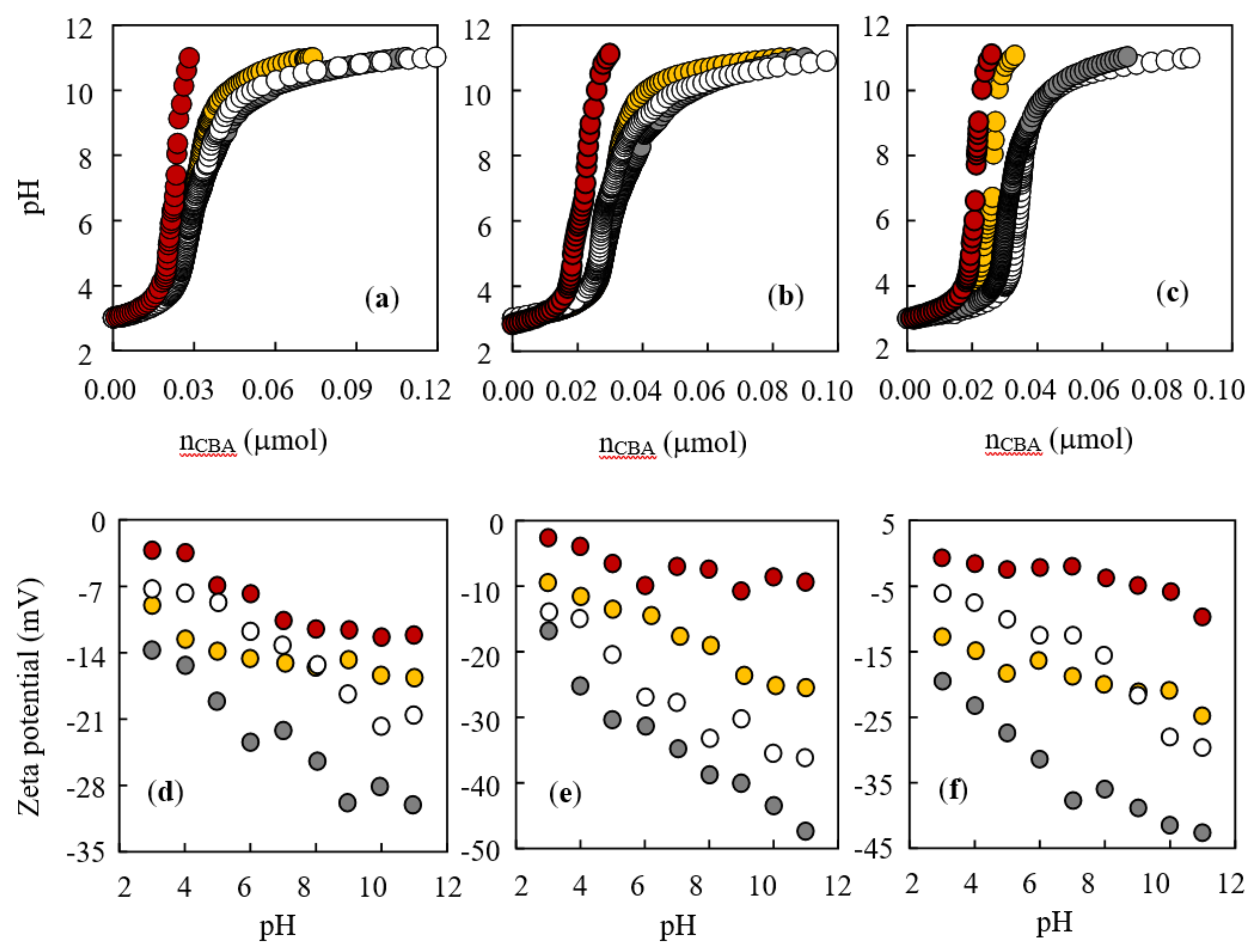
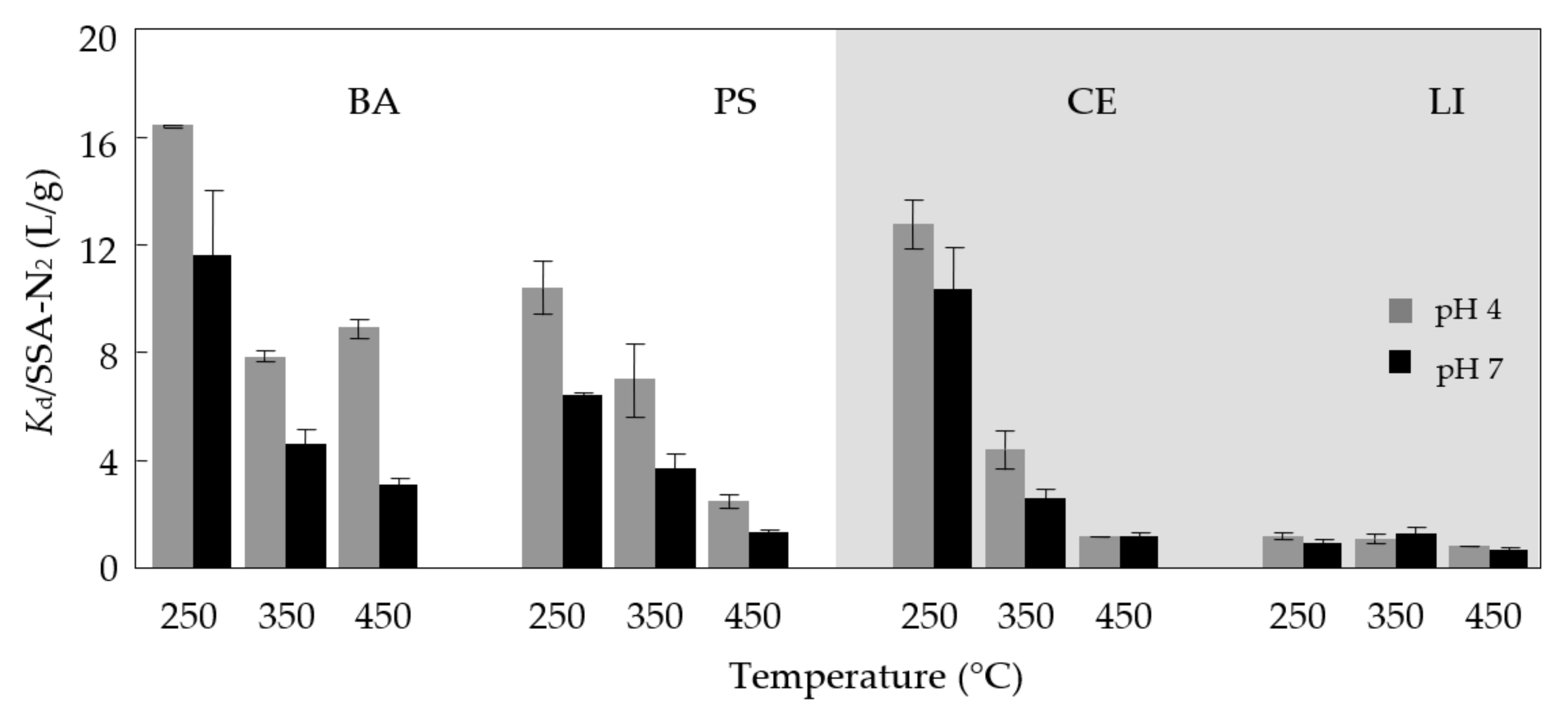
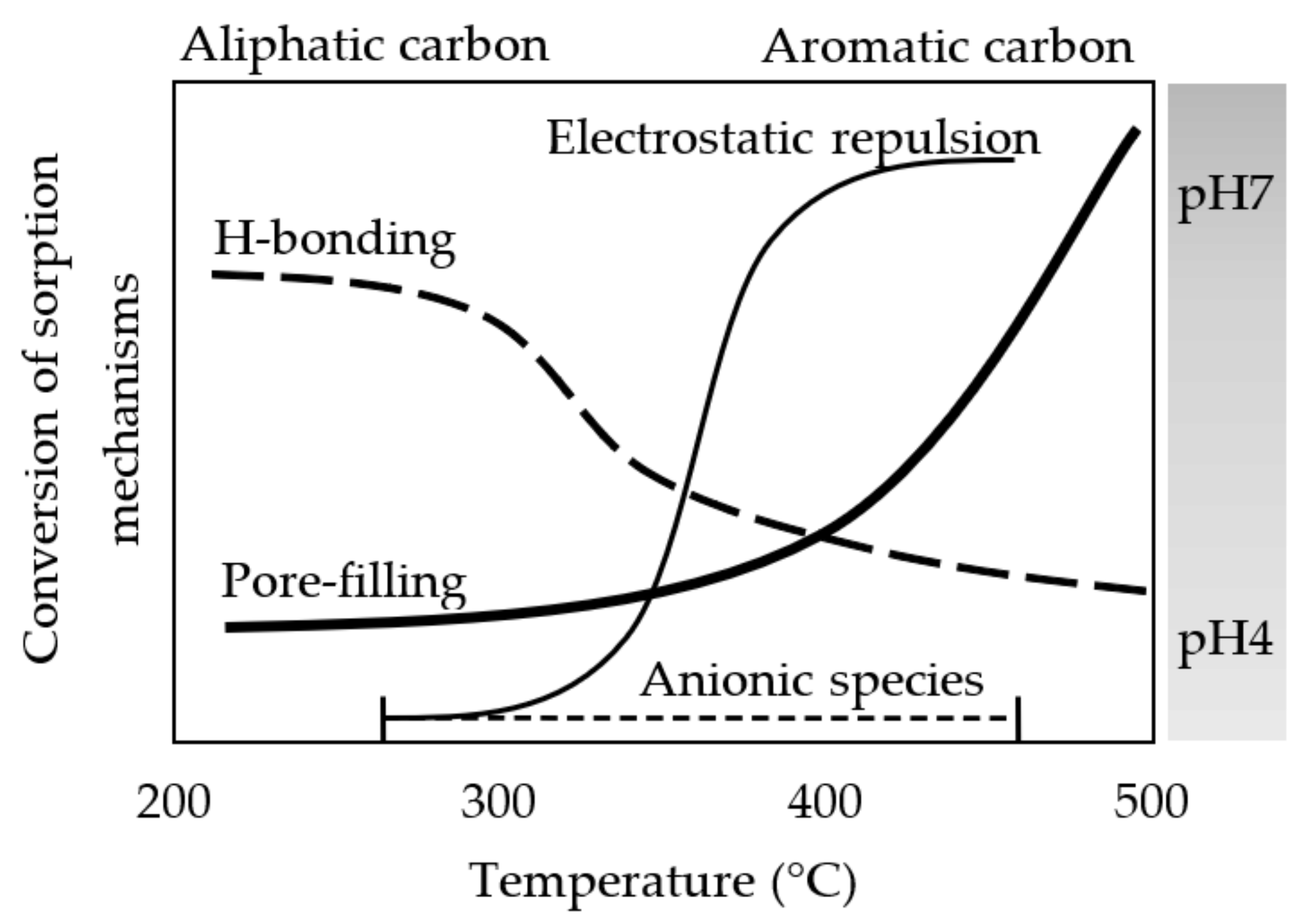
3.5. Sorption as Affected by pH Values
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Jiang, P.; Zhang, H.; Yuan, W. Biochar production and applications in agro and forestry systems: A review. Sci. Total Environ. 2020, 723, 137775. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Zhao, J.; Liu, Y.; Lang, D.; Wu, M.; Pan, B.; Steinbergd, C.E.W. The relative importance of different carbon structures in biochars to carbamazepine and bisphenol a sorption. J. Hazard. Mater. 2019, 373, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A Critical Review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Mitch, W.A.; Xu, W. Activity and reactivity of pyrogenic carbonaceous matter toward organic compounds. Environ. Sci. Technol. 2017, 51, 8893–8908. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B. Black carbon (biochar) in water/soil environments: Molecular structure, sorption, stability, and potential risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef]
- Torres-Sciancalepore, R.; Fernandez, A.; Asensio, D.; Riveros, M.; Fabani, M.P.; Fouga, G.; Rodriguez, R.; Mazza, G. Kinetic and thermodynamic comparative study of quince bio-waste slow pyrolysis before and after sustainable recovery of pectin compounds. Energy Convers. Manag. 2022, 252, 115076. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Ortiz, L.R.; Torres, E.; Zalazar, D.; Zhang, H.; Rodriguez, R.; Mazza, G. Influence of pyrolysis temperature and bio-waste composition on biochar characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Parthasarathy, P.; Fernandez, A.; Al-Ansari, T.; Mackey, H.R.; Rodriguez, R.; Mazza, G.; McKay, G. Thermal degradation characteristics and kinetic study of camel manure pyrolysis. J. Environ. Chem. Eng. 2021, 9, 106071. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Sjöström, E. Lignin in Wood Chemistry; Academic Press: San Diego, CA, USA, 1993; pp. 71–89. [Google Scholar]
- Harvey, O.R.; Kuo, L.-J.; Zimmerman, A.R.; Louchouarn, P.; Amonette, J.E.; Herbert, B.E. An index-based approach to assessing recalcitrance and soil carbon sequestration potential of engineered black carbons (biochars). Environ. Sci. Technol. 2012, 46, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Kang, M.; Sun, K.; Pan, Z.; Wu, F.; Xing, B. Properties of biochar-amended soils and their sorption of imidacloprid, isoproturon, and atrazine. Sci. Total. Environ. 2016, 550, 504–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keiluweit, M.; Kleber, M. Molecular-level interactions in soils and sediments: The role of aromatic pi-systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, L.; Sun, K.; Jin, J.; Ro, K.S.; Libra, J.A.; Liu, X.; Xing, B. Sorption of four hydrophobic organic contaminants by biochars derived from maize straw, wood dust and swine manure at different pyrolytic temperatures. Chemosphere 2016, 144, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Chen, L.; Que, C.; Yang, K.; Deng, F.; Deng, X.; Shi, G.; Xu, G.; Wu, M. Adsorption of antibiotics on graphene and biochar in aqueous solutions induced by pi-pi interactions. Sci. Rep. 2016, 6, 31920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Liang, G.; Zhang, X.; Cai, X.; Li, R.; Xie, X.; Wang, Z. Coating magnetic biochar with humic acid for high efficient removal of fluoroquinolone antibiotics in water. Sci. Total. Environ. 2019, 688, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Lu, C.; Liao, Q.H.; Gudda, F.O.; Ling, W.T. Antibiotics in animal manure and manure-based fertilizers: Occurrence and ecological risk assessment. Chemosphere 2020, 255, 127006. [Google Scholar] [CrossRef]
- Ben, W.W.; Pan, X.; Qiang, Z.M. Occurrence and partition of antibiotics in the liquid and solid phases of swine wastewater from concentrated animal feeding operations in Shandong Province, China. Environ. Sci. Process. Impacts 2013, 15, 870–875. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, D.; Zhang, J.; Li, F.; Chu, G.; Wu, M.; Pan, B.; Steinberg, C.E.W. The contrasting role of minerals in biochars in bisphenol A and sulfamethoxazole sorption. Chemosphere 2021, 264, 128490. [Google Scholar] [CrossRef]
- Luo, B.; Huang, G.; Yao, Y.; An, C.; Zhang, P.; Zhao, K. Investigation into the influencing factors and adsorption characteristics in the removal of sulfonamide antibiotics by carbonaceous materials. J. Clean. Prod. 2021, 319, 128692. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J. A comparative investigation into the formation behaviors of char, liquids and gases during pyrolysis of pinewood and lignocellulosic components. Bioresour. Technol. 2014, 170, 262–269. [Google Scholar] [CrossRef]
- Rocha, G.J.d.; Nascimento, V.M.; Goncalves, A.R.; Silva, V.F.N.; Martin, C. Influence of mixed sugarcane bagasse samples evaluated by elemental and physical-chemical composition. Ind. Crop. Prod. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, Y.; Lu, X.; Li, Q. Energy consumption and product release characteristics evaluation of oil shale non-isothermal pyrolysis based on TG-DSC. J. Pet. Sci. Eng. 2020, 187, 106812. [Google Scholar] [CrossRef]
- Han, L.; Ro, K.S.; Sun, K.; Jin, J.; Libra, J.A.; Xing, B. New evidence for high sorption capacity of hydrochar for hydrophobic organic pollutants. Environ. Sci. Technol. 2016, 50, 13274–13282. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, D.; Yang, Y.; Ran, Y.; Mao, J.; Chu, W.; Cao, X. Effects of the chemical structure, surface, and micropore properties of activated and oxidized black carbon on the sorption and desorption of phenanthrene. Environ. Sci. Technol. 2019, 53, 7683–7693. [Google Scholar] [CrossRef]
- Zhuo, C.; Hu, S.; Yang, Y.; Ran, Y. Effects of the structures and micropores of sedimentary organic matter on the oxidative degradation of benzo(a)pyrene by Na2S2O8. Water Res. 2020, 174, 115635. [Google Scholar] [CrossRef]
- Hu, S.; Xu, D.; Kong, X.; Gong, J.; Yang, Y.; Ran, Y.; Mao, J. Effect of the structure and micropore of activated and oxidized black carbon on the sorption and desorption of nonylphenol. Sci. Total Environ. 2021, 761, 144191. [Google Scholar] [CrossRef]
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E.W. Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 2018, 240, 1–9. [Google Scholar] [CrossRef]
- Ma, H.Z.; Allen, H.E.; Yin, Y.J. Characterization of isolated fractions of dissolved organic matter from natural waters and a wastewater effluent. Water Res. 2001, 35, 985–996. [Google Scholar] [CrossRef]
- Xiao, F.; Pignatello, J.J. Interactions of triazine herbicides with biochar: Steric and electronic effects. Water Res. 2015, 80, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, L.; Wan, Y.; Zheng, S.; Zhu, D. Adsorption of tetracycline and sulfamethoxazole on crop residue-derived ashes: Implication for the relative importance of black carbon to soil sorption. Environ. Sci. Technol. 2011, 45, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Pignatello, J.J. Effects of post-pyrolysis air oxidation of biomass chars on adsorption of neutral and ionizable compounds. Environ. Sci. Technol. 2016, 50, 6276–6283. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.D.; Guo, D.W.; Zhang, Y.; Sun, S.Z.; Zhao, Y.J.; Shang, Q.; Sun, H.L.; Wu, J.Q.; Tan, H.P. Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem. Eng. J. 2021, 410, 127707. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.Y.; Zhao, J.; Herbert, S.; Xing, B.S. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ. Pollut. 2013, 181, 60–67. [Google Scholar] [CrossRef]
- Ling, C.; Li, X.; Zhang, Z.; Liu, F.; Deng, Y.; Zhang, X.; Li, A.; He, L.; Xing, B. High adsorption of sulfamethoxazole by an amine-modified polystyrene-divinylbenzene resin and its mechanistic insight. Environ. Sci. Technol. 2016, 50, 10015–10023. [Google Scholar] [CrossRef]
- Wu, M.; Pan, B.; Zhang, D.; Xiao, D.; Li, H.; Wang, C.; Ning, P. The sorption of organic contaminants on biochars derived from sediments with high organic carbon content. Chemosphere 2013, 90, 782–788. [Google Scholar] [CrossRef]

| Samples | Bulk Elemental Composition (%) | Ali a | Aro a | SSA (m2/g) b | |||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | H | H/C | O/C | (%) | (%) | N2 | CO2 | |
| BA250 | 53.50 | 39.84 | 5.60 | 1.26 | 0.56 | 68.84 | 31.16 | 20.27 | 106.94 |
| BA350 | 67.49 | 24.58 | 3.91 | 0.70 | 0.27 | 51.65 | 48.35 | 19.86 | 291.50 |
| BA450 | 71.45 | 21.01 | 3.53 | 0.59 | 0.22 | 45.93 | 54.07 | 91.95 | 370.24 |
| PS250 | 58.37 | 33.62 | 5.33 | 1.10 | 0.43 | 66.44 | 33.56 | 15.37 | 116.96 |
| PS350 | 71.89 | 22.63 | 4.08 | 0.68 | 0.24 | 49.05 | 50.95 | 15.13 | 269.35 |
| PS450 | 76.24 | 20.54 | 3.70 | 0.58 | 0.20 | 45.25 | 54.75 | 70.52 | 341.24 |
| CE250 | 48.72 | 48.87 | 6.52 | 1.61 | 0.75 | 79.16 | 20.84 | 15.87 | 47.39 |
| CE350 | 70.23 | 28.22 | 3.91 | 0.67 | 0.30 | 53.06 | 46.94 | 17.31 | 339.65 |
| CE450 | 78.09 | 18.63 | 3.46 | 0.53 | 0.18 | 42.78 | 57.22 | 40.19 | 436.11 |
| LI250 | 66.41 | 25.48 | 5.53 | 1.00 | 0.29 | 55.16 | 44.84 | 17.64 | 58.43 |
| LI350 | 69.93 | 23.81 | 3.95 | 0.68 | 0.26 | 46.72 | 53.28 | 16.07 | 276.68 |
| LI450 | 74.99 | 19.56 | 3.47 | 0.55 | 0.20 | 41.83 | 58.19 | 53.17 | 385.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, G.; Han, Z.; Wang, Z.; Kong, D.; Qin, W.; Si, Y.; Wang, G.; Steinberg, C.E.W. The Sorption of Sulfamethoxazole by Aliphatic and Aromatic Carbons from Lignocellulose Pyrolysis. Agronomy 2022, 12, 476. https://doi.org/10.3390/agronomy12020476
Chu G, Han Z, Wang Z, Kong D, Qin W, Si Y, Wang G, Steinberg CEW. The Sorption of Sulfamethoxazole by Aliphatic and Aromatic Carbons from Lignocellulose Pyrolysis. Agronomy. 2022; 12(2):476. https://doi.org/10.3390/agronomy12020476
Chicago/Turabian StyleChu, Gang, Zifeng Han, Zimo Wang, Defeng Kong, Wenxiu Qin, Youbin Si, Guozhong Wang, and Christian E. W. Steinberg. 2022. "The Sorption of Sulfamethoxazole by Aliphatic and Aromatic Carbons from Lignocellulose Pyrolysis" Agronomy 12, no. 2: 476. https://doi.org/10.3390/agronomy12020476
APA StyleChu, G., Han, Z., Wang, Z., Kong, D., Qin, W., Si, Y., Wang, G., & Steinberg, C. E. W. (2022). The Sorption of Sulfamethoxazole by Aliphatic and Aromatic Carbons from Lignocellulose Pyrolysis. Agronomy, 12(2), 476. https://doi.org/10.3390/agronomy12020476