In Vitro Organogenesis of Critically Endangered Lachenalia viridiflora
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Biometrical Observations
2.3. Statistical Analysis
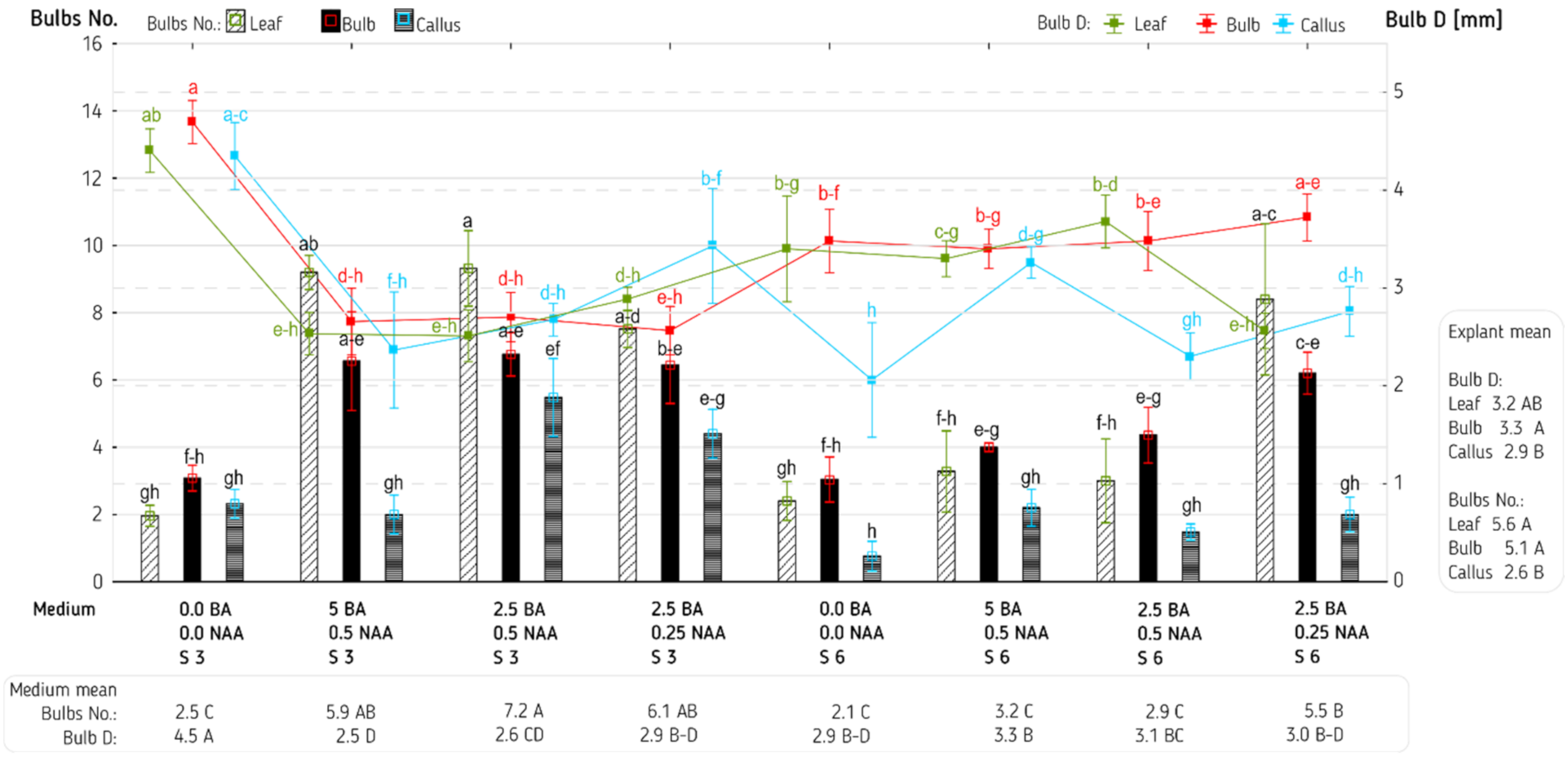
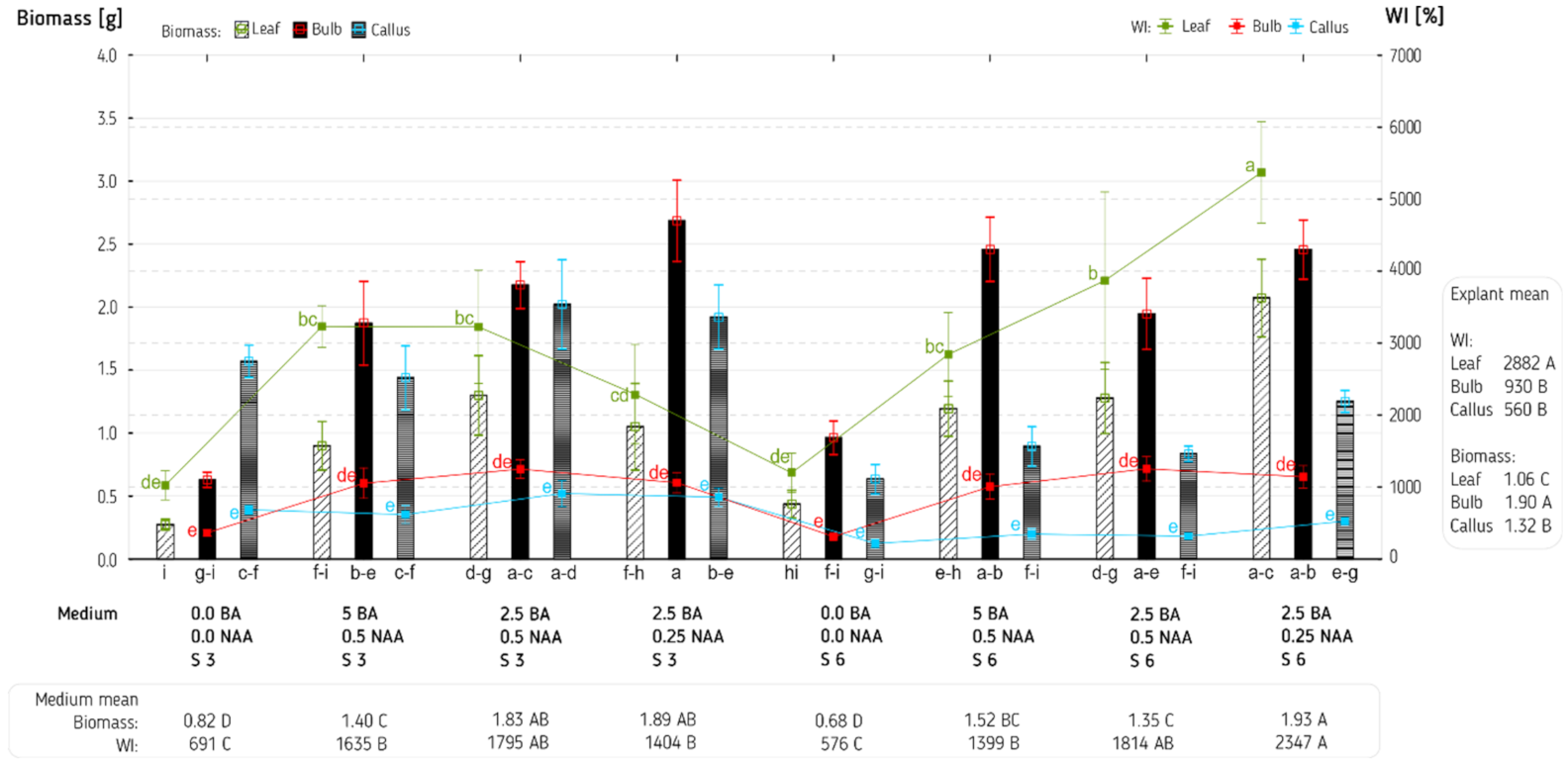
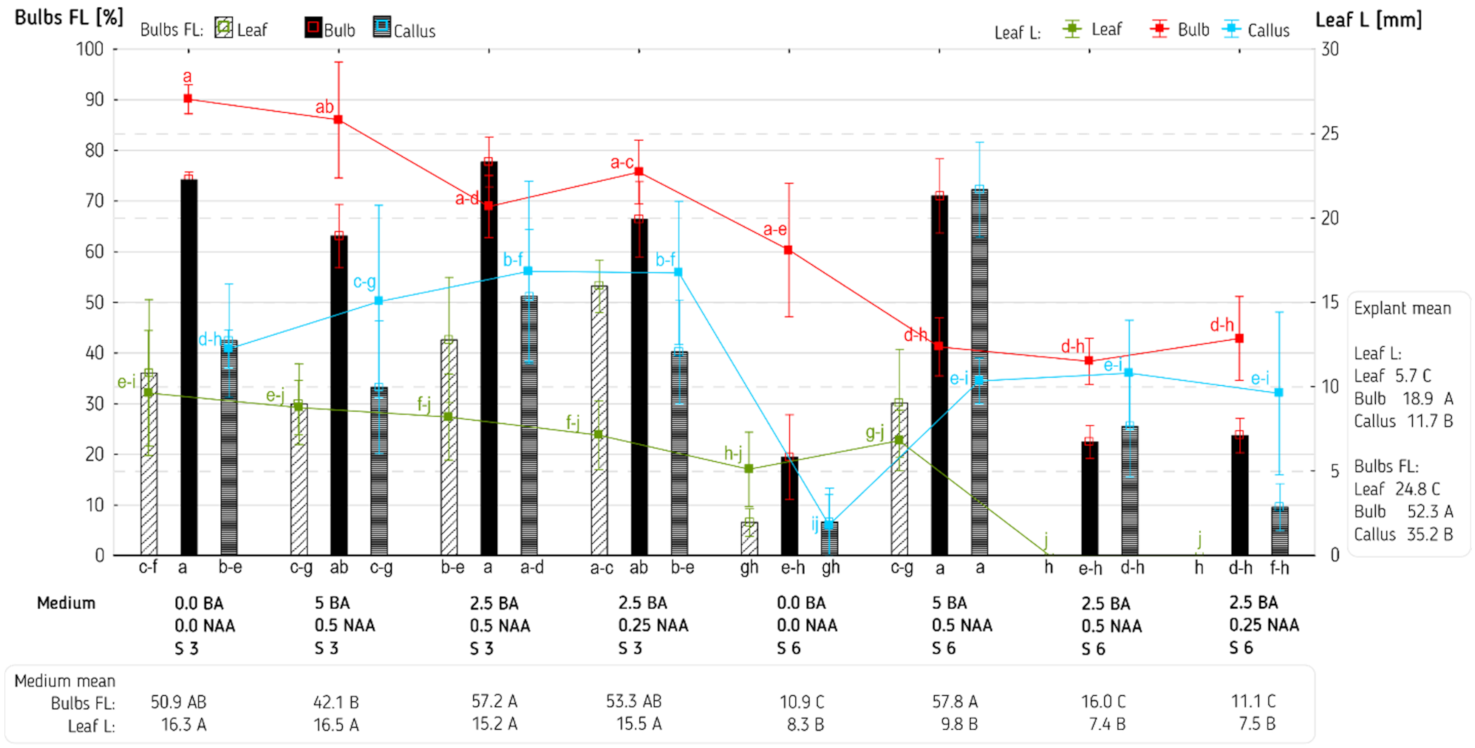
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duncan, G.D. The Genus Lachenalia: Botanical Magazine Monograph, 1st ed.; Royal Botanic Gardens: Kew, UK, 2012; ISBN 9781842463826. [Google Scholar]
- Duncan, G.D. Two new species, two rediscoveries and a range extension in Lachenalia (Asparagaceae: Scilloideae) from southern and western South Africa. Phytotaxa 2017, 316, 261–270. [Google Scholar] [CrossRef]
- Raimondo, D.; von Staden, L.; Foden, W.; Victor, J.; Helme, N.; Turner, R.; Kamundi, D.; Manyama, P. Red List of South African Plants.Series: Strelitzia; South African National Biodiversity Institute (SANBI Publishing): Pretoria, South African, 2009; Volume 25, ISBN 78-1-919976-52-5. [Google Scholar]
- Kleynhans, R. Lachenalia spp. In Flower Breeding and Genetics: Issues, Challenges and Opportunities for the 21st Century; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 491–516. [Google Scholar]
- Kleynhans, R. Lachenalia Jacq. f. ex Murray Named “Aqua Lady”. U.S. Plant Patent Application No. US 2017/0112037 P1, 20 April 2017. [Google Scholar]
- Nel, D.D. Rapid propagation of Lachenalia hybrids in vitro. S. Afr. J. Bot. 1983, 2, 245–246. [Google Scholar] [CrossRef][Green Version]
- Ault, J.R. In vitro rooting and greenhouse acclimatization of Lachenalia shoots. HortScience 1995, 30, 1304–1305. [Google Scholar] [CrossRef]
- McCartan, S.A.; van Staden, J. Micropropagation of members of the Hyacinthaceae with medicinal and ornamental potential—A review. S. Afr. J. Bot. 1999, 65, 361–369. [Google Scholar] [CrossRef][Green Version]
- Grace, O.M.; van Staden, J. A horticultural history of Lachenalia (Hyacinthaceae): Review article. S. Afr. J. Sci. 2003, 99, 526–531. [Google Scholar]
- Moyo, M.; Bairu, M.W.; Amoo, S.O.; van Staden, J. Plant biotechnology in South Africa: Micropropagation research endeavours, prospects and challenges. S. Afr. J. Bot. 2011, 77, 996–1011. [Google Scholar] [CrossRef][Green Version]
- Aremu, A.O.; Plačková, L.; Masondo, N.A.; Amoo, S.O.; Moyo, M.; Novák, O.; Doležal, K.; Van Staden, J. Regulating the regulators: Responses of four plant growth regulators during clonal propagation of Lachenalia montana. Plant Growth Regul. 2017, 82, 305–315. [Google Scholar] [CrossRef]
- Bach, A.; Kapczyńska, A.; Dziurka, K.; Dziurka, M. Phenolic compounds and carbohydrates in relation to bulb formation in Lachenalia ‘Ronina’ and ‘Rupert’ in vitro cultures under different lighting environments. Sci. Hortic. 2015, 188, 23–29. [Google Scholar] [CrossRef]
- Bach, A.; Kapczyńska, A.; Dziurka, K.; Dziurka, M. The importance of applied light quality on the process of shoot organogenesis and production of phenolics and carbohydrates in Lachenalia sp. cultures in vitro. S. Afr. J. Bot. 2018, 114, 14–19. [Google Scholar] [CrossRef]
- Niederwieser, J.G.; Vcelar, B.M. Regeneration of Lachenalia species from leaf explants. HortScience 1990, 25, 684–687. [Google Scholar] [CrossRef]
- Niederwieser, J.G.; van Staden, J. Interaction between benzyladenine, naphthaleneacetic acid and tissue age on adventitious bud formation on leaf sections of Lachenalia hybrids. S. Afr. J. Bot. 1992, 58, 13–16. [Google Scholar] [CrossRef][Green Version]
- Van Staden, J.; Drewes, F.E. The effect of benzyladenine and its glucosides on adventitious bud formation on Lachenalia leaf sections. S. Afr. J. Bot. 1994, 60, 191–192. [Google Scholar] [CrossRef][Green Version]
- Slabbert, M.M.; Niederwieser, J.G. In vitro bulblet production of Lachenalia. Plant Cell Rep. 1999, 18, 620–624. [Google Scholar] [CrossRef]
- Niederwieser, J.G.; Ndou, A.M. Review on adventitious bud formation in Lachenalia. Acta Hortic. 2002, 570, 135–140. [Google Scholar] [CrossRef]
- Bach, A.; Kapczyńska, A. The initiation of the in vitro cultures of some Lachenalia cultivars. Ann. Warsaw Univ. Life Sci.—SGGW Hortic. Landsc. Archit. 2013, 34, 13–20. [Google Scholar]
- Ascough, G.D.; van Staden, J.; Erwin, J.E. In vitro Storage Organ Formation of Ornamental Geophytes. Hortic. Rev. 2008, 417–445. [Google Scholar] [CrossRef]
- Kapczyńska, A. Propagation of Lachenalia cultivars with leaf cuttings. Acta Sci. Pol. Hortorum Cultus 2019, 18, 189–195. [Google Scholar] [CrossRef]
- Podwyszyńska, M. The mechanisms of in vitro storage organ formation in ornamental geophytes. Floric. Ornam. Biotechnol. 2012, 6, 9–23. [Google Scholar]
- Fennell, C.W.; van Staden, J.; Bornman, C.H. Biotechnology of southern African bulbs. S. Afr. J. Bot. 2004, 70, 37–46. [Google Scholar] [CrossRef]
- Kumar, S.; Awasthi, V.; Kanwar, J.K. Influence of growth regulators and nitrogenous compounds on in vitro bulblet formation and growth in oriental lily. Hortic. Sci. 2007, 34, 77–83. [Google Scholar] [CrossRef]
- Malik, M.; Bach, A. Morphogenetic pathways from Narcissus L. ‘Carlton’ in vitro cultures of PC stage flower bud explants according to cytokinin and auxin rations. Acta Sci. Pol. Hortorum Cultus 2016, 15, 101–111. [Google Scholar]
- Qadir, J.; Singh, S.; Kaloo, Z.A. A review on in vitro propagation of some medicinally important plant species of family Asparagaceae. Int. J. Biosci. Technol. 2017, 10, 1–12. [Google Scholar]
- Prokopiuk, B.; Cioć, M.; Maślanka, M.; Pawłowska, B. Effects of light spectra and benzyl adenine on in vitro adventitious bulb and shoot formation of Lilium regale E.H. Wilson. Propag. Ornam. Plants 2018, 18, 12–18. [Google Scholar]
- Niederwieser, J.G.; van Staden, J.; Upfold, S.J.; Drewes, F.E. Metabolism of 6-benzyladenine by leaf explants of Lachenalia during adventitious bud formation. S. Afr. J. Bot. 1992, 58, 236–238. [Google Scholar] [CrossRef]
- Bach, A.; Sochacki, D. Propagation of ornamental geophytes: Physiology and management systems. In Ornamental Geophytes: From Basic Science to Sustainable Production; Kamenetsky, R., Okubo, H., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 261–286. [Google Scholar]
- Van Rensburg, J.G.J.; Vcelar, B.M. The effect of the sucrose concentration on the initiation and growth of adventitious buds from leaf tissue of Lachenalia. S. Afr. J. Bot. 1989, 55, 117–121. [Google Scholar] [CrossRef]
- Bach, A.; Pawlowska, B.; Pulczynska, K. Utilization of soluble carbohydrates in shoot and bulb regeneration of Hyacinthus orientalis L. in vitro. Acta Hortic. 1992, 325, 487–492. [Google Scholar] [CrossRef]
- Niederwieser, J.G.; Bornman, C.H. Role of biotechnology in the development and production of Lachenalia and Ornithogalum cultivars in South Africa. S. Afr. J. Bot. 2004, 70, 47–51. [Google Scholar] [CrossRef]
- Pearson, M.N.; Cohen, D.; Cowell, S.J.; Jones, D.; Blouin, A.; Lebas, B.S.M.; Shiller, J.B.; Clover, G.R.G. A survey of viruses of flower bulbs in New Zealand. Australas. Plant Pathol. 2009, 38, 305–309. [Google Scholar] [CrossRef]
- Pence, V.C. Evaluating costs for the in vitro propagation and preservation of endangered plants. Vitr. Cell. Dev. Biol. Plant 2011, 47, 176–187. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Micropropagation. In Plant Tissue Culture: An Introductory Text; Springer: New Delhi, India, 2013; pp. 245–274. [Google Scholar]
- Hesami, M.; Naderi, R.; Yoosefzadeh-Najafabadi, M.; Maleki, M. In vitro culture as a powerful method for conserving Iranian ornamental geophytes. BioTechnologia 2018, 99, 73–81. [Google Scholar] [CrossRef]
- Kumar, V.; Moyo, M.; Van Staden, J. Enhancing plant regeneration of Lachenalia viridiflora, a critically endangered ornamental geophyte with high floricultural potential. Sci. Hortic. 2016, 211, 263–268. [Google Scholar] [CrossRef]
- Maślanka, M.; Kapczyńska, A. Cold treatment promotes in vitro germination of two endangered Lachenalia species. S. Afr. J. Bot. 2021, 142, 430–432. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Statistica 13.3; Data Analysis Software System. TIBCO Software Inc.: Palo Alto, CA, USA, 2017.
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 8th ed.; Cengage: Hampshire, UK, 2019. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis, Springer Series in Statistics, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95442-2. [Google Scholar]
- De Klerk, G.J.M. Micropropagation of bulbous crops: Technology and present state. Floric. Ornam. Biotechnol. 2012, 6, 1–8. [Google Scholar]
- Askari, N.; Visser, R.G.F.; De Klerk, G.-J. Growth of lily bulblets in vitro, a review. Int. J. Hortic. Sci. Technol. 2018, 5, 133–143. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, Z.; Gao, C.; Sun, M.; Li, S.; Min, R.; Wu, J.; Li, D.; Wang, X.; Wei, Y.; et al. Change in sucrose cleavage pattern and rapid starch accumulation govern lily shoot-to-bulblet transition in vitro. Front. Plant Sci. 2021, 11, 564713. [Google Scholar] [CrossRef]
- Maślanka, M.; Bach, A. Induction of bulb organogenesis in in vitro cultures of tarda tulip (Tulipa tarda Stapf.) from seed-derived explants. Vitr. Cell. Dev. Biol. Plant 2014, 50, 712–721. [Google Scholar] [CrossRef]
- Evenor, D.; Levi-Nissim, A.; Afgin, L.; Lilien-Kipnis, H.; Watad, A.A. Regeneration of plantlets and bulblets from explants and callus of Allium aflatunense cultivars and selection from indigenous Israeli Allium ampeloprasum. Acta Hortic. 1997, 430, 325–330. [Google Scholar] [CrossRef]
- Bakhshaie, M.; Babalar, M.; Mirmasoumi, M.; Khalighi, A. Somatic embryogenesis and plant regeneration of Lilium ledebourii (Baker) Boiss., an endangered species. Plant Cell Tissue Organ Cult. 2010, 102, 229–235. [Google Scholar] [CrossRef]
- Kleynhans, R.; Niederwieser, J.G.; Hancke, F.L. Lachenalia: Development and commercialization of a new flower bulb crop. Acta Hortic. 2002, 570, 81–85. [Google Scholar] [CrossRef]
- Gheisari, M.; Miri, S.M. In vitro callus induction and bulblet regeneration of hyacinth (Hyacinthus orientalis L.). Plant Cell Biotechnol. Mol. Biol. 2017, 18, 145–155. [Google Scholar]
- Maślanka, M.; Prokopiuk, B. Bulb organogenesis of Tulipa tarda in vitro cultures in relation to light environment. Acta Agric. Scand. Sect. B 2019, 69, 398–404. [Google Scholar] [CrossRef]
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Azadi, P.; Khosh-Khui, M. Micropropagation of Lilium ledebourii (Baker) Boiss as affected by plant growth regulators, sucrose concentrations, harvesting season and cold treatments. Electron. J. Biotechnol. 2007, 10, 582–591. [Google Scholar] [CrossRef]
- Nhut, D.T. Micropropagation of lily (Lilium longiflorum) via in vitro stem node and pseudo-bulblet culture. Plant Cell Rep. 1998, 17, 913–916. [Google Scholar] [CrossRef]
- Ozel, C.A.; Khawar, K.M.; Unal, F. Factors affecting efficient in vitro micropropagation of Muscari muscarimi Medikus using twin bulb scale. Saudi J. Biol. Sci. 2015, 22, 132–138. [Google Scholar] [CrossRef]
- Podwyszyńska, M. Effect of carbohydrates on shoot multiplication and bulb formation of tulip in vitro. Roczniki Akademii Rolniczej w Poznaniu Ogrodnictwo 2001, 33, 119–126. [Google Scholar]
- Taeb, A.G.; Alderson, P.G. Effect of low temperature and sucrose on bulb development and on the carbohydrate status of bulbing shoots of tulip in vitro. J. Hortic. Sci. 1990, 65, 193–197. [Google Scholar] [CrossRef]
- Kumar, S.; Kashyap, M.; Sharma, D.R. In vitro regeneration and bulblet growth from lily bulbscale explants as affected by retardants, sucrose and irradiance. Biol. Plant. 2005, 49, 629–632. [Google Scholar] [CrossRef]
- Bach, A. Micropropagation of hyacinths (Hyacinthus orientalis L.). In High-Tech and Micropropagation, Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 1992; pp. 144–159. [Google Scholar]
- Chung, C.H.; Chung, Y.M.; Yang, S.J.; Ko, E.K.; Jeong, S.J.; Nam, J.S.; Kim, G.T.; Yi, Y.B. Effects of storage temperature and sucrose on bulblet growth, starch and protein contents in in vitro cultures of Hyacinthus orientalis. Biol. Plant. 2006, 50, 346–351. [Google Scholar] [CrossRef]
- Squires, W.M.; Langton, F.A.; Fenlon, J.S. Factors influencing the transplantation success of micropropagated narcissus bulbils. J. Hortic. Sci. 1991, 66, 661–671. [Google Scholar] [CrossRef]
- Cheesman, L.; Finnie, J.F.; van Staden, J. Eucomis zambesiaca baker: Factors affecting in vitro bulblet induction. S. Afr. J. Bot. 2010, 76, 543–549. [Google Scholar] [CrossRef]
- Rahimi, M.; Daneshvar, M.H.; Heidari, M. Propagation and bulb formation of Fritillaria (Fritillaria imperialis L.) via in vitro culture. Int. J. Plant Anim. Environ. Sci. 2014, 4, 707–710. [Google Scholar]
- Kizil, S.; Sogut, T.; Acay, U.; Sarihan, B.; Sesiz, U.; Khawar, K.M. Effects of sucrose concentrations on increase in bulb size of in vitro regenerated Hyacinth (Hyacinthus orientalis L.) bulblets. Sci. Bull. Ser. F Biotechnol. 2017, 2285, 51–55. [Google Scholar]
- Juan-Vicedo, J.; Pavlov, A.; Ríos, S.; Casas, J.L. In vitro culture and micropropagation of the Baetic-Moroccan endemic plant Lapiedra martinezii Lag. (Amaryllidaceae). Vitr. Cell. Dev. Biol. Plant 2019, 55, 725–732. [Google Scholar] [CrossRef]
- Sutlana, J.; Sutlana, N.; Siddique, M.; Islam, A.; Hossain, M.; Hossain, T. In vitro bulb production in Hippeastrum (Hippeastrum hybridum). J. Cent. Eur. Agric. 2010, 11, 469–474. [Google Scholar] [CrossRef]
- Iezzoni, A.F.; Pritts, M.P. Applications of Principal Component Analysis to Horticultural Research. HortScience 1991, 26, 334–338. [Google Scholar] [CrossRef]
- Rotaru, A.S.; Pop, I.D.; Vatcă, A.; Cioban, A. Usefulness of principal components analysis in agriculture. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2012, 69, 54–59. [Google Scholar]
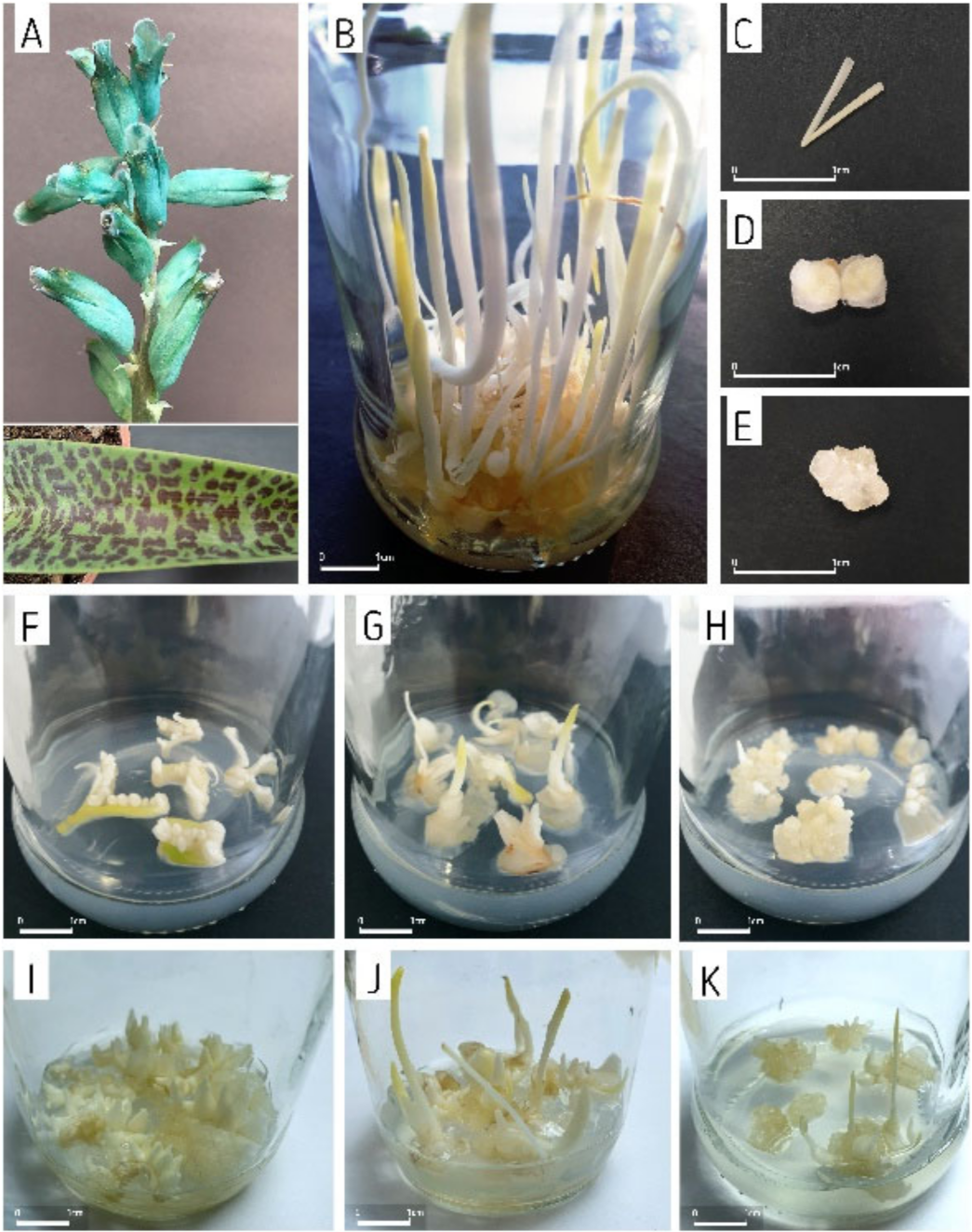
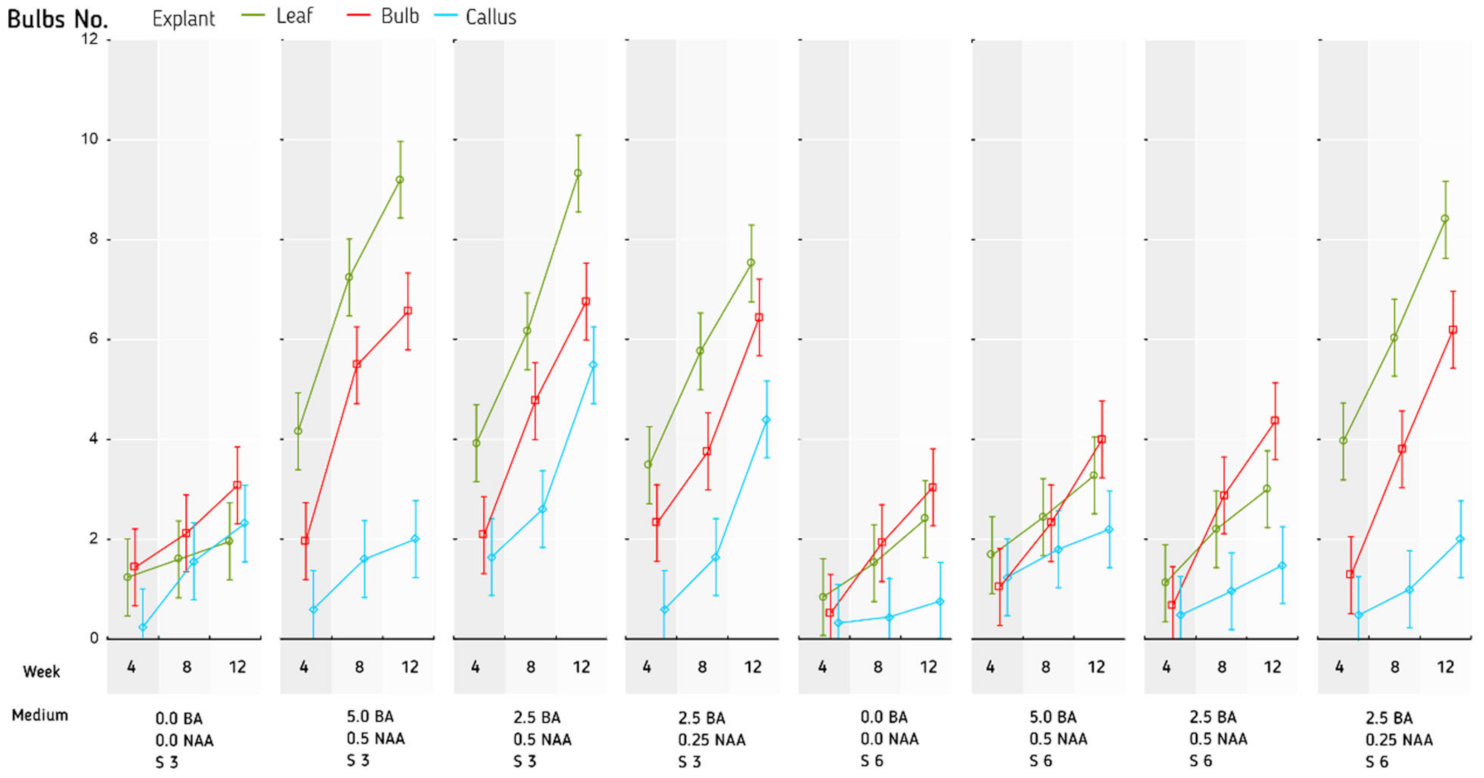
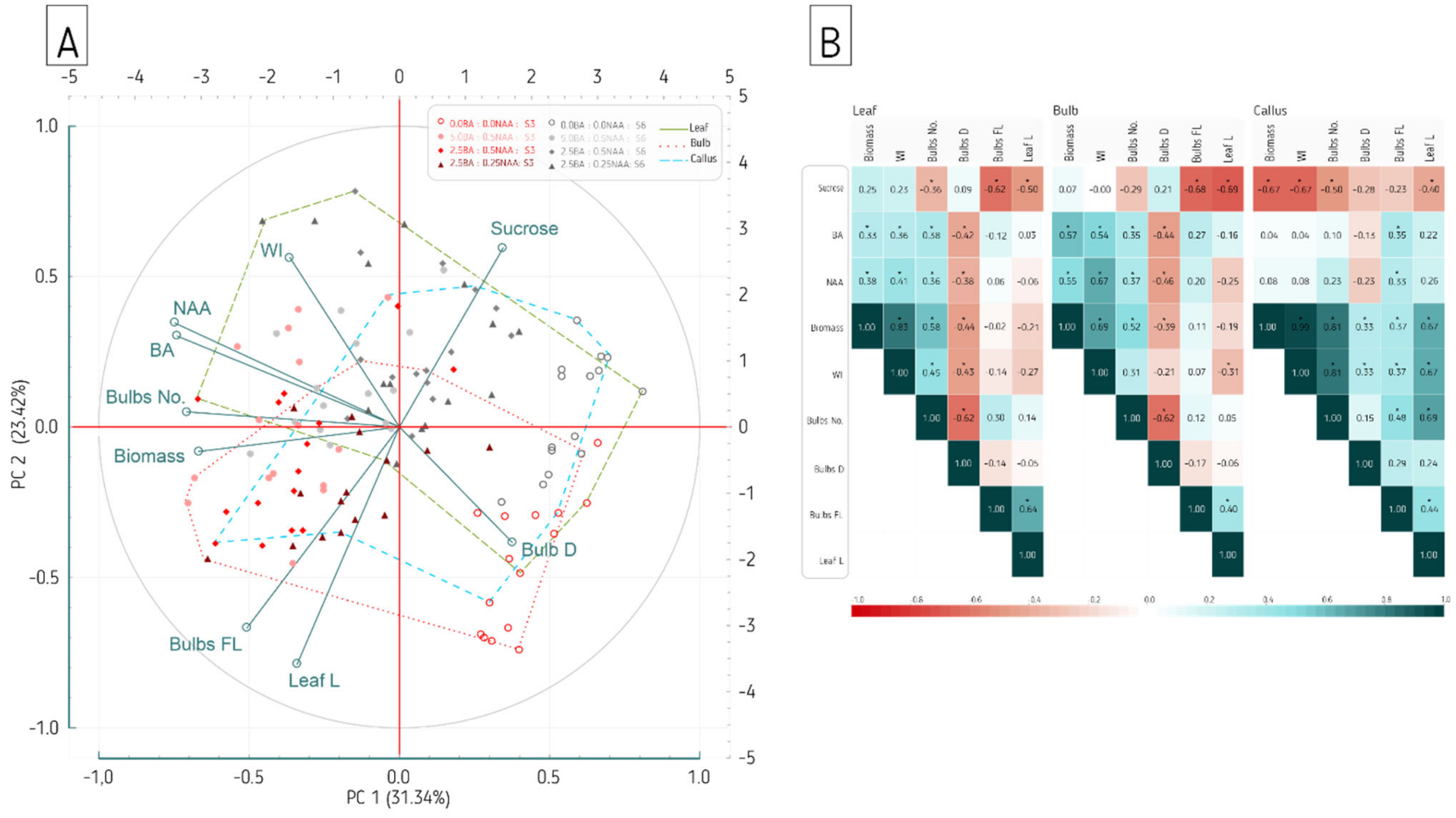
| Sucrose (%) | Plant Growth Regulators (µM) | |
|---|---|---|
| BA | NAA | |
| 3 | 0 | 0 |
| 5 | 0.5 | |
| 2.5 | 0.5 | |
| 2.5 | 0.25 | |
| 6 | 0 | 0 |
| 5 | 0.5 | |
| 2.5 | 0.5 | |
| 2.5 | 0.25 | |
| Source of Variation | df | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| Biomass (g) | WI | Bulbs No. | Bulb D (mm) | Bulbs FL (%) | Leaf L (mm) | ||
| Explant (E) | 2 | 7.32 *** | 62,252,188 *** | 105.30 *** | 1.93 * | 7689.6 *** | 1743.53 *** |
| Medium (M) | 7 | 3.35 *** | 5,219,339 *** | 58.28 *** | 5.48 *** | 6673.0 *** | 260.01 *** |
| E × M | 14 | 1.12 *** | 2,928,211 *** | 9.80 ** | 1.04 * | 489.9 NS | 60.87 NS |
| Error | 96 | 0.27 | 808,766 | 3.98 | 0.53 | 347.1 | 41.65 |
| Characters | Principal Components | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Biomass | −0.668545 | −0.08133 | 0.220848 |
| WI | −0.364687 | 0.563526 | 0.594663 |
| Bulbs No. | −0.707269 | 0.050211 | 0.560183 |
| Bulb D | 0.376447 | −0.384364 | 0.160915 |
| Bulbs FL | −0.507503 | −0.668136 | −0.148332 |
| Leaf L | −0.340757 | −0.788754 | −0.084753 |
| Sucrose | 0.345083 | 0.595031 | −0.255695 |
| BA | −0.739398 | 0.302067 | −0.479799 |
| NAA | −0.747778 | 0.345689 | −0.440573 |
| Eigen Value | 2.82 | 2.12 | 1.26 |
| Percentage of Variance | 31.34 | 23.42 | 14.01 |
| Cumulative % of Variance | 31.33 | 54.75 | 68.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maślanka, M.; Mazur, J.; Kapczyńska, A. In Vitro Organogenesis of Critically Endangered Lachenalia viridiflora. Agronomy 2022, 12, 475. https://doi.org/10.3390/agronomy12020475
Maślanka M, Mazur J, Kapczyńska A. In Vitro Organogenesis of Critically Endangered Lachenalia viridiflora. Agronomy. 2022; 12(2):475. https://doi.org/10.3390/agronomy12020475
Chicago/Turabian StyleMaślanka, Małgorzata, Justyna Mazur, and Anna Kapczyńska. 2022. "In Vitro Organogenesis of Critically Endangered Lachenalia viridiflora" Agronomy 12, no. 2: 475. https://doi.org/10.3390/agronomy12020475
APA StyleMaślanka, M., Mazur, J., & Kapczyńska, A. (2022). In Vitro Organogenesis of Critically Endangered Lachenalia viridiflora. Agronomy, 12(2), 475. https://doi.org/10.3390/agronomy12020475






