Abstract
White button mushroom—Agaricus bisporus (J.E.Lange) Imbach—is among the most popular cultivated mushrooms worldwide. The most serious challenge in industrial mushroom production is the green mold disease caused by Trichoderma species. Our aim was to isolate and examine bacterial strains from mushroom casing material for their potential use as biocontrol agents. Twenty-seven bacterial strains were isolated and tested against mold pathogens of white button mushroom. The Bacillus velezensis strain SZMC 25431 was selected for further examination and tested under simulated Agaricus cultivation conditions against T. aggressivum SZMC 23834 in a 1200-L Fitotron SGC120 standard plant growth chamber. Our results showed that the bacterial treatment was effective against the pathogen in all cases, but the best results were achieved at an application concentration of 105 cells mL−1. Industrial-scale experiments were also carried out in Agaricus growing houses with a bearing surface of 480 m2: the bacterial suspension was mixed in water tanks applied for daily irrigation. The results suggest that the bacterial treatment may even increase the crop yield of A. bisporus. Based on our results, we concluded that the selected B. velezensis strain may potentially be used for biological and integrated treatment in Agaricus cultivation.
1. Introduction
White button mushroom (Agaricus bisporus) is among the most popular cultivated mushrooms in the world. In industrial mushroom production, a large number of fungi, bacteria, viruses, and nematodes can cause infections [1]. Trichoderma aggressivum f. europaeum (formerly known as Trichoderma harzianum Rifai Th2) is the main fungal pathogen of A. bisporus responsible for the green mold epidemics in Europe [2]. It is able to colonize mushroom compost and prevent the growth of A. bisporus, leading to a drastic reduction in crop yield [3]. The symptoms are easily recognizable by the green color of the cropping surface, covering large regions; in some cases, it covers the entire mushroom bed. When fruiting bodies are formed, brown necrotic lesions often develop [2]. Green mold has been present in mushroom production for decades and is unquestionably the most severe infection, sometimes causing entire crop losses [4]. In the 1950s, it was described as a minor problem due to the poor quality of mushroom growing material and lack of hygiene, but this characterization changed after the first outbreaks in the 1980s [3]. According to reports about the epidemics in the British Isles during 1985–1986 and in late 1990 and 1991, green mold caused crop losses of about GBP 3–4 million [3]. Later, a similar epidemic caused a loss of over USD 30 million in North America [5]. The disease later spread in France, Spain, Hungary, Poland, Croatia, Mexico, and Australia [4]. The infection spreads so aggressively that a single contaminated grain spawn in a 45 kg tray of compost resulted in a crop loss of 12–46% [6]. The North American green mold biotype, T. aggressivum f. aggressivum (formerly known as Trichoderma harzianum Th4), was first reported in Europe by Hatvani et al. [7] from a Hungarian mushroom farm, where it caused nearly 100% crop loss.
Cobweb disease is a very characteristic infection caused by several Cladobotryum species, including C. dendroides (teleomorph: Hypomyces rosellus (Alb. & Schwein.) Tul.) and C. mycophilum (teleomorph: Hypomyces odoratus G.R.W. Arnold) [8]. As the name suggests, symptoms appear as cobweb-like white mycelium over the surface of the casing material and fruiting bodies. Infected mushrooms ultimately darken, turn brown, and start to rot [9]. These pathogens can infect several mushroom species, including wild mushrooms, in their natural habitats; however, the white button mushroom is the most frequently reported host [10,11]. Another important pathogen of the cultivated white button mushroom is Lecanicillium fungicola Preuss (syn: Verticillium fungicola), which causes dry bubble disease. The severity of this disease may vary and manifest as a necrotized cap, blow-out, and undifferentiated mycelium called dry bubble [12]. In either case, the symptomatic fruiting bodies are unsuitable for the market, which may result in remarkable economic losses [13,14]. Wet bubble disease caused by Hypomyces perniciosus Magnus (syn: Mycogone perniciosa) is also considered a serious pathogen of the white button mushroom. The most common source of the infection is the casing material [15].
The prevention of fungal diseases relies primarily on maintaining strict hygiene in mushroom production facilities [16,17], but standard procedures for disease control also include chemical treatments. Due to the extensive use of chemical products in agriculture, pesticides are often initially present in the mushroom growing material before the composting process. Pesticide residues are also frequently detected in mushroom fruiting bodies [18]. Furthermore, as a consequence of the accumulation of chemicals used in agriculture, numerous pathogens adapted and became resistant to many commercially available pesticides [19]. Fungicides in the EU are strictly regulated. Only two fungicides, prochloraz and metrafenone, are allowed in white button mushroom cultivation in Europe, while in North America, chlorothalonil, thiabendazole, and thiophanate-methyl are also approved [20,21,22]. The European Union also approved sodium chloride (table salt) as an antifungal agent for spot treatment. When applied, salt acts as a desiccant, removing water from the pathogen, thus preventing the spread of the disease [23].
In contrast to chemicals known to pollute natural ecosystems [24,25], the biological control of plant and mushroom diseases based on the exploitation of natural microbial antagonists against pathogens is a safe and environmentally friendly alternative. The market is dominated by Bacillus-based microbial biopesticides used in plant and mushroom protection [26,27,28,29]. The popularity of biocontrol products based on Bacillus strains is largely due to their low production costs, as cheap industrial by-products can be used as substrates for their fermentation [30].
The bacterial community in the mushroom growing material is of great importance, as the amount and type of bacteria might influence sporophore formation [31]. Although some Bacillus species with antagonistic activity against Trichoderma aggressivum also negatively affect white button mushroom [32], biological fungicides based on the Bacillus velezensis strain QST 713 [33] (formerly known as B. subtilis QST 713) and other isolates were previously tested against green mold, showing promising results. The application of B. velezensis QST 713 was examined as a coating for mushroom grain spawn and as compost additive [34], while a B. subtilis strain was tested as a spray applied to mushroom beds [35]. The results of these studies suggested that a biofungicide based on Bacillus species could serve as a harmless alternative to synthetic fungicides in mushroom production [34,36,37,38].
Considering all of the above-mentioned methods, the application of Bacillus spp. strains may provide an environmentally friendly, low-cost, and effective solution to mold problems in mushroom cultivation. The aim of this study was to identify potential bioeffectors for white button mushroom cultivation and to create a treatment plan that is simple to apply, is neutral for white button mushroom, and has antagonistic potential against harmful molds.
2. Materials and Methods
2.1. Isolation and Identification of Bacillus Strains
Samples from mushroom casing material were collected from white button mushroom growing houses in September 2016 and September 2017. One gram of soil sample was suspended in 10 mL of 0.9% NaCl solution and gently mixed for 15 min. A 10-fold dilution series was made from the supernatant, and each dilution step was incubated at 90 °C for 15 min. Heat treatment was followed by spreading 50 µL of each dilution step on the surface of yeast extract–sucrose (YES) medium (3 g L−1 yeast extract, 10 g L−1 sucrose, 20 g L−1 agar, supplemented with 15-15 μg mL−1 nystatin and carbendazim). The plates were incubated at room temperature for 48 h. Afterwards, single colonies were randomly picked and separated until homogeneous on YES medium. The isolated strains were deposited in the Szeged Microbiology Collection (SZMC, www.szmc.hu, accessed on 9 February 2022).
In order to identify bacterial isolates, DNA was extracted using E.Z.N.A.® Bacterial DNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s instructions. The strains were identified by sequence analysis of a fragment of the 16S RNA gene—the universal marker for the identification of bacteria [39]—targeted by the Eub-8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and Eub-534R (5′-ATTACCGCGGCTGCTGG-3′) primers. In the case of the Bacillus strain selected for further experiments, identification was also based on additional loci with discrimination power within the B. subtilis group: a fragment of the DNA gyrase alpha subunit (gyrA) [40] targeted by the GyrA-F (5′-CAGTCAGGAAATGCGTACGTCCTT-3′) and GyrA-R (5′-CAAGGTAATGCTCCAGGCATTGCT-3′) primers, as well as a fragment of the rpoB gene [41] targeted by the RpobR (5′-GTCCTACATTGGCAAGATCGTATC-3′) and RpobF (5′-AGGTCAACTAGTTCAGTATGGACG 3′) primers. PCR conditions for EuB were as follows: initial denaturation at 95 °C for 2 min; 30 cycles of denaturation at 94 °C for 30 s, annealing at 51 °C for 30 s, and elongation at 72 °C for 1 min. The initial denaturation for the gyrA region was set to 95 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 45 s, and elongation at 72 °C for 1 min. In the case of rpoB, the initial denaturation was at 95 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s, and elongation at 72 °C for 1 min. The final extension step after the last cycle was at 72 °C for 2 min in all reactions. Sequences were determined by Sanger dideoxy sequencing at the sequencing service of the Biological Research Centre, Szeged, Hungary, on a 3500 Genetic Analyzer 8-Capillary Array (Applied Biosystems™) platform.
The DNA sequences were submitted to NCBI GenBank (accession numbers OM320465-OM320477, OM326895, and OM469331) and analyzed by the Basic Local Alignment Search Tool (National Center for Biotechnology Information [42]).
2.2. Confrontation Assays
To test the antagonistic ability of isolated bacteria against mushroom pathogenic fungi, in vitro confrontation assays were carried out on YES medium at 20 °C. The pathogenic fungi tested were T. aggressivum f. europaeum SZMC 1811, SZMC 23227, and T. aggressivum f. aggressivum SZMC 1813. For the assays, 10 µL of overnight bacterial culture grown in liquid YES medium and 5 mm diameter disks of 3–4-day-old fungal colonies grown on YES medium were used. The tested bacterium and the mushroom pathogenic fungal strain were inoculated in spots 25 mm apart on the surface of the medium. After three days of incubation, the radius of the mushroom pathogen was measured. The bacterial strains that could inhibit the growth of all examined Trichoderma strains by 20% were further tested against additional mushroom pathogens (T. aggressivum f. aggressivum SZMC 23035 and SZMC 23834, T. aggressivum f. europaeum SZMC 1746 and SZMC 1811, and C. mycophilum SZMC 20792 and SZMC 20793).
The antagonistic properties of B. velezensis SZMC 25431 were also tested on a continuous layer of fungal pathogens. To create a consistent mycelial coating, 700 μL of the conidial suspensions of the fungi was spread evenly on the surface of PDA medium.
The optimal concentration of colony-forming units (CFU, conidia mL−1) to create a continuous fungal layer on the surface of the medium for 700 μL inoculum was determined for all strains (Table 1). After the inoculum was spread evenly on the surface of PDA medium (39 g L−1 potato dextrose agar; VWR, Debrecen, Hungary), cultures were placed in a laminar hood to dry the surface. Bacterial cultures were prepared by inoculation in YEG broth (5 g L−1 glucose, 3 g L−1 yeast extract) and incubation overnight. The following day, 10 μL of the bacterial culture (105, 106, and 107 CFU mL−1) was pipetted onto the center of the PDA plates. Each combination was applied in three replicates. The plates were incubated at 24 °C for three days, and then the inhibition zones were measured.

Table 1.
List of fungal strains used in the confrontation experiments.
2.3. Extracellular Enzyme and Lipopeptide Production of B. velezensis SZMC 25431
The bacterial strain was cultivated in 50 mL of nutrient solution (5 g L−1 glucose, 3 g L−1 yeast extract) at 25 °C for five days. From the liquid culture, 1 mL was transferred to Eppendorf tubes and centrifuged (Thermo Scientific, Fresco™ 17 Microcentrifuge, Osterode am Harz, Germany) at 5000× g for 5 min. During the experiments, six types of substrates were used to measure enzyme activities: N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide for chymotrypsin-like protease, N-alpha-benzoyl-L-arginine p-nitroanilide for trypsin-like protease, 4-nitrophenyl N-acetyl-β-D-glucosaminide for exochitinase, 4-nitrophenyl-β-D-glucopyranoside for β-glucosidase, 4-nitrophenyl-β-D-cellobioside for cellobiohydrolase, and 4-nitrophenyl-palmitate for lipase/esterase enzyme activities (Sigma-Aldrich, Budapest, Hungary). The chromogenic substrates were dissolved in dimethyl-sulfoxide to a concentration of 3 mM. To measure optical densities, 50 µL of substrate solution, 50 µL of supernatant, and 50 µL of phosphate buffer (pH 6.6) were transferred to the wells of 96-well microtiter plates. The measurements were carried out with a Spectrostar Nano Microplate Reader (BMG Labtech, Ortenberg, Germany) at 405 nm. Enzyme activities were calculated in units, with 1 unit of enzyme activity defined as the amount of enzyme required to release 1 µmol p-nitrophenol or p-nitroaniline min−1 under the determined reaction conditions [44].
To investigate the lipopeptide-producing properties of B. velezensis SZMC 25431, overnight culture of the bacterial strain grown on glucose–yeast extract agar medium (5 g L−1 glucose, 3 g L−1 yeast extract, 20 g L−1 agar) was used to inoculate nutrient solution. This solution was inoculated with a 25 mL suspension containing 2.5×107 CFU, and the culture was incubated for five days at 25 °C. After the incubation period, the culture was centrifuged at 8000× g for 10 min, and the aqueous phase was removed and transferred to a new tube. The pH of the supernatant was adjusted to 2 by adding 500 µL of hydrochloric acid (HCl) to precipitate the lipopeptide compounds. The suspension was incubated at 4 °C overnight and centrifuged (Thermo Scientific, Fresco™ 17 Microcentrifuge, Osterode am Harz, Germany) at 8500× g for 10 min. The aqueous phase was discarded, and the precipitate was resuspended in 1 mL of ethanol and centrifuged again at 8000× g for 5 min. The lipopeptide content of the supernatant was determined by HPLC–MS. HPLC was performed using a Thermo UltiMate 3000 UHPLC (Thermo Fisher Scientific, Waltham, MA, USA) device. Separation was performed using a Gemini column (150 × 2.0 mm, 3 µm) (Phenomenex, Torrance, CA, USA). The column temperature was maintained at 30 °C. The injection volume was 5 µL. The mobile phase used to separate metabolites consisted of water containing 0.1% acetic acid (A) and methanol–acetonitrile 50/50 (V/V) with 0.1% acetic acid (B). The flow rate was 0.2 mL min−1. The composition of solvent B varied as follows: 0–4 min, held at 5%; 4 min, held at 80%; 24 min, held at 95%; 28 min, held at 95%; then immediate reduction to 5%.
Mass spectrometry was performed using a Thermo OrbiTrap Q Exactive Plus HRMS spectrometer (Thermo Scientific, San Jose, CA, USA). The instrument was operated as follows: ion-spray voltage, 3000 V; ion source, HESI; capillary temperature, 280 °C, source temperature, 175 °C; sheath gas flow rate, 20 units; auxiliary gas flow rate, 15 units. The flow rate units are arbitrarily specified by the Thermo Scientific™ TraceFinder™ software. The standards used during the measurements were iturin A (I1774), fengycin (SMB00292), and surfactin (S3523) from Sigma-Aldrich (Budapest, Hungary).
2.4. Experiments in Plant Growth Chamber
A Fitotron SGC120 standard plant growth chamber (Weiss Gallenkamp, Loughborough, UK) was previously optimized for mushroom growing [45]. A pilot experiment was performed on mushroom compost to determine whether infecting the compost, the casing material, or both with green mold has an influence on the yield. The strain used in the experiment was T. aggressivum f. aggressivum SZMC 23834. The pilot experiment was carried out in the Fitotron SGC120 standard plant growth chamber in 15 × 15 × 10 cm flower pots in three replicates. The growing material applied in our experiments was commercial spawned phase III compost used in the same manner as in the mushroom houses. Half of the commercial casing material was sterilized in an autoclave for 40 min, while the other half remained untreated. For each pot, 500 g of compost and 500 g of casing material, provided by Új Champignons Ltd. (Kerecsend, Hungary), were used. The treatments were carried out in the combinations listed in Supplementary Table S1.
The compost added at casing (CAC-ing) technique was applied to promote colonization. The importance of this step is that properly mixing a small portion of phase III compost with the casing material provides notable benefits, and most importantly, it reduces the growing cycle and increases mushroom yield [46]. Mushroom crops were picked and weighed throughout the cultivation period.
The most effective Bacillus strain that significantly inhibited the growth of the green mold agents in vitro was selected for pot experiments, which were performed in the Fitotron SGC120 standard plant growth chamber. Four consecutive experiments were performed with B. velezensis applied at gradually decreasing concentrations from 107 to 105 cells mL−1 to determine the optimal concentration. The treatments were carried out at the concentrations listed in Table 2.

Table 2.
The concentrations of bacterial cells and fungal conidia in the pot experiments.
Before the confrontation assays in plant pots, the B. velezensis SZMC 25431 isolate was inoculated with a loop in 100 mL Erlenmeyer flasks containing 50 mL of YEG broth (2 g L−1 yeast extract, 2 g L−1 glucose) and cultured overnight. The casing material was sterilized in an autoclave and left to cool down. Conidial suspensions of the T. aggressivum f. aggressivum SZMC 23834 isolate were prepared by washing the surface of 5-day-old fungal cultures grown on PDA medium with 0.9% NaCl solution. A Bürker chamber was used to count the conidia and cells in the stock solution and then diluted to reach the relevant cell concentrations.
The mushroom compost and the sterilized casing material were weighed before all experiments and placed into 15 × 15 × 10 cm (first experiment) and 15 × 15 × 20 cm (second, third, and fourth experiments) flower pots. The treatments were carried out by pouring 50 mL of the solutions on top of the growing material.
The crops were harvested in 3 flushes and weighed throughout the cultivation period (36 days), the data were summarized and analyzed at the end of the experiment, and averages with standard deviations were calculated for each flush per treatment.
2.5. Experiments in Mushroom Growing House
The location of the on-site experiments was the Új Champignons Ltd. (Kerecsend, Hungary). In the facilities, the white button mushroom is grown on a shelf system, with each growing house containing 15 shelves with a total bearing surface of 480 m2. According to the disease history records of the farm, the most frequent and important problem is Trichoderma green mold, followed by dry bubble and cobweb diseases. The B. velezensis SZMC 25431 stock solution was prepared at a concentration of 108 cells mL−1. Fermentation was carried out in a yeast extract–glucose nutrient solution (glucose 10 g L−1, yeast extract 5 g L−1). Stock solutions were added to water tanks used for daily irrigation (Supplementary Figure S1).
During the treatments, 6.25 L of diluted suspension (105 cell mL−1) per square meter was applied to the growing trays. Two successive industrial experiments were carried out in mushroom growing houses. In both experiments, the first mushroom house was treated only with B. velezensis, and in the second, B. velezensis was combined with fungicides (1.1 g/m2 SPORGON® 50 WP, BASF; 0.66 mL/m2 VIVANDO®, BASF), while the third treatment included the use of the above-mentioned fungicides (which is the standard treatment on the farm) without the application of bacterial suspension. Throughout the mushroom growing period, mushrooms were harvested and weighed in the same manner as the regular crops.
3. Results
3.1. Isolation and Identification of Bacillus Strains
In this study, 27 Bacillus strains were isolated from different white button mushroom casing samples collected from the mushroom growing houses of Új Champignons Ltd. (Kerecsend, Hungary). The strains were identified by sequence analysis of a fragment of the 16S rRNA gene, which was supplemented with the gyrA and rpoB loci in the case of the Bacillus strain selected for the biocontrol application (Supplementary Table S2).
3.2. Selection of Potential Biocontrol Strains
The bacterial isolates were subjected to confrontation assays against T. aggressivum (Figure 1) as well as C. mycophilum strains (Figure 2). Among four Bacillus isolates (SZMC 25295, SZMC 25431, SZMC 25442, and SZMC 25449) that showed clear inhibitory activity on T. aggressivum strains, the most effective strain against the tested pathogens of white button mushroom was SZMC 25431, identified as Bacillus velezensis based on its gyrA and rpoB sequences.
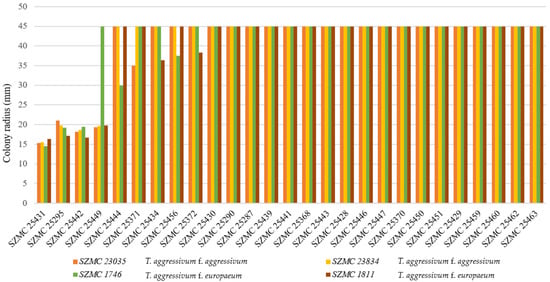
Figure 1.
Confrontation assay performed between Trichoderma aggressivum strains and Bacillus isolates. A colony radius of 45 mm means no inhibition and full coverage of the yeast extract–sucrose agar (YES) plate by the fungal colony after 3 days of incubation at 20 °C.
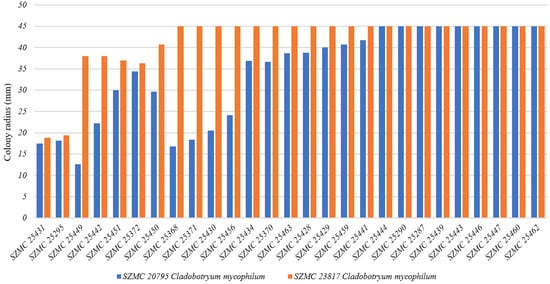
Figure 2.
Confrontation assay performed between Cladobotryum mycophilum strains and Bacillus isolates. A colony radius of 45 mm means no inhibition and full coverage of the yeast extract–sucrose (YES) agar plate by the fungal colony after 3 days of incubation at 20 °C.
Inhibition zones on the continuous fungal layer are shown in Figure 3. Based on our findings, the treatment with the B. velezensis SZMC 25431 isolate could inhibit all tested mushroom pathogenic mold strains, although in different concentrations and with varying efficiency. The bacterial cell concentrations had no effect on the size of the inhibition zones in the cases of T. aggressivum f. aggressivum SZMC 23035, Trichoderma aggressivum f. europaeum SZMC 1746, and the two C. mycophilum strains (SZMC 20795 and SZMC 20817). The Trichoderma strains seemed to react to a similar extent with relatively small differences. The T. aggressivum f. europaeum isolates (SZMC 1811 and SZMC 1746) proved to be more susceptible than the T. aggressivum f. aggressivum strains (SZMC 23834 and SZMC 23035). The bacterial suspension also inhibited the mycelial growth of the oyster mushroom pathogens T. pleuroti SZMC 12454 and T. pleuroticola SZMC 1729 strains. The T. aggressivum f. europaeum isolates displayed the largest inhibition zones with an average of 5 mm in all replicates.
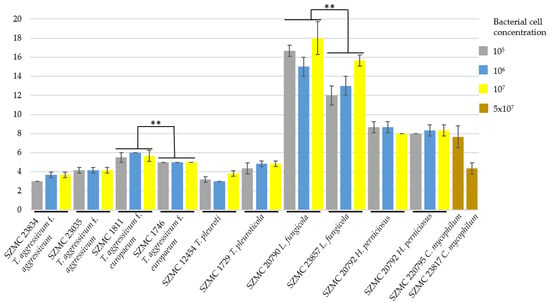
Figure 3.
Effectiveness of treatment with Bacillus velezensis SZMC 25431 on the continuous layer of different fungal pathogens of white button mushroom and oyster mushroom. Columns show the radius of the inhibition zone. The order of fungal strains (from left to right) is as follows: Trichoderma aggressivum f. aggressivum, SZMC 23834 and 23035; T. aggressivum f. europaeum, SZMC 1811 and 1746; T. pleuroti, SZMC 12454; T. pleuroticola, SZMC 1729; Lecanicillium fungicola, SZMC 20790 and SZMC 23857; Hypomyces perniciosus, SZMC 20792 and SZMC 20793; Cladobotryum mycophilum, SZMC 20795 and SZMC 23817. The p value calculated with paired t-tests was p < 0.01 **.
The B. velezensis suspension was also highly efficient against the tested L. fungicola strains (SZMC 20790 and SZMC 23857), and the most effective concentration was 107 cells mL−1. The mycovirus-containing L. fungicola strain SZMC 23857 appeared to be less sensitive to the bacterium. The inhibition zones formed in the fungal layer of the two H. perniciosus strains had sizes between those formed by the Trichoderma and Lecanicillium strains. However, the tests encountered difficulties with both C. mycophilum strains due to complete inhibition of bacterial growth, except for the SZMC 20795 isolate with 107 CFU mL−1 bacterial cell suspension. Even in this case, the bacteria grew on the inoculated area in only one of the three plates. On the other hand, on the plate where bacterial growth was successful, an inhibition zone of 7 mm was formed in the fungal layer. We assume that the spore suspension of C. mycophilum strains contained metabolites that were able to inhibit the growth of B. velezensis at these concentrations. The experiment was repeated with the undiluted bacterial cell suspension (5 × 107 CFU mL−1), where the bacteria were able to grow and also formed inhibition zones in all cases. The C. mycophilum SZMC 20795 strain appeared to be more sensitive, as the radius of the inhibition zones was almost twice that in the case of C. mycophilum SZMC 23817.
3.3. Enzyme Activities and Lipopeptide Production of B. velezensis SZMC 25431
The extracellular enzyme activity data of the investigated strain are presented in Figure 4. According to our results, this bacterial strain produces every type of enzyme activity investigated, with lipase activity being the most dominant.
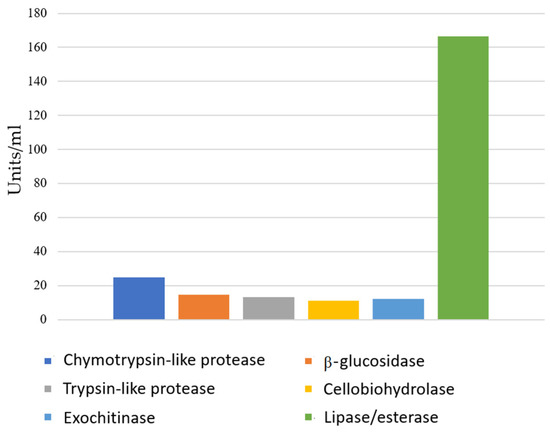
Figure 4.
Extracellular enzyme activities of Bacillus velezensis SZMC 25431.
The B. velezensis SZMC 25431 strain was found to produce several variants of surfactins, iturins, and fengycins, which were determined based on the detected m/z values corresponding to the protonated molecular ions [M + H]+ of the lipopeptides. Comparing the proportions in which these antibiotics were produced, surfactin levels proved to exceed those of the other two (Figure 5 and Figure 6).
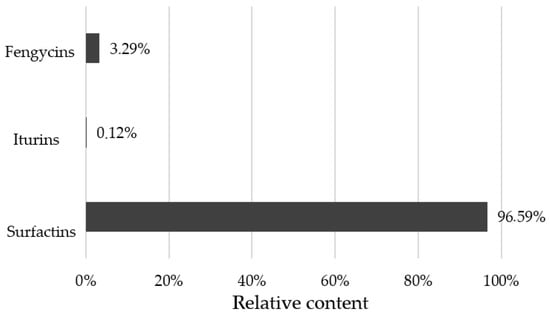
Figure 5.
Relative lipopeptide production of Bacillus velezensis SZMC 25431 expressed as percentages of the total amount.
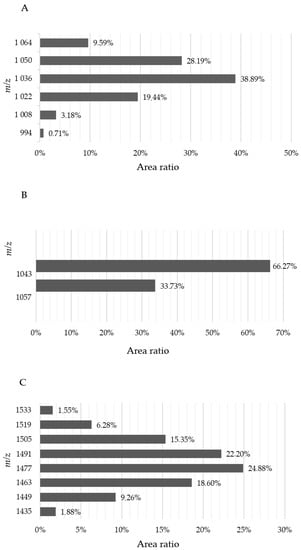
Figure 6.
Relative lipopeptide production of Bacillus velezensis SZMC 25431 expressed as percentages according to the molecular weights of their protonated molecular ions ([M + H]+). (A) Surfactins, (B) iturins, and (C) fengycins. m/z: Mass-to-charge ratio.
3.4. Green Mold Infection and Biocontrol Treatment in Plant Growth Chamber
According to our pilot study, the inoculation of the casing material (Treatment 5) and mixing the conidia of T. aggressivum in the compost and the casing material (Treatment 6) both markedly reduced the yield (Supplementary Figure S2). Based on the results, we concluded that inoculating the casing material with 50 mL/pot of T. aggressivum conidial suspension (107 conidia mL−1) is sufficient for the artificial infection of the mushroom growing material.
In the first and second experiments of the biocontrol setup, pots treated with T. aggressivum conidial suspension revealed the lowest yield (Figure 7). The symptoms of green mold infection appeared only during the final days of the cultivation period. The 50 mL/pot bacterial cell suspensions applied at 107 and 106 cells mL−1 concentrations had a negative effect on the crop yield: lower quantities of mushrooms were harvested from the pots treated with the B. velezensis suspension. In the second experiment, pots infected with T. aggressivum but also treated with B. velezensis resulted in higher total yields compared to pots inoculated with T. aggressivum alone (Figure 7B).
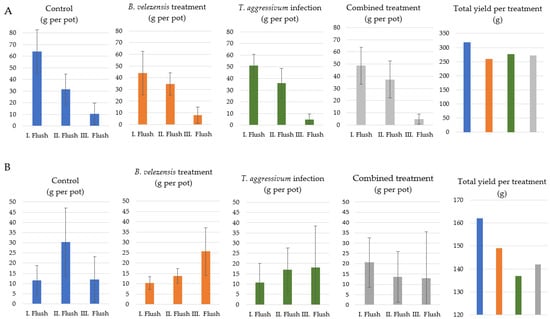
Figure 7.
Effect of different treatments on the crop yield of Agaricus bisporus grown in pots during the 1st (A) and 2nd (B) experiments in plant growth chamber. Control: without Bacillus/Trichoderma inoculation; Bacillus velezensis only at 106 (A) and 105 (B) cells g−1 of casing; Trichoderma aggressivum at 105 (A) and 106 (B) conidia g−1 of casing; combined treatment: B. velezensis + T. aggressivum at 106 + 105 (A) and 105 + 106 (B) cells g−1 of casing. Total yields for each treatment represent the sum of yields of the 3 replicates across 3 flushes.
Based on our results, we concluded that lowering the concentration of bacterial cells might have a beneficial effect on cropping. Two consecutive experiments were carried out to evaluate the possible consequences, and the results are shown in Figure 8. Decreasing the cell concentration of the bacterial suspension to 104 cells g−1 of compost mL−1 had a positive effect on cropping. We also found that the pots infected with the T. aggressivum conidial suspension produced the lowest yield. An increase in total yield was observed in cases when the mushroom growing material was infected with T. aggressivum but also treated with B. velezensis suspension, suggesting that the bacterium shows biocontrol activity under the simulated growth conditions.
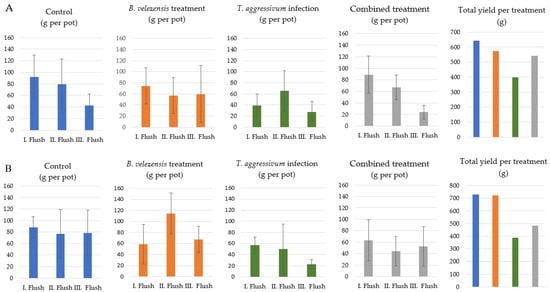
Figure 8.
Effect of different treatments on the crop yield of Agaricus bisporus grown in pots during the 3rd (A) and 4th (B) experiments in plant growth chamber. Control: without Bacillus/Trichoderma inoculation; Bacillus velezensis only at 104 cells g−1 of casing (A,B); Trichoderma aggressivum at 106 cells g−1 of casing (A,B); combined treatment: B. velezensis + T. aggressivum at 104 + 106 cells g−1 of casing. Total yields for each treatment represent the sum of yields of the 3 replicates across 3 flushes.
3.5. Influence of B. velezensis SZMC 25431 on the Yield of A. bisporus in Mushroom Growing Houses
The mushroom yields from the first and second flushes and the total yield recorded in the growing house experiments are shown in Table 3. According to the results, the tested bacterial strain did not seem to hinder the development of white button mushroom, since the application of the treatment did not result in crop reduction. The results also suggest that treatment with the B. velezensis SZMC 25431 suspension may even have a positive effect on fruiting body formation, both in the combined treatment (bacteria and fungicides) and in mushroom houses treated with B. velezensis SZMC 25431 only. In our first on-site experiment, mushroom houses receiving the combined treatment reached a yield increase of 34.6%, while in the second experiment, it was 17.4%.

Table 3.
Mushroom yield in two consecutive growing house experiments.
4. Discussion
The aim of our study was to isolate, select, and investigate effective bacterial strains against the most important pathogens of white button mushroom. The experiments provided insights into the effect of the bacterial treatment of white button mushroom in vitro under simulated growth conditions and in mushroom growing houses.
Mushroom growing materials have been shown to be valuable sources of antagonistic Bacillus strains. The ability to form endospores ensures the survival of Bacillus strains under unfavorable environmental conditions, their persistence in the ecosystem, and the production of dry, stable Bacillus-based products with a long shelf-life [47]. In France, B. velezensis strain QST 713 is used against T. aggressivum in industrial white button mushroom production [48]. In the present study, we successfully isolated and investigated bacteria from Agaricus casing material. Two series of confrontation assays were conducted to test the antagonistic potential of the isolates: the first one to select the most effective bacterial strain against the most common fungal pathogens, and another one to investigate whether the effectiveness is concentration-dependent. During the confrontation assays on a continuous layer of pathogens, the B. velezensis strain SZMC 25431 showed antagonistic activity against all tested Trichoderma species, as well as against L. fungicola, to a much greater extent than other bacteria. Other screening efforts were also aimed at the selection of Bacillus strains with biocontrol potential against harmful molds in mushroom cultivation. Bhat et al. [38] reported the in vitro antagonistic activity of five Bacillus strains isolated from compost and the casing layer against L. fungicola. A native B. subtilis strain from mushroom compost showed considerable suppression of T. aggressivum f. europaeum and T. harzianum both in vitro and in vivo: the B. subtilis strain B-38 reduced disease incidence in plots infected with T. harzianum T54 and T. aggressivum f. europaeum T77 as well, although statistically significant differences were not observed [49]. Stanojević et al. [29] reported 22 Bacillus spp. strains that inhibited the growth of T. aggressivum f. europaeum, T. harzianum, and T. koningii, while only 13 isolates inhibited the growth of T. atroviride. Molecular analyses showed that these strains were from the B. subtilis, B. amyloliquefaciens, B. licheniformis, and B. pumilus species. Liu et al. [50] isolated the B. velezensis B154 strain from Agaricus mushroom compost and tested it against several fungal pathogens, including T. harzianum, which causes green mold, and Neurospora sitophila, which causes red bread mold and was previously isolated from a commercial Agaricus mushroom factory in China.
Bacillus strains were studied in oyster mushroom production as well: several bacterial species were isolated from wheat-straw substrates that were later tested for antifungal activity against Trichoderma. Bacillus spp., Pseudomonas spp., and Actinomycetes showed inhibition, but Paenibacillus polymyxa inhibited the growth of T. harzianum to a much greater extent than other isolates and stimulated defense mechanisms through the production of laccases [51].
The mechanism of antagonism in the case of Bacillus strains is not fully elucidated, although they are known to produce a significant number of secondary metabolites; thus, the most probable mechanism is antibiosis. The investigation of the antifungal activity of B. velezensis B154 revealed a new type of fengycin with a molecular mass of 1498.7633 Da [50]. According to the results of genome analyses, Bacillus spp. possess genes involved in the production of important lipopeptides, such as iturin, fengycin [52], surfactin, bacilysin, difficidin [53], bacilysocin, and bacillomycin [54]. The mechanism behind the biocontrol effects of B. velezensis strain QST 713 was reported to be the overexpression of genes related to surfactin and fengycin production in the presence of the pathogen [48]. In the present study, the B. velezensis strain SZMC 25431 was found to produce several variants of lipopeptides, predominantly surfactins, which might form the basis of the antagonistic properties of this strain. Based on the recent knowledge and nomenclature of surfactins [55,56], in B. velezensis SZMC25431, the amount of this lipopeptide family constituted variants possessing m/z values of 994 (C12-[Sur], Rt = 22.29 min; C13-[Val7], Rt = 24.69 min), 1008 (C13-[Sur], Rt = 23.71 min; C14-[Val7], Rt = 27.00 min), 1022 (C14-[Sur], Rt = 24.34 min; C14-[Sur], Rt = 25.53 min; C14-[Sur], Rt = 25.95 min; C15-[Val7], Rt = 28.35 min), 1036 (C14-[AME5], Rt = 24.91 min; C15-[Sur], Rt = 26.58 min; C15-[Sur], Rt = 27.25 min; C15-[Val7], Rt = 28.61 min), 1036 (C14-[AME5], Rt = 24.91 min; C15-[Sur], Rt = 26.58 min; C15-[Sur], Rt = 27.25 min; C16-[Val7], Rt = 28.61 min), 1050 (C15-[AME5], Rt = 25.63 min; C15-[AME5], Rt = 26.16 min; C16-[Sur], Rt = 27.82 min), and 1064 (C16-[AME5], Rt = 26.83 min). Of these components, variants detected at m/z 1036 were produced in the highest proportion (Figure 6A). In the case of iturins, one C14-iturin A (Rt = 9.11 min; m/z 1043) and two C15-iturin A compounds (Rt = 8.9 min and Rt = 9.68 min; m/z 1057) were detected and identified according to the work of Yang et al. [57]. The variant linked to the C14 fatty acid was present in the highest proportion among the iturins (Figure 6B). In accordance with recent reports on fengycins [57], the strain produced both A and B types of fengycins containing 4-4 variants. The A variants were detected at m/z 1435 (C14-fengycin A; Rt = 8.31 min and Rt = 9.71 min), at m/z 1449 (C15-fengycin A; Rt = 8.77 min), at m/z 1463 (C16-fengycin A; Rt = 7.88 min, Rt = 8.56 min, and Rt = 9.13 min), and at m/z 1477 (C17-fengycin A; Rt = 7.61 min, Rt = 8.03 min, Rt = 8.87 min, Rt = 9.23 min, and Rt = 9.49 min), while the B variants were determined at m/z 1491 (C16-fengycin B; Rt = 7.72 min, Rt = 8.24 min, Rt = 9.28 min, and Rt = 9.54 min), at m/z 1505 (C17-fengycin B; Rt = 7.92 min, Rt = 8.39 min, Rt = 9.33 min, and Rt = 10.06 min), at m/z 1519 (C18-fengycin B; Rt = 8.02 min, Rt = 8.7 min, and Rt = 9.9 min), and at m/z 1533 (C19-fengycin B; Rt = 8.2 min). Among the fengycins, the C17-fengycin A and C16-fengycin B variants were produced in the highest proportions by the B. velezensis strain SZMC 25431 (Figure 6C).
Using plant growth chambers to simulate the growth conditions in mushroom houses is a practical way to conduct small-scale experiments. The Fitotron SGC120 standard plant growth chamber (Weiss Gallenkamp, Loughborough, UK) is universal, and it can also be used for other purposes, for example, plant cultivation [58] and insect rearing [59], and a simulated mushroom growing environment can also be achieved [45]. In our study, the bacterial treatment was highly effective against the T. aggressivum f. aggressivum SZMC 25834 strain under the mushroom growing conditions simulated in the plant growth chamber. We also concluded that the effectiveness of the treatment rose as the applied concentration of the strain was decreased from 106 to 104 cells g−1 of casing.
According to the findings of Liu et al. [50], treatment with B. velezensis B145 significantly increased the yield of white button mushroom. During our farm experiments, the bacterial suspension of B. velezensis was mixed in 1000 L water tanks; thus, the treatment was carried out at a concentration of 6.25 L/m2 with a suspension of 105 cells mL−1 in a mushroom growing house with a 480 m2 total bearing surface. This concentration promoted the growth of the white button mushroom, reaching yield increases of 26% and 17.9%, as well as 34.6% and 17.4% excess yield in combined treatments during the first and second experiments, respectively, which seems to be very promising. However, this needs further confirmation, as due to the difficulties of arranging large-scale experiments in industrial mushroom farms, a limitation of this trial is that it was performed at a single farm with only two replications.
5. Conclusions
The application of the treatment with the B. velezensis SZMC 25431 strain had a positive effect on mushroom yield, both alone and in combination with commercial fungicides. Therefore, it has the potential to be applied as a tool for biological and integrated treatments. The treatment suggested in this study can be incorporated into the watering routine of mushroom farms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12020467/s1, Table S1: List of treatments during the pilot experiment; Table S2: Bacterial strains isolated and identified during the study; Figure S1. Application of the Bacillus velezensis suspension in the mushroom growing house using water tanks; Figure S2. Effect of artificial Trichoderma aggressivum infection on the crop yield of Agaricus bisporus grown in pots in a plant growth chamber.
Author Contributions
Conceptualization, L.K., C.C. and C.V.; data curation, A.V., L.H., L.K., A.S., C.C. and C.V.; investigation, R.B., M.V., S.V., A.V., A.B., A.S., H.A., J.B., N.B.-B., A.M. and C.C.; methodology, A.B. and H.A.; project administration, C.C. and L.K., resources, C.C. and C.V.; software, A.S., supervision, L.K., L.H., C.C. and C.V.; validation, A.B. and A.S.; visualization, A.B., A.S. and R.B.; writing—original draft, R.B., M.V. and L.K.; writing—review and editing, C.C., L.H. and L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hungarian Government and the European Union within the frame of the Széchenyi 2020 Programme through grant GINOP-2.2.1-15-2016-00006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
DNA sequences are available in the NCBI GenBank Nucleotide database (https://www.ncbi.nlm.nih.gov, accessed on 9 February 2022) under accession numbers OM320465-OM320477, OM326895, and OM469331).
Acknowledgments
We would like to express our appreciation to Új Champignons Ltd. for providing mushroom growing materials for plant growth chamber experiments and access to mushroom houses for on-site experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hatvani, L.; Antal, Z.; Manczinger, L.; Szekeres, A.; Druzhinina, I.S.; Kubicek, C.P.; Nagy, A.; Nagy, E.; Vágvölgyi, C.; Kredics, L. Green mold disease of Agaricus bisporus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathology 2007, 97, 532–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Largeteau, M.L.; Savoie, J.M. Microbially induced diseases of Agaricus bisporus: Biochemical mechanisms and impact on commercial mushroom production. Appl. Microbiol. Biotechnol. 2010, 86, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kredics, L.; García-Jimenez, L.; Naeimi, S.; Czifra, D.; Urbán, P.; Manczinger, L.; Vágvölgyi, C.; Hatvani, L. A challenge to mushroom growers: The green mould disease of cultivated champignons. In Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2010; pp. 295–305. ISBN 978-84-614-6194-3. [Google Scholar]
- Kredics, L.; Naeimi, S.; Hatvani, L.; Vágvölgyi, C.; Cai, F.; Druzhinina, I.S.; Manczinger, L. ‘The Good, the Bad and the Ugly’ in the shades of green: The genus Trichoderma in the spotlight. Indian Phytopathol. 2021, 74, 403–411. [Google Scholar] [CrossRef]
- Samuels, G.; Dodd, S.; Castlebury, L.; Petrini, O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 2002, 94, 146–170. [Google Scholar] [CrossRef]
- Grogan, H.M.; Noble, R.; Gaze, R.H.; Fletcher, J.T. Control of Trichoderma harzianum—A weed mould of mushroom cultivation. In Proceedings of the Brighton Crop Protection Conference, Brighton, UK, 18–21 November 1996; Volume 1, pp. 337–342, ISBN 0-948404-99-X. [Google Scholar]
- Hatvani, L.; Kredics, L.; Allaga, H.; Manczinger, L.; Vágvölgyi, C.; Kuti, K.; Geösel, A. First report of Trichoderma aggressivum f. aggressivum green mold on Agaricus bisporus in Europe. Plant Dis. 2017, 101, 1052–1056. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.; Grogan, H.; Fitzpatrick, D.; Kavanagh, K. Analysis of the effect of Bacillus velezensis culture filtrate on the growth and proteome of Cladobotryum mycophilum. Fungal Biol. 2022, 126, 11–19. [Google Scholar] [CrossRef]
- White, P.F.; Smith, J. Pests. In Mushroom Pest and Disease Control: A Colour Handbook; Fletcher, J., Gaze, R., Eds.; Manson Publishing: London, UK, 2007; pp. 140–159. ISBN 978-1-84076-083-4. [Google Scholar]
- Carrasco, J.; Navarro, M.J.; Gea, F.J. Cobweb, a serious pathology in mushroom crops: A review. Spanish J. Agricult. 2017, 15, e10R01. [Google Scholar] [CrossRef] [Green Version]
- Lakkireddy, K.; Khonsuntia, W.; Kües, U. Mycoparasite Hypomyces odoratus infests Agaricus xanthodermus fruiting bodies in nature. AMB Express 2020, 10, 141. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Baars, J.J.; Kalkhove, S.I.C.; Lugones, L.G.; Wösten, H.A.B.; Bakker, P.A.H.M. Lecanicillium fungicola: Causal agent of dry bubble disease in white-button mushroom. Mol. Plant. Pathol. 2010, 11, 585–595. [Google Scholar] [CrossRef]
- Soković, M.; Van Griensven, L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Mills, P.R.; Thomas, J.; Sergeant, M.R.; Costa, A.; Collopy, P.D.; Bailey, A.M.; Foster, G.D.; Challen, M.P. Interactions between Agaricus bisporus and the pathogen Verticillium fungicola. In Stress in Yeasts and Filamentous Fungi, 1st ed.; Avery, S.V., Stratford, M., van West, P., Eds.; Academic Press: London, UK, 2008; Volume 27, pp. 1–17. ISBN 978-0-12-374184-4. [Google Scholar]
- Kouser, S.; Shah, S.; Ahmed, M.; Shah, M.D.; Sheikh, P.A. Morphological characteristics of wet bubble disease (Mycogone perniciosa) isolated from button mushroom (Agaricus bisporus) and assessment of factors affecting disease development and spread. Afr. J. Microbiol. Res. 2015, 9, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Romaine, C.P.; Royse, D.J.; Schlagnhaufer, C. Superpathogenic Trichoderma resistant to topsin M found in Pennsylvania and Delaware. Mushr. News 2005, 53, 6–9. [Google Scholar]
- Pecchia, J.A.; Beyer, D.M. Pest management on US commercial mushroom farms. Outlooks Pest Manag. 2013, 24, 28–29. [Google Scholar] [CrossRef]
- Dhar, B.; Ahlawat, O.P.; Sharma, R.K.; Dubey, J.K.; Patiyal, S.K.; Thakur, M. Organic button mushroom (Agaricus bisporus) production, quality produce and pesticide residue analysis. In Mushroom Biology and Mushroom Products, Proceedings of the Sixth International Conference on Mushroom Biology and Mushroom Products, Bonn, Germany, 29 September–3 October 2008; Lelley, J.I., Buswell, J.A., Eds.; Gamu GmbH, Institut für Pilzforschung: Krefeld, Germany, 2008; pp. 203–211. [Google Scholar]
- Hjeljord, L.; Tronsmo, A. Trichoderma and Gliocladium in biological control: An overview. In Trichoderma and Gliocladium, Enzymes, Biological Control and Commercial Applications, 1st ed.; Harman, G.E., Kubicek, C.P., Eds.; CRC Press: Boca Raton, FL, USA, 1998; Volume 2. [Google Scholar] [CrossRef]
- Grogan, H.M.; Gaze, R.H. Fungicide resistance among Cladobotryum spp.—Causal agents of cobweb disease of the edible mushroom Agaricus bisporus. Mycol. Res. 2000, 104, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Kosanović, D.; Potočnik, I.; Vukojević, J.; Stajić, M.; Rekanović, E.; Stepanović, M.; Todorović, B. Fungicide sensitivity of Trichoderma spp. from Agaricus bisporus farms in Serbia. J. Environ. Sci. Health Part B 2015, 50, 607–613. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- European Commission, EFSA (European Food Safety Authority). Technical report on the outcome of the consultation with Member States and EFSA on the basic substance application for (sea) salt (sodium chloride) for use in plant protection as fungicide and insecticide. EFSA Supporting Publ. 2017, 14, 56. [Google Scholar] [CrossRef] [Green Version]
- Di Poi, C.; Costil, K.; Bouchart, V.; Halm-Lemeille, M.P. Toxicity assessment of five emerging pollutants, alone and in binary or ternary mixtures, towards three aquatic organisms. Environ. Sci. Pollut. Res. 2017, 25, 6122–6134. [Google Scholar] [CrossRef] [Green Version]
- Uwizeyimana, H.; Wang, M.; Chen, W.; Khan, K. The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil. Environ. Toxicol. Pharmacol. 2017, 55, 20–29. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Thakore, Y. The biopesticide market for global agricultural use. Ind. Biotech. 2006, 2, 194–208. [Google Scholar] [CrossRef]
- Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Bacillus-based Biological Control of Plant Diseases. In Pesticides in the Modern World: Pesticides Use and Management; Stoytcheva, M., Ed.; InTechOpen: London, UK, 2011; pp. 273–302. [Google Scholar] [CrossRef] [Green Version]
- Stanojević, O.; Berić, T.; Potočnik, I.; Rekanović, E.; Stanković, S.; Milijasević-Marić, S. Biological control of green mould and dry bubble diseases of cultivated mushroom (Agaricus bisporus L.) by Bacillus spp. Crop Prot. 2019, 126, 104944. [Google Scholar] [CrossRef]
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Torres, R.; Solsona, C.; Teixidó, N. Production of the postharvest biocontrol agent Bacillus subtilis CPA-8 using low cost commercial products and by-products. Biol. Control 2012, 60, 280–289. [Google Scholar] [CrossRef]
- Cai, W.M.; Yao, H.Y.; Feng, W.L.; Jin, Q.L.; Liu, Y.Y.; Li, N.Y.; Zheng, Z. Microbial community structure of casing soil during mushroom growth. Pedosphere 2009, 19, 446–452. [Google Scholar] [CrossRef]
- Kim, W.G.; Weon, H.Y.; Seok, S.J.; Lee, K.H. In vitro antagonistic characteristics of bacilli isolates against Trichoderma spp. and three species of mushrooms. Mycobiology 2008, 36, 266–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandin, C.; Le Coq, D.; Deschamps, J.; Védie, R.; Rousseau, T.; Aymerich, S.; Briandet, R. Complete genome sequence of Bacillus velezensis QST713: A biocontrol agent that protects Agaricus bisporus crops against the green mould disease. J. Biotechnol. 2018, 278, 10–19. [Google Scholar] [CrossRef]
- Potočnik, I.; Todorović, B.; Rekanović, E.; Luković, J.; Paunović, D.; Milijašević-Marčić, S. Impact of Bacillus subtilis QST713 mushroom grain spawn treatment on yield and green mould control. Pestic. Phytomed. 2018, 33, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Potočnik, I.; Rekanović, E.; Todorović, B.; Luković, J.; Paunović, D.; Stanojević, O.; Milijašević-Marčić, S. The effects of casing soil treatment with Bacillus subtilis Ch-13 biofungicide on green mould control and mushroom yield. Pest. Phytomed. 2019, 34, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Anastassiadou, M.; Arena, M.; Auteri, D.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; Chiusolo, A.; Crivellente, F.; De Lentdecker, C.; et al. Peer review of the pesticide risk assessment of the active substance Bacillus amyloliquefaciens strain QST 713 (formerly Bacillus subtilis strain QST 713). EFSA J. 2021, 19, 6381–6401. [Google Scholar] [CrossRef]
- Aydoğdu, M.; Sülü, S.M.; Kurbetli, İ.; Sülü, G. In vitro and in vivo biological control of the green mold using different bacteria in button mushroom cultivation. Egypt. J. Biol. Pest Cont. 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Bhat, M.A.; Simon, S.; Munshi, N.A.; Bhat, Z.A. In vitro efficacy of casing and compost isolated bacterial inoculants against Verticillium fungicola (Preuss) Hassebrauk and Agaricus bisporus (Lange) Imbach. J. Biol. Control 2010, 24, 137–141. [Google Scholar] [CrossRef]
- Drancourt, M.; Bollet, C.; Carlioz, A.; Martelin, R.; Gayral, J.P.; Raoult, D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J. Clin. Microbiol. 2000, 38, 3623–3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.-H.; Park, B.-S.; Kim, H.-Y.; Lee, K.-H.; Kim, K.S. Antagonistic and plant growth-promoting effects of Bacillus velezensis BS1 isolated from rhizosphere soil in a pepper field. Plant Pathol. J. 2021, 37, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ki, J.S.; Zhang, W.; Qian, P.Y. Discovery of marine Bacillus species by 16S rRNA and rpoB comparisons and their usefulness for species identification. J. Microbiol. Meth. 2009, 77, 48–57. [Google Scholar] [CrossRef]
- NCBI Blast, Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 2004. Available online: https://www.blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 29 December 2021).
- Kartali, T.; Shahab, D.; Nyilasi, I.; Hatvani, L.; Kredics, L.; Vágvölgyi, C.; Papp, T. Double-stranded RNA elements in Lecanicil-lium and Mycogene strains infecting Agaricus bisporus. Mikol. Közl. Clusiana 2017, 56, 103–105. [Google Scholar]
- Kedves, O.; Kocsubé, S.; Bata, T.; Andersson, M.A.; Salo, J.M.; Mikkola, R.; Salonen, H.; Szűcs, A.; Kedves, A.; Kónya, Z.; et al. Chaetomium and Chaetomium-like species from European indoor environments include Dichotomopilus finlandicus sp. nov. Pathogens 2021, 10, 1133. [Google Scholar] [CrossRef] [PubMed]
- Allaga, H.; Büchner, R.; Hatvani, L.; Szekeres, A.; Vágvölgyi, C.; Kredics, L.; Manczinger, L. Adaptation of a plant growth chamber for the experimental cultivation of champignons (Agaricus bisporus). In Proceedings of the 21st Danube-Kris-Mures-Tisza (DKMT) Euroregional Conference on Environment and Health, Novi Sad, Serbia, 6–8 June 2019; Škrbić, B., Ed.; University of Novi Sad, Faculty of Technology: Novi Sad, Serbia, 2019; pp. 51–59. [Google Scholar]
- Zied, D.C.; Minhoni, M.T.A.; Pardo-Gonzalez, J.E.; Pardo-Gimenez, A. A study of compost added to a casing technique in Agaricus bisporus cultivation from phase III bulk compost. Hort. Sci. 2010, 45, 1649–1653. [Google Scholar] [CrossRef] [Green Version]
- Pertot, I.; Alabouvette, C.; Esteve, H.E.; França, S. Mini-Paper—The Use of Microbial Biocontrol Agents against Soil-Borne Diseases; Eip-Agri: Brussels, Belgium, 2015; Available online: http://ec.europa.eu/eip/agriculture/sites/agrieip/files/8_eip_sbd_mp_biocontrol_final.pdf (accessed on 9 February 2022).
- Pandin, C.; Darsonval, M.; Mayeur, C.; Le Coq, D.; Aymerich, S.; Romain, B. Biofilm formation and synthesis of antimicrobial compounds by the biocontrol agent Bacillus velezensis QST713 in an Agaricus bisporus compost micromodel. Appl. Environ. Microbiol. 2019, 85, e00327-19. [Google Scholar] [CrossRef] [Green Version]
- Milijašević-Marčić, S.; Stepanović, S.; Todorović, B.; Duduk, B.; Stepanović, J.; Rekanović, E.; Potočnik, I. Biological control of green mould on Agaricus bisporus by a native Bacillus subtilis strain from mushroom compost. Eur. J. Plant. Pathol. 2017, 148, 509–519. [Google Scholar] [CrossRef]
- Liu, C.; Sheng, J.; Chen, L.; Zheng, Y.; Lee, D.Y.W.; Yang, Y.; Xu, M.; Shen, L. Biocontrol activity of Bacillus subtilis isolated from Agaricus bisporus mushroom compost against pathogenic fungi. J. Agricul. Food Chem. 2015, 63, 6009–6018. [Google Scholar] [CrossRef]
- Velázquez-Cedeño, M.; Farnet, A.M.; Mata, G.; Savoie, J.M. Role of Bacillus spp. in antagonism between Pleurotus ostreatus and Trichoderma harzianum in heat-treated wheat-straw substrates. Bioresour. Technol. 2008, 99, 6966–6973. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Kiesewalter, H.T.; Kovács, R.; Frisvad, J.C.; Weber, T.; Larsen, T.O.; Kovács, Á.T.; Ding, L. Depiction of secondary metabolites and antifungal activity of Bacillus velezensis DTU001. Synth. Syst. Biotechnol. 2019, 4, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Q.; Li, Q.L.; Hu, J.C. The complete genome sequence of Bacillus velezensis 9912D reveals its biocontrol mechanism as a novel commercial biological fungicide agent. J. Biotechnol. 2017, 247, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Bóka, B.; Manczinger, L.; Kecskeméti, A.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N.S.; Vágvölgyi, C.; Szekeres, A. Ion trap mass spectrometry of surfactins produced by Bacillus subtilis SZMC 6179J reveals novel fragmentation features of cyclic lipopeptides. Rapid Commun. Mass Spectrom. 2016, 30, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Kecskeméti, A.; Bartal, A.; Bóka, B.; Kredics, L.; Manczinger, L.; Shine, K.; Alharby, N.S.; Khaled, J.M.; Varga, M.; Vágvölgyi, C.; et al. High-frequency occurrence of surfactin monomethyl isoforms in the ferment broth of a Bacillus subtilis strain revealed by ion trap mass spectrometry. Molecules 2018, 23, 2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Li, X.; Li, X.; Yu, H.; Shen, Z. Identification of lipopeptide isoforms by MALDI-TOF-MS/MS based on the simultaneous purification of iturin, fengycin, and surfactin by RP-HPLC. Anal. Bioanal. Chem. 2015, 407, 2529–2542. [Google Scholar] [CrossRef]
- Calvo, O.C.; Quaglia, G.; Mohiley, A.; Cesarini, M.; Fangmeier, A. Assessing potential aquatic toxicity of airport runoff using physicochemical parameters and Lemna gibba and Aliivibrio fischeri bioassays. Environ. Sci. Pollut. Res. 2020, 27, 40604–40617. [Google Scholar] [CrossRef]
- Yan, Y.; Ziemek, J.; Schetelig, M.F. CRISPR/Cas9 mediated disruption of the white gene leads to pigmentation deficiency and copulation failure in Drosophila suzukii. J. Insect Physiol. 2020, 126, 104091. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).