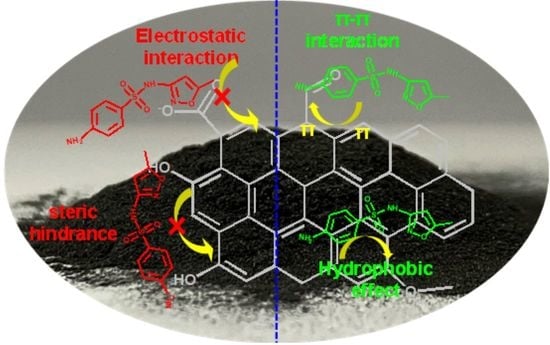
The Effects of Chemical Oxidation and High-Temperature Reduction on Surface Functional Groups and the Adsorption Performance of Biochar for Sulfamethoxazole Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbents
2.3. Characterization of Adsorbents
2.4. Adsorption Experiments
3. Results
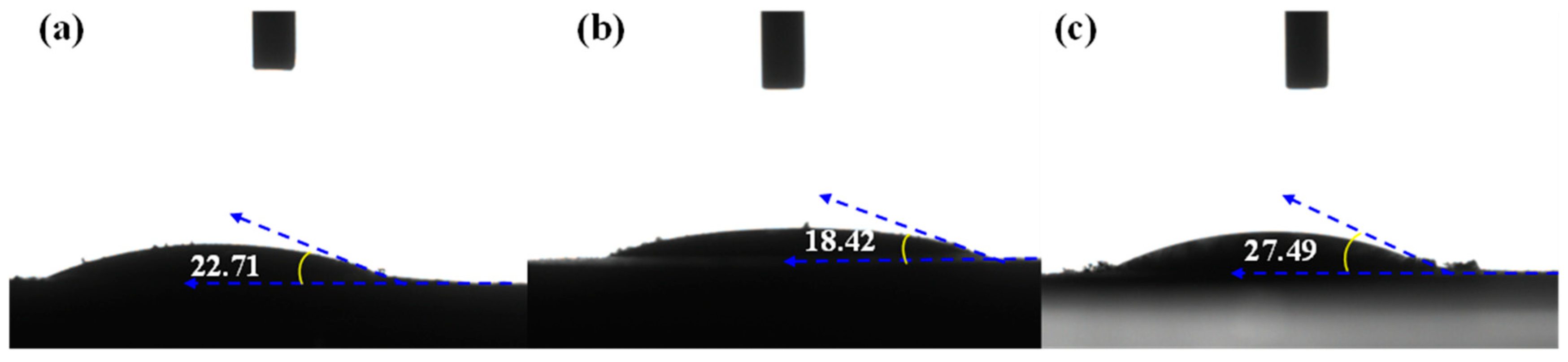
3.1. Characterization of Adsorbents
3.2. Adsorption Isotherms and Adsorption Kinetics
3.3. The Effect of Coexisting Interferents on SMX Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nam, S.W.; Jung, C.; Li, H.; Yu, M.; Flora, J.R.V.; Boateng, L.K.; Her, N.; Zoh, K.-D.; Yoon, Y. Adsorption characteristics of diclofenac and sulfamethoxazole to graphene oxide in aqueous solution. Chemosphere 2015, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, Y.T.; Zhu, Y.H.; Yu, H.; Peng, Y.Z. Adsorption and co-adsorption of tetracycline and doxycycline by one-step synthesized iron loaded sludge biochar. Chemosphere 2019, 236, 1224254. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhang, G.D.; Liu, Y.; Lu, S.Y.; Qin, P.; Guo, X.C.; Bi, B.; Wang, L.; Xi, B.D.; Wu, F.C.; et al. Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing. China Environ. Pollut. 2019, 246, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Rahele, R.; Hassan, B. A comparative adsorption study of sulfamethoxazole onto graphene and graphene oxide nanosheets through equilibrium, kinetic and thermodynamic modeling. Process Saf. Environ. 2016, 102, 20–29. [Google Scholar]
- Guan, Y.D.; Wang, B.; Gao, Y.; Liu, W.; Zhao, X.; Huang, X.; Yu, J.H. Occurrence and fate of antibiotics in the aqueous environment and their removal by constructed wetlands in China: A review. Pedosphere 2017, 27, 42–51. [Google Scholar] [CrossRef]
- Wang, T.; Ai, S.; Zhou, Y.; Luo, Z.; Dai, C.; Yang, Y.; Zhang, J.C. Adsorption of agricultural wastewater contaminated with antibiotics, pesticides and toxic metals by functionalized magnetic nanoparticles. J. Environ. Chem. Eng. 2018, 6, 6468–6478. [Google Scholar] [CrossRef]
- Mandu, I.; Gao, B.; Zimmerman, A.; Zhang, M.; Hao, C. Synthesis, characterization, and dye sorption ability of carbon nanotube–biochar nanocomposites. Chem. Eng. J. 2014, 236, 39–46. [Google Scholar]
- Jiang, S.F.; Sheng, G.P.; Jiang, H. Advances in the characterization methods of biomass pyrolysis products. J. ACS Sustain. Chem. Eng. 2019, 7, 12639–12655. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. J. Bioresour. Technol. 2017, 46, 150–159. [Google Scholar] [CrossRef]
- Greiner, B.G.; Shimabuku, K.K.; Summers, R.S. Influence of biochar thermal regeneration on sulfamethoxazole and dissolved organic matter adsorption. Environ. Sci. Water Res. Technol. 2018, 4, 169–174. [Google Scholar] [CrossRef]
- Teixidó, M.; Pignatello, J.J.; Beltrán, J.L.; Granados, M.; Peccia, J. Speciation of the Ionizable Antibiotic Sulfamethazine on Black Carbon (Biochar). Environ. Sci. Technol. 2011, 45, 10020–10027. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Pan, B.; Zhang, D.; Xiao, D.; Li, H.; Wang, C.; Ning, P. The sorption of organic contaminants on biochars derived from sediments with high organic carbon content. Chemosphere 2013, 90, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.; Dallmeyer, I.; Garcia-Perez, M. Modification of biochar surface by air oxidation: Role of pyrolysis temperature. Biomass Bioenergy 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Chen, D.Y.; Yu, X.Z.; Song, C.; Pang, X.L.; Huang, J.; Li, Y.J. Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresour. Technol. 2016, 218, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.Y.; Sun, J.X.; Chu, L.; Cui, L.Q.; Quan, G.X.; Yan, J.L.; Hussain, Q.; Lqbal, M. Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef]
- Yang, J.-C.E.; Zhu, M.-P.; Dionysiou, D.D.; Yuan, B.L.; Fu, M.-L. Interplay of bicarbonate and the oxygen-containing groups of carbon nanotubes dominated the metal-free activation of peroxymonosulfate. Chem. Eng. J. 2022, 430, 133102. [Google Scholar] [CrossRef]
- Ntzoufra, P.; Vakros, J.; Frontistis, Z.; Tsatsos, S.; Kyriakou, G.; Kennou, S.; Manariotis, I.D.; Mantzavinos, D. Effect of sodium persulfate treatment on the physicochemical properties and catalytic activity of biochar prepared from spent malt rootlets. J. Environ. Chem. Eng. 2021, 9, 105071. [Google Scholar] [CrossRef]
- Hu, P.D.; Long, M.C. Cobalt-catalyzed sulfate radical-based advanced oxidation:A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Wang, H.; Xia, W.; Lu, P. Study on adsorption characteristics of biochar on heavy metals in soil. Korean J. Chem. Eng. 2017, 34, 1867–1873. [Google Scholar] [CrossRef]
- Tao, Q.; Chen, Y.X.; Zhao, J.W.; Li, B.; Li, Y.H.; Tao, S.Y.; Li, M.; Li, Q.Q.; Xu, Q.; Li, Y.B.; et al. Enhanced Cd removal from aqueous solution by biologically modified biochar derived from digestion residue of corn straw silage. Sci. Total Environ. 2019, 674, 213–222. [Google Scholar] [CrossRef]
- Qian, L.B.; Zhang, W.Y.; Yan, J.C.; Han, L.; Gao, W.G.; Liu, R.Q.; Chen, M.F. Effective removal of heavy metal by biochar colloids under different pyrolysis temperatures. Bioresour. Technol. 2016, 206, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Liu, J.W.; Zhang, X.; Xu, K.; He, L.M.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Raman spectroscopy of biochar from the pyrolysis of three typical Chinese biomasses: A novel method for rapidly evaluating the biochar property. Energy 2020, 202, 117644. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, Y.; Yan, J.C.; Qian, L.B.; Han, L.; Chen, M.F. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: Important role of biochar defect structures. Chem. Eng. J. 2019, 370, 614–624. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, P.; Gong, B.; Lu, Y.; Zhu, N.; Hu, Z. Preservation of Fe complexes into layered double hydroxides improves the efficiency and the chemical stability of Fe complexes used as heterogeneous photo-Fenton catalysts. Appl. Surf. Sci. 2013, 286, 371–378. [Google Scholar] [CrossRef]
- Fan, S.S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Pal, D.; Maiti, S.K. Abatement of cadmium (Cd) contamination in sediment using tea waste biochar through meso-microcosm study. J. Clean. Prod. 2019, 212, 986–996. [Google Scholar] [CrossRef]
- Huang, H.; Niu, Z.; Shi, R.R.; Tang, J.C.; Lv, L.; Wang, J.; Fan, Y.M. Thermal oxidation activation of hydrochar for tetracycline adsorption: The role of oxygen concentration and temperature. Bioresour. Technol. 2020, 306, 123096. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.M.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/ TPR Study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Wang, Y.F.; Gao, Z.X.; Shang, Y.X.; Qi, Z.Y.; Zhao, W.; Peng, Y.Z. Proportional modulation of zinc-based MOF/carbon nanotube hybrids for simultaneous removal of phosphate and emerging organic contaminants with high efficiency. Chem. Eng. J. 2021, 417, 128063. [Google Scholar] [CrossRef]
- Qi, C.L.; Ma, X.L.; Ning, G.Q.; Song, X.Y.; Chen, B.; Lan, X.Y.; Li, Y.F.; Zhang, X.; Gao, J.S. Aqueous slurry of S-doped carbon nanotubes as conductive additive for lithium ion batteries. Carbon 2015, 92, 245–253. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhang, H.; Song, X.X.; Han, X.K.; Bao, J.J.; Zhang, N.; He, G.H. Co3O4-based catalysts derived from natural wood with hierarchical structure for elemental mercury oxidation. J. Energy. Inst. 2021, 94, 285–293. [Google Scholar] [CrossRef]
- Cho, H.H.; Smith, B.A.; Wnuk, J.D.; Fairbrother, D.H.; Ball, W.P. Influence of surface oxides on the adsorption of naphthalene onto multiwalled carbon nanotubes. Environ. Sci. Technol. 2008, 42, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.B.; He, M.J.; Xu, X.Y.; Cao, X.D.; Tsang, D.C.W. Impacts of different activation processes on the carbon stability of biochar for oxidation resistance. Bioresour. Technol. 2021, 338, 125555. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, Z.; Zhao, J.; Herbert, S.; Xing, B. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ. Pollut. 2013, 181, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Lian, F.; Bao, Q.; Liu, Z.; Song, Z.; Zhu, L. Impact of low molecular weight organic acids (LMWOAs) on biochar micropores and sorption properties for sulfamethoxazole. Environ. Pollut. 2016, 214, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kan, E. Effects of pyrolysis temperature on the physicochemical properties of alfalfa-derived biochar for the adsorption of bisphenol A and sulfamethoxazole in water. Chemosphere 2019, 218, 741–748. [Google Scholar] [CrossRef]
- Zhang, X.; Guang, D.D.; Zhang, J.J.; Lei, X.B.; Lian, Q.Y.; Holmes, W.E.; Zappi, M.e.; Yao, H. Insight into the activation mechanisms of biochar by boric acid and its application for the removal of sulfamethoxazole. J. Hazard. Mater. 2022, 424, 127333. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, Y.; Lee, G.; Kim, Y.; Han, J.; Park, C.M. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4-biochar composite. Bioresour. Technol. 2019, 281, 179–187. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Mehala, R.; Sharumitha, T.G.; Surendhar, D. Enhanced adsorptive removal of sulfamethoxazole from water using biochar derived from hydrothermal carbonization of sugarcane bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.H.; Li, L.Y.; Wang, Y.; Liu, N.; Smith, R.L., Jr. Removal of hydrophilic ionic liquids from aqueous solutions by adsorption onto high surface area oxygenated carbonaceous material. Chem. Eng. J. 2014, 256, 407–414. [Google Scholar] [CrossRef]
- Li, Q.; Yu, W.; Guo, L.W.; Wang, Y.H.; Zhao, S.Y.; Zhou, L.; Jiang, X.H. Sorption of sulfamethoxazole on inorganic acid solution etched biochar derived from Alfalfa. Materials 2021, 14, 1033. [Google Scholar] [CrossRef]
- Ji, L.L.; Chen, W.; Zheng, S.R.; Xu, Z.Y.; Zhu, D.Q. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir 2009, 25, 11608–11613. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Li, D.Y.; Zhang, Z.H.; Logan, B.E.; Liu, G.H.; Sun, M.C.; Dai, C.C.; Feng, Y.J. Unveiling the correlation of Fe3O4 fractions upon the adsorption behavior of sulfamethoxazole on magnetic activated carbon. Sci. Total Environ. 2021, 757, 143717. [Google Scholar] [CrossRef] [PubMed]
- Daifullah, A.; Girgis, B.S.; Gad, H. A study of the factors affecting the removal of humic acid by activated carbon prepared from biomass material. Colloid. Surf. A 2004, 235, 1–10. [Google Scholar] [CrossRef]
- Yang, D.X.; Li, J.; Luo, L.; Deng, R.Y.; He, Q.; Chen, Y. Exceptional levofloxacin removal using biochar-derived porous carbon sheets: Mechanisms and density-functional-theory calculation. Chem. Eng. J. 2020, 387, 124103. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The role of variable surface charge and surface complexation in the adsorption of humic acid on magnetite. Colloid. Surf. A 2004, 230, 99–109. [Google Scholar] [CrossRef]
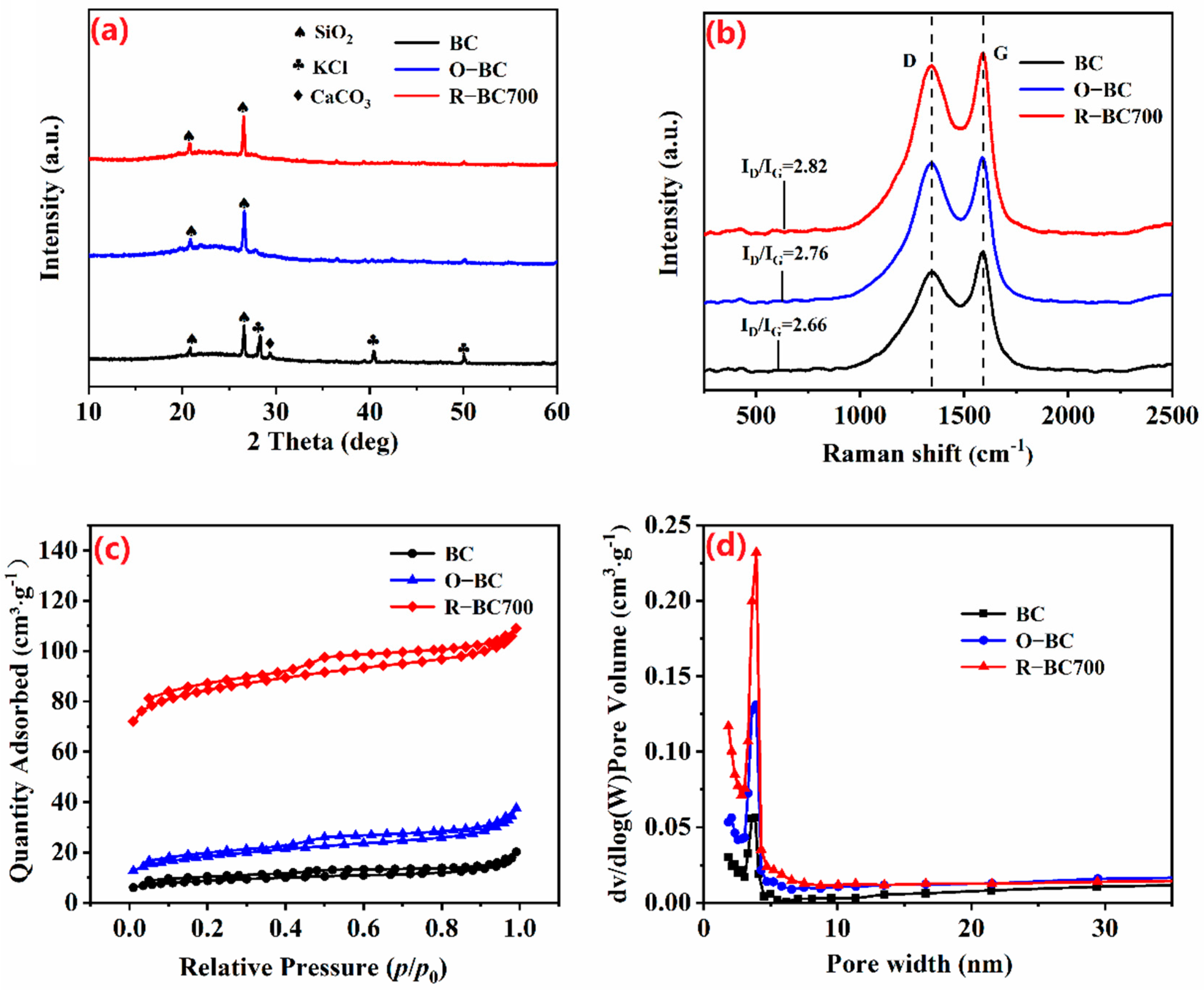




| Biochars | BET Surface Area (SBET a, m2·g−1) | Pore Volume | Average Pore Width (L0, nm) | ||
|---|---|---|---|---|---|
| Total Pore (Vt b, cm3·g−1) | Micropore (Vmicro c, cm3·g−1) | Micropore (VMic, %) | |||
| BC | 31 | 0.031 | 0.005 | 16.12 | 3.96 |
| O-BC | 65 | 0.058 | 0.010 | 17.24 | 3.52 |
| R-BC700 | 294 | 0.168 | 0.096 | 57.14 | 2.29 |
| Biochars | Elemental Analysis (%) | XPS (Atomic%) | |||
|---|---|---|---|---|---|
| C | H | H/C | C | O | |
| BC | 52.35 | 1.53 | 0.029 | 85.10 | 14.90 |
| O-BC | 57.70 | 1.76 | 0.030 | 79.33 | 20.67 |
| R-BC600 | 62.87 | 0.77 | 0.012 | 87.07 | 12.93 |
| R-BC700 | 61.40 | 0.86 | 0.014 | 87.30 | 12.70 |
| R-BC800 | 61.75 | 0.20 | 0.003 | 87.43 | 12.57 |
| Biochars | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax (mg·g−1) | KL (L·mg−1) | R2 | KF (L·g−1) | n | R2 | |
| BC | 0.16 | 2.435 | 0.88 | 0.109 | 0.187 | 0.92 |
| O-BC | 4.91 | 0.424 | 0.93 | 1.800 | 0.307 | 0.98 |
| R-BC600 | 6.64 | 4.235 | 0.78 | 4.612 | 0.138 | 0.99 |
| R-BC700 | 14.66 | 0.153 | 0.95 | 2.969 | 0.447 | 0.99 |
| R-BC800 | 6.71 | 3.951 | 0.97 | 4.220 | 0.172 | 0.88 |
| Adsorbents | qmax (mg·g−1) | Equilibrium Time (min) | Conditions | References | ||
|---|---|---|---|---|---|---|
| C0 (mg·L−1) | pH | Adsorbent Dosage (g·L−1) | ||||
| Giant reed BC (300 °C) | 4.99 | Not mentioned | - | - | - | [35] |
| Giant reed BC (600 °C) | 1.93 | |||||
| Rice straw BC (300 °C) | 4.21 | Not mentioned | - | - | - | [36] |
| Wheat straw BC (300 °C) | 6.75 | |||||
| Pine Sawdust BC | 13.80 | 30 | 20.5 | 4.0 | 2.0 | [37] |
| Alfalfa-derived biochar | 90.00 | 180 | 100 | 5.0 | 0.1 | [38] |
| Wood-derived biochar (boric acid-activated) | 212.87 | 240 | 50 | 5.0 | 0.5 | [39] |
| CuZnFe2O4-Bamboo BC | 212.87 | 30 | 20 | 7.0 | 0.2 | [40] |
| R-BC700 | 14.66 | 20 | 15 | 5.3 | 0.5 | This work |
| Biochars | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | ||||
|---|---|---|---|---|---|---|
| qe (mg·g−1) | K1 (min−1) | R2 | qe (mg·g−1) | K2 (g·(mg·min)−1) | R2 | |
| O-BC | 3.62 | 0.048 | 0.88 | 3.81 | 0.020 | 0.95 |
| R-BC700 | 9.60 | 0.040 | 0.83 | 10.17 | 0.012 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Yu, J.; Li, W.; He, X.; Li, X. The Effects of Chemical Oxidation and High-Temperature Reduction on Surface Functional Groups and the Adsorption Performance of Biochar for Sulfamethoxazole Adsorption. Agronomy 2022, 12, 510. https://doi.org/10.3390/agronomy12020510
Hou J, Yu J, Li W, He X, Li X. The Effects of Chemical Oxidation and High-Temperature Reduction on Surface Functional Groups and the Adsorption Performance of Biochar for Sulfamethoxazole Adsorption. Agronomy. 2022; 12(2):510. https://doi.org/10.3390/agronomy12020510
Chicago/Turabian StyleHou, Jifei, Jialin Yu, Wenxuan Li, Xiudan He, and Xuede Li. 2022. "The Effects of Chemical Oxidation and High-Temperature Reduction on Surface Functional Groups and the Adsorption Performance of Biochar for Sulfamethoxazole Adsorption" Agronomy 12, no. 2: 510. https://doi.org/10.3390/agronomy12020510
APA StyleHou, J., Yu, J., Li, W., He, X., & Li, X. (2022). The Effects of Chemical Oxidation and High-Temperature Reduction on Surface Functional Groups and the Adsorption Performance of Biochar for Sulfamethoxazole Adsorption. Agronomy, 12(2), 510. https://doi.org/10.3390/agronomy12020510