Intercropping Tuber Crops with Teak in Gunungkidul Regency, Yogyakarta, Indonesia
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site and Time
2.2. Research Method and Data Collection
2.3. Data Analysis
3. Results
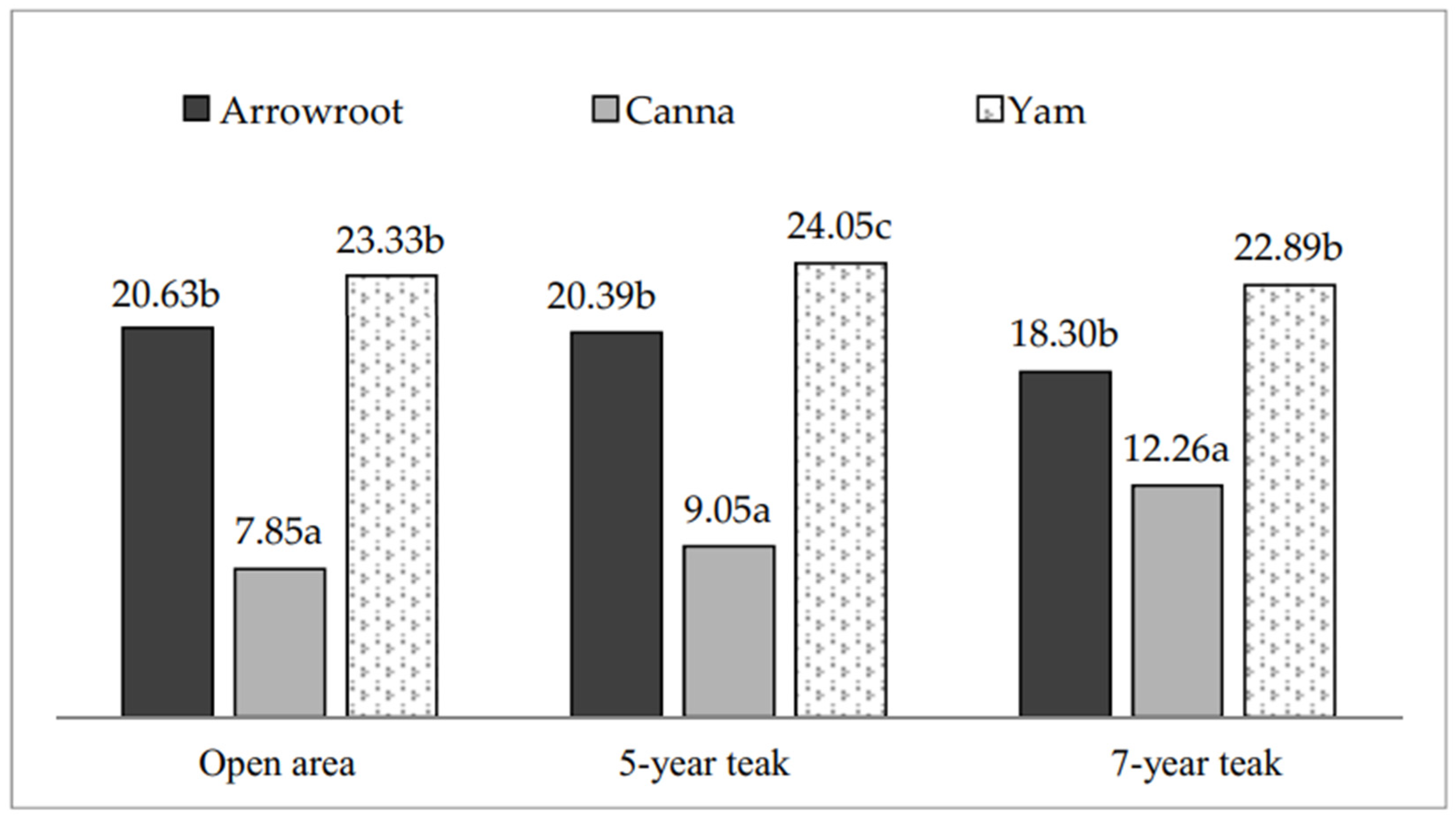
Effect of Cropping Pattern on the Growth and Yield of Tuber Plants
4. Discussion
4.1. Growth and Yield of Arrowroot (Maranta arundinacea L.)
4.2. Growth and Yield of Canna (Canna edulis Kerr.)
4.3. Growth and Yield of Yam (Dioscorea esculenta L.)
| Site Characteristics | Soil Type | Elevation (m a.s.l.) | Light Intensity (%) | Soil pH | Relative Air Humidity (%) | Rainfall (mm/Year) | Temp. (°C) |
|---|---|---|---|---|---|---|---|
| Site trial | Litosol | 230–325 | 5-year teak (45.13) dan 7-year teak (38.76) | 5–6.5 | 79–84 | 2.327 | 17.3–35.5 |
| Maranta arundinacea | Grumusol [72] | 605–1.351 [32] | 30.56–56.05 [55] 58 [35] | 6.7 [72] | 50–75 [55] | 1.202 [58] | 25–34 [55] |
| Canna edulis | Alluvial, Yellow-red podzolic [73] Ultisol, Sandy-Clay [74] | 0–250 [36] 250–300 [75] | 75 (for tuber weight) and 50 (for vegetative propagation) [65] 30–40 [35] 50 [36] 42 [76] | 4.5–8 [74] | 80.2 [35] 68–80.2 [36] | 1120–2664 [36] Resistant to dry land and efficient in the use of N [77] | 28.3 [35] Resistant to various air temperatures in the tropics [76] |
| Dioscorea esculenta | 500–2000 [34] 103–240 [70] | 50–70% [67] 60–70% [34] | 5.5–6.5 [34] 6.8 [70] | 40% [34] 50–79% [70] | 1.000–1.500 [34] 1.970–3.425 [70] | 20–30 [34] 29–34 [36] |
4.4. Enabling Smallholder of Agroforestry Practices
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | F-Calculated | |||||
|---|---|---|---|---|---|---|
| Arrow Root | Canna | Yam | ||||
| 1 MAP | 6 MAP | 1 MAP | 6 MAP | 1 MAP | 6 MAP | |
| Plant Height (cm) | 9.06 * | 115.08 ** | 6.79 ns | 3.73 ns | 3.88 ns | 11.51 * |
| Plant Diameter (mm) | 24.35 ** | 46.62 ** | 11.39 * | 2.44 ns | 1.83 ns | 5.73 ns |
| Number of shoots (pieces) | 2.35 ns | 16.51 ns | 0.43 ns | 0.44 ns | 1.60 ns | 1.25 ns |
| Number of leaves (sheets) | 28.67 ** | 2.38 ns | 8.72 * | 12.19 * | 15.85 * | 17.44 * |
| No. | Variables | F-Calculated | ||
|---|---|---|---|---|
| Arrow Root | Canna | Yam | ||
| 1 | Tuber weight/clump (g) | 79.65 ** | 4.11 ns | 32.46 ** |
| 2 | Tuber weight/piece (g) | 74.94 ** | - | 26.68 ** |
| 3 | Tuber weight/ha (Mg/ha) | 80.00 ** | 4.02 ns | 32.15 ** |
| 4 | Tuber length (cm) | 44.96 ** | 3.57 ns | 76.44 ** |
| 5 | Tuber diameter (cm) | 0.75 ns | 24.49 ** | 0.43 ns |
| 6 | Number of tubers/clump (pieces) | 26.38 ** | - | 6.22 ns |
| 7 | Starch content (%) | 2.36 ns | 2.31 ns | 0.58 ns |
| F-Calculated Treatments | ||
|---|---|---|
| Shades | Species | Shades × Species |
| 0.28 ns | 138.77 ** | 3.17 * |
References
- Mayrowani, H.; Ashari, N. Pengembangan agroforestry untuk mendukung ketahanan pangan dan pemberdayaan petani sekitar hutan. Forum Penelit. Èkonomi 2011, 29. [Google Scholar] [CrossRef]
- Tan, Z.; Lal, R.; Wiebe, K.D. Global soil nutrient Depletion and yield reduction. J. Sustain. Agric. 2005, 26, 123–146. [Google Scholar] [CrossRef]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil science. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef] [PubMed]
- Abdurachman, A.; Dariah, A.; Mulyani, A. Strategi dan teknologi pengelolaan lahan kering mendukung pengadaan pangan nasional. J. Litbang Pertan. 2008, 27, 43–49. [Google Scholar]
- Ellison, D.; Morris, C.E.; Locatelli, B.; Sheil, D.; Cohen, J.; Murdiyarso, D.; Gutierrez, V.; van Noordwijk, M.; Creed, I.F.; Pokorny, J.; et al. Trees, forests and water: Cool insights for a hot world. Glob. Environ. Chang. 2017, 43, 51–61. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Bernard, F.; van Noordwijk, M.; Luedeling, E.; Villamor, G.B.; Sileshi, G.W.; Namirembe, S. Social actors and unsustainability of agriculture. Curr. Opin. Environ. Sustain. 2014, 6, 155–161. [Google Scholar] [CrossRef]
- Van Noordwijk, M. Agroforestry as nexus of sustainable development goals. In Proceeding of the 3rd International Conference in Agroforestry—Adopting Modern Agroforestry toward Smart Social Forestry Program, Yogyakarta, Indonesia, 16–17 October 2019. [Google Scholar] [CrossRef]
- Elagib, N.A.; Al-Saidi, M. Balancing the benefits from the water-energy-land-food nexus through agroforestry in the Sahel. Sci. Total Environ. 2020, 742, 140509. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Caihong, Z.; Ekanayake, E.M.B.P. Livelihood improvement through agroforestry compared to conventional farming system: Evidence from Northern Irrigated Plain, Pakistan. Land 2021, 10, 645. [Google Scholar] [CrossRef]
- Quandt, A.; McCabe, J.T. “You can steal livestock but you can’t steal trees.” The livelihood benefits of agroforestry during and after violent conflict. Hum. Ecol. 2017, 45, 463–473. [Google Scholar] [CrossRef]
- Essa, M.; Nizami, S.M.; Mirza, S.N.; Khan, I.A.; Athar, M. Contribution of agroforestry in farmers’ livelihood and its impact on natural forest in northern areas of Pakistan. Afr. J. Biotechnol. 2011, 10, 15529–15537. [Google Scholar] [CrossRef]
- Garrity, D.P.; Akinnifesi, F.K.; Ajayi, O.C.; Weldesemayat, S.G.; Mowo, J.G.; Kalinganire, A.; Larwanou, M.; Bayala, J. Evergreen agriculture: A robust approach to sustainable food security in Africa. Food Secur. 2010, 2, 197–214. [Google Scholar] [CrossRef]
- Charles, R.L.; Munishi, P.; Nzunda, E.F. Agroforestry as adaptation strategy under climate change in Mwanga District, Kilimanjaro, Tanzania. Int. J. Environ. Prot. 2013, 3, 29–38. [Google Scholar]
- Batish, D.R.; Kohli, R.K.; Jose, S.; Singh, H.P. Ecological Basis of Agroforestry; CRC Press: Boca Raton, FL, USA, 2007; ISBN 0-429-13914-4. [Google Scholar]
- Samadhi, N.; Mallipu, A. A Reviving Local Food Achieving Sustainable Food System. The Jakarta Post, 16 October 2021. [Google Scholar]
- Van Noordwijk, M.; Duguma, L.A.; Dewi, S.; Leimona, B.; Catacutan, D.C.; Lusiana, B.; Öborn, I.; Hairiah, K.; Minang, P.A. SDG synergy between agriculture and forestry in the food, energy, water and income nexus: Reinventing agroforestry? Curr. Opin. Environ. Sustain. 2018, 34, 33–42. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Kumar, T.J. Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. Int. J. Food Sci. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Deswina, P.; Priadi, D. Development of arrowroot (Maranta arundinacea L.) as functional food based of local resource. In Proceedings of the 6th International Simposium of Innovative Bioproduction Indonesia on Biotechnology and Bioengineering (ISIBIO), Tangerang, Indonesia, 23–24 October 2019. [Google Scholar] [CrossRef]
- Yazid, N.S.M.; Abdullah, N.; Muhammad, N.; Matias-Peralta, H.M. Application of starch and starch-based products in food industry. J. Sci. Technol. 2018, 10, 144–174. [Google Scholar] [CrossRef]
- Lai, K.; Tsai, Y.; Wang, T. Studies on the edible canna in Taiwan: Botanical characteristics and economical use of edible canna. J. Agric. Assoc. China 1980, 1–14. [Google Scholar]
- Nugraheni, N.F.; Karyadi, J.N.W.; Tarwaca, S.N.; Indrasari, Y.P.; Albyan, R.; Setiyadi, I.; Ayuni, D. Effect of various drying methods on the physical characteristics of purple yam powder. In Proceeding of the International Conference in Agricultural Technology, Engineering, and Environmental Science (ICATES), Banda Aceh, Indonesia, 21 September 2020. [Google Scholar] [CrossRef]
- Harijono, H.; Estiasih, T.; Saputri, D.S.; Kusnadi, J. Effect of blanching on properties of water yam (Dioscorea alata) flour. Adv. J. Food Sci. Technol. 2013, 5, 1342–1350. [Google Scholar] [CrossRef]
- Prameswari, R.D.; Estiasih, T. Pemanfaatan tepung gembili (Dioscorea esculenta L.) dalam pembuatan cookies. J. Pangan Agroind. 2013, 1, 115–128. [Google Scholar]
- Sabda, M.; Wulanningtyas, H.S.; Ondikeleuw, M.; Baliadi, Y. Karakterisasi potensi gembili (Dioscorea esculenta L.) lokal asal papua sebagai alternatif bahan pangan pokok. Bul. Plasma Nutfah 2019, 25, 25–32. [Google Scholar] [CrossRef]
- Oktalina, S.N.; San Afri Awang, P.S.; Hartono, S. Strategi petani hutan rakyat dan kontribusinya terhadap penghidupan di kabupaten Gunungkidul. J. Kawistara 2015, 5, 221–328. [Google Scholar]
- Seruni, A.P.; Aguilar, F.X.; Cai, Z.; Gold, M.A.; Roshetko, J.M. Parcelized cut-and-carry agroforestry systems for confined livestock. Small-Scale For. 2021, 20, 119–143. [Google Scholar] [CrossRef]
- Roshetko, J.M.; Rohadi, D.; Perdana, A.; Sabastian, G.; Nuryartono, N.; Pramono, A.A.; Widyani, N.; Manalu, P.; Fauzi, M.A.; Sumardamto, P.; et al. Teak agroforestry systems for livelihood enhancement, industrial timber production, and environmental rehabilitation. For. Trees Livelihoods 2013, 22, 241–256. [Google Scholar] [CrossRef]
- Sudomo, A.; Maharani, D. Aplication of plant diversity through coplex agrofresty on three land use system. In Advant in Environement Research; Nova Publisher: New York, NY, USA, 2020; pp. 55–77. [Google Scholar]
- Sabastian, G.E.; Yumn, A.; Roshetko, J.M.; Manalu, P.; Martini, E.; Perdana, A. Adoption of silvicultural practices in smallholder timber and NTFPs production systems in Indonesia. Agrofor. Syst. 2019, 93, 607–620. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2019–2028; Food & Agriculture Organization: Rome, Italy, 2019; ISBN 92-5-131374-1. [Google Scholar]
- Rohandi, A. Productivity and quality of three varieties of ginger on many light intensity levels under stand of pine. J. Agrofor. Indones. 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Oktafani, M.B.; Supriyono; Budiastuti, M.S.; Purnomo, D. Performance of Arrowroot (Marantha arundinacea) in various light intensities. In Proceedings of the 4th International Conference on Sustainable Agriculture and Environment (4th ICSAE), Surakarta, Indonesia, 10–12 August 2017. [Google Scholar] [CrossRef]
- Miller, J.H. Nonnative Invasive Plants of Southern Forests: A Field Guide to Identification and Control; Southern Research Station: Asheville, NC, USA, 2003; Volume 62.
- Azis, S.N. Pengaruh Naungan Sengon (Falcataria moluccana L.) Dan Pemupukan Terhadap Pertumbuhan Ganyong Putih (Canna edulis Ker.). Bachelor’s Thesis, Institut Pertanian Bogor, Bogor, Indonesia, 2013. [Google Scholar]
- Utami, N.W.; Diyono, D. Respon pertumbuhan dan produksi 4 varian ganyong (Canna edulis) terhadap intensitas naungan dan umur panen yang berbeda. J. Teknol. Lingkung. 2016, 12, 333–343. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, H.; Zhao, F.; Chen, C.; Liu, W.; Yang, B.; Zhang, W. Recognizing the role of plant species composition in the modification of soil nutrients and water in rubber agroforestry systems. Sci. Total Environ. 2020, 723, 138042. [Google Scholar] [CrossRef] [PubMed]
- Gunungkidul, B.P.S.K. (Ed.) Kabupaten Gunungkidul Dalam Angka 2021. © BPS Kabupaten Gunungkidul: Yogyakarta, Indonesia, 2021; ISBN 0215-5230. [Google Scholar]
- Heryani, N.; Sawiyo, N. Pemberian irigasi suplementer pada lahan kering berbasis kearifan lokal untuk meningkatkan produktivitas lahan. In Proceedings of the Seminar Nasional Matematika, Sain, dan Teknologi, Yogyakarta, Indonesia, 9 November 2013; Volume 4, pp. D.58–D.71. [Google Scholar]
- Wardhana, W.; Sartohadi, J.; Rahayu, L.; Kurniawan, A. Analisis transisi lahan di kabupaten gunungkidul dengan citra penginderaan jauh multi temporal. J. Ilmu Kehutanan 2012, 6, 89–102. [Google Scholar]
- Nurlaela, N. Pemberdayaan masyarakat petani kakao melalui pengembangan kelembagaan (studi kasus di desa putat, kecamatan patuk, kabupaten gunung kidul). J. AgroSainTa Widyaiswara Mandiri Membangun Bangsa 2017, 1, 52–60. [Google Scholar]
- Oktalina, S.N.; Awang, S.A.; Hartono, S.; Suryanto, P. Pemetaan aset penghidupan petani dalam mengelola hutan rakyat di kabupaten Gunungkidul (The farmer livelihood asset mapping on community forest management in Gunungkidul district). J. Mns. Lingkung. 2016, 23, 58–65. [Google Scholar] [CrossRef][Green Version]
- Bahri, S.; Julaikha, S. Nilai-Nilai Gotong-Royong Dalam Masyarakat Petani Padi Sawah Di Desa Sungai Siput Kecamatan Siak Kecil Kabupaten Bengkalis. Jom FISIP. 2014, 1, 1–13. [Google Scholar]
- Maryudi, A.; Nawir, A.A.; Sekartaji, D.A.; Sumardamto, P.; Purwanto, R.H.; Sadono, R.; Suryanto, P.; Soraya, E.; Soeprijadi, D.; Affianto, A.; et al. Smallholder farmers’ knowledge of regulations governing the sale of timber and supply chains in Gunungkidul district, Indonesia. Small-Scale For. 2017, 16, 119–131. [Google Scholar] [CrossRef]
- Roshetko, J.M.; Manurung, G.E. Smallholder teak production systems in Gunungkidul, Indonesia. In Proceedings of the 2nd World Congress of Agroforestry—The Future of Global Land Use, Nairobi, Kenya, 24–28 August 2009. [Google Scholar]
- Simon, H. Metode Inventore Hutan; Pustaka Pelajar: Yogyakarta, Indonesia, 2007; ISBN 978-979-1277-17-4. [Google Scholar]
- Aqil, M.; Efendi, R. Aplikasi SPSS Dan SAS Untuk Perancangan Percobaan: Aplikasi Pertanian, Aplikasi Peternakan, Aplikasi Kehutanan Dan Aplikasi MIPA; Absolute Media: Yogyakarta, Indonesia, 2021; ISBN 602-1083-07-5. [Google Scholar]
- Nair, P.K.R. An Introduction to Agroforestry; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1993; ISBN 0-7923-2134-0. [Google Scholar]
- De Souza, D.C.; Costa, P.A.; Silva, L.F.L.E.; Guerra, T.S.; Resende, L.V.; Pereira, J. Productivity of rhizomes and starch quantification in cultures of different vegetative propagules of arrowroot. J. Agric. Sci. 2019, 11, 419–425. [Google Scholar] [CrossRef]
- De Souza, D.C.; Silva, R.D.J.; Guerra, T.S.; Silva, L.F.L.E.; Resende, L.V.; Pereira, J. Characterization of arrowroot starch in different agronomic managements. Rev. Ceres 2019, 66, 323–332. [Google Scholar] [CrossRef]
- Wickramasinghe, H.A.M.; Noda, T. Physicochemical properties of starches from Sri Lankan rice varieties. Food Sci. Technol. Res. 2008, 14, 49–54. [Google Scholar] [CrossRef][Green Version]
- Wiyono, W.; Lestari, P.; Hidayat, R.; Oktalina, S.N.; Utomo, S.; Prasetyo, E.; Ngadianto, A.; Nugroho, P. Penerapan teknik silvikultur intensif pada pengelolaan hutan rakyat di kabupaten gunungkidul. J. Pengabdi. Pengemb. Masy. 2018, 1, 57–69. [Google Scholar] [CrossRef]
- Rohadi, D.; Kallio, M.; Krisnawati, H.; Manalu, P. Economic incentives and household perceptions on smallholder timber plantations: Lessons from case studies in Indonesia. In Proceedings of the International Conference on Smallholder and Community Forest Management—Taking Stock of Smallholder and Community Forestry: Where do we go from here? Montpellier Conference, Montpellier, France, 24–26 March 2010; pp. 1–14. [Google Scholar]
- Roshetko, J.M.; Astho, A.; Rohadi, D.; Widyani, N.; Manurung, G.S.; Fauzi, A.; Sumardamto, P. Smallholder teak systems on Java, Indonesia: Income for families, timber for industry. In Proceedings of the IUFRO 3.08.00 Small-Scale Forestry Conference 2012: Science for Solutions, Amherst, MA, USA, 24–27 September 2012; pp. 162–167. [Google Scholar]
- Rohandi, A.; Budiadi, B.; Hardiwinoto, S.; Harmayani, E.; Sudrajat, D.J. Variability in morpho-physiology, tuber yield and starch content of several arrowroot populations in Garut district. AGRIVITA J. Agric. Sci. 2017, 39, 311–323. [Google Scholar] [CrossRef]
- Lakitan, B. Dasar-Dasar: Fisiologi Tumbuhan; Raja Grafindo Persada: Jakarta, Indonesia, 2015; ISBN 979-421-377-2. [Google Scholar]
- Purwaningrahayu, R.D.; Sebayang, H.T.; Syekhfani, S.; Aini, N. Resistance level of some soybean (Glycine max L. Merr) genotypes toward salinity stress. Berk. Penelit. Hayati 2015, 20, 7–14. [Google Scholar] [CrossRef]
- Murniyanto, E.; Badami, K. Karakteristik agroekologi garut (Marantha arundinaceae L.) pulau madura. Agrovigor J. Agroekoteknol. 2009, 2, 59–66. [Google Scholar]
- Sastra, D.R. Analisis Keragaman Genetik Dan Tanggap Tanaman Garut (Maranta arundinacea L.) Terhadap Intensitas Cahaya Matahari. Ph.D. Theses, Bogor Agricultural Institute, Bogor, Indonesia, 2002. [Google Scholar]
- Miftakhussolikhah, M.; Ariani, D.; Ervika, R.; Angwar, M.; Wardah, W.; Karlina, L.L.; Pranoto, Y. Cooking characterization of arrowroot (Maranta arundinaceae) noodle in various arenga starch substitution [Karakteristik pemasakan mie garut (Maranta arundinaceae) pada variasi subtitusi pati aren]. Berita Biol. 2017, 15, 141–148. [Google Scholar]
- Djaafar, T.F.; Pustika, A.B. Pengembangan budi daya tanaman garut dan teknologi pengolahannya untuk mendukung ketahanan pangan. J. Penelit. Dan Pengemb. Pertan. 2010, 29, 25–33. [Google Scholar]
- Prehaten, D.; Hardiwinoto, S.; Naâ, M.; Supriyo, H.; Widiyatno, W.; Rodiana, D. Productivity of arrowroots and taro grown under superior teak clones with several levels of stand density. Biosaintifika J. Biol. Biol. Educ. 2021, 13, 51–57. [Google Scholar] [CrossRef]
- Handayani, T.; Wijayanto, N.; Wulandari, A.S. Analisis pertumbuhan mindi (Melia azedarach L.) dan produktivitas umbi garut (Maranta arundinacea dan Maranta linearis L.) dalam sistem agroforestri. J. Trop. Silvic. 2018, 9, 144–150. [Google Scholar] [CrossRef]
- Gardner, F.P.; Pearce, R.B.; Mitchell, R.L. Fisiologi Tanaman Budidaya; UI Press: Jakarta, Indonesia, 1991; ISBN 979-456-088-x. [Google Scholar]
- Muhartini, S. Pertumbuhan dan hasil ganyong (Canna edulis Ker.) pada beberapa tingkat naungan dan takaran pupuk kandang. Caraka Tani J. Sustain. Agric. 2009, 24, 114–120. [Google Scholar] [CrossRef][Green Version]
- Sudomo, A.; Hani, A. Produktivitas talas (Colocasia esculenta L. Shott) di bawah tiga jenis tegakan dengan sistem agroforestri di lahan hutan rakyat. J. Ilmu Kehutan. 2014, 8, 100–107. [Google Scholar]
- Lestari, P.; Utami, N.W.; Wawo, A.H. Adaptasi intensitas cahaya rendah gembili (Dioscorea esculenta) pada naungan atrtifisial [Adaptation on low light intensity of lesser yam under artificial shading]. Pros. Semin. Nas. Masy. Biodivers. Indones. 2019, 5, 374–382. [Google Scholar] [CrossRef]
- Sawitri. Adaptasi anatomi dan morfologi tanaman gembili (Dioscorea esculenta) terhadap pola tanam dan intensitas cahaya relatif di hutan rakyat Kabupaten Sukoharjo. Bachelor’s Theses, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2015. [Google Scholar]
- Budiman, B.; Arisoesilaningsih, E. Predictive model of Amorphophallus muelleri growth in some agroforestry in East Java by multiple regression analysis. Biodivers. J. Biol. Divers. 2012, 13, 18–22. [Google Scholar] [CrossRef]
- Fatma, L.Y.; Jumari, J.; Utami, S. Keanekaragaman Dioscorea spp dan habitatnya di Kabupaten Kudus, Jawa Tengah Diversity and habitat of Dioscorea spp in Kudus, Central Java. Bioma Berk. Ilm. Biol. 2018, 20, 17–24. [Google Scholar] [CrossRef]
- Maharani, D. Adaptasi Morfosiologi Dan Produktivitas Tanaman Gembili (Dioscorea esculenta (Lour.) Burk.) Di Bawah Jenis Tegakan Berbeda Di Wanagama I. Master’s Theses, Pasca Sarjana Fakultas, Kehutanan Universitas Gadjah Mada, Yogyakarta, Indonesia, 2017. [Google Scholar]
- Patola, L.N.P.; Supriyono, S.; Pardjanto, P. Effect use biofertilizer and differences type soil on growth and yield arrowroot. SAINS Tanah-J. Soil Sci. Agroclimatol. 2017, 14, 29–35. [Google Scholar] [CrossRef][Green Version]
- Fu, L.; Sun, H.; Sharma, R.P.; Lei, Y.; Zhang, H.; Tang, S. Nonlinear mixed-effects crown width models for individual trees of Chinese fir (Cunninghamia lanceolata) in south-central China. For. Ecol. Manag. 2013, 302, 210–220. [Google Scholar] [CrossRef]
- Rosmiah, R.; Gusmiatun; Pebriana, P. Ganyong dan pupuk kandang. Klorofil 2014, IX-2, 89–93. [Google Scholar]
- Yulfia, Y.; Puji, H.; Prasetyo, P. Keragaan pertumbuhan ganyong (Canna edulis Kerr) pada berbagai ketinggian tempat bedasarkan ciri morfologi di kabupaten bengkulu selatan. NATURALIS 2012, 1, 85–88. [Google Scholar]
- Lingga, P.; Sarwono, B.; Rahardi, F.; Rahardja, P.; Afriastini, J.; Wudianto, R.; Apriadji, W. Bertanam ubi-Ubian; Penebar Swadaya: Jawa Brat, Indonesia, 1986. [Google Scholar]
- Hermann, M.; Quynh, N.; Peters, D. Reappraisal of edible canna as a high-value starch crop in Vietnam. CIP Prog. Rep. 1997, 98, 415–424. [Google Scholar]
- Rahman, M.A.; Rahman, A.; Miah, M.G.; Hoque, M.A.; Rahman, M.M. Productivity and profitability of jackfruit-eggplant agroforestry system in the terrace ecosystem of Bangladesh. Turk. J. Agric. Food Sci. Technol. 2018, 6, 124–129. [Google Scholar]
- Ceunfin, S.; Prajitno, D.; Suryanto, P.; Putra, E.T.S. Assessment of competition and the benefits of intercropping of maize-soybeans under eucalyptus stands. Savana Cendana 2017, 2, 1–3. [Google Scholar] [CrossRef]
- Rachmadyanto, B. Analisis Usahatani Ganyong Dan Permasalahannya Di Desa Jatisari Kecamatan Tajinan Kabupaten Malang. Bachelor’s Theses, University of Muhammadiyah Malang, Jawa Timur, Indonesia, 2011. [Google Scholar]
- Baluk, M.S.N. Analisis Pendapatan Usahatani Garut Dan Nilai Tambah Pati Garut Di Desa Waringinanom, Kecamatan Poncokusumo, Kabupaten Malang. Bachelor’s Theses, Widya Karya Malang Catholic University, Jawa Timur, Indonesia, 2021. [Google Scholar]
- Tatay, P.; Widiastuti, M.M.D.; Untari, U. Analisis pendapatan budidaya dan pengolahan hasil gembili (Dioscorea esculenta) sebagai sumber pangan alternatif bagi keluarga di kampung yanggandur. Musamus J. Agribus. 2018, 1, 32–40. [Google Scholar] [CrossRef]
- Nugroho, P.; Soedjoko, S.A.; Kusumandari, A.; Marhaento, H. Adaptasi dan mitigasi bencana tanah longsor melalui penguatan kapasitas masyarakat dan peningkatan produktivitas lahan melalui sistem agroforestri. In Proceedings of the Prosiding Seminar Nasional Agroforestri 2013—Agroforestry untuk Pangan dan Lingkungan yang lebih Baik, Malang, Indonesia, 21 May 2013; p. 381. [Google Scholar]
- Dhyani, S.K.; Tripathi, R.S. Biomass and production of fine and coarse roots of trees under agrisilvicultural practices in north-east India. Agrofor. Syst. 2000, 50, 107–121. [Google Scholar] [CrossRef]
- Ishii, H. Response of a clonal teak plantation to thinning and pruning in Java, Indonesia. J. Trop. For. Sci. 2017, 29, 44–53. [Google Scholar]
- Seta, G.W.; Widiyatno; Hidayati, F.; Na’iem, M. Impact of thinning and pruning on tree growth, stress wave velocity, and pilodyn penetration response of clonal teak (Tectona grandis) plantation. For. Sci. Technol. 2021, 17, 57–66. [Google Scholar]
- Kallio, M.; Kanninen, M.; Rohadi, D. Farmers’ tree planting activity in Indonesia—Case studies in the provinces of central Java, Riau, and South Kalimantan. For. Trees Livelihoods 2011, 20, 191–209. [Google Scholar] [CrossRef]
- Huxley, P. Tropical Agroforestry; Blackwell Science Ltd.: Oxford, UK, 1999; ISBN 0-632-04047-5. [Google Scholar]
- Saptono, M. Growth and yield of cassava in agro forestry system using crown tree management: Crown pruning for optimization light interception. AGRIVITA J. Agric. Sci. 2011, 33, 22–31. [Google Scholar]
- Bertomeu, M.; Roshetko, J.M.; Rahayu, S. Optimum pruning intensity for reducing crop suppression in a Gmelina-maize smallholder agroforestry system in Claveria, Philippines. Agrofor. Syst. 2011, 83, 167–180. [Google Scholar] [CrossRef]
- Purnomo, D.; Sitompul, S.M. Irradiasi pada sistem agroforestri berbasis jati dan pinus serta pengaruhnya terhadap pertumbuhan tanaman kedelai. Biodiversitas 2006, 7, 251–255. [Google Scholar]
- Suryanto, P.; Widyastuti, S.M.; Sartohadi, J.; Awang, S.A. Traditional knowledge of homegarden-dry field agroforestry as a tool for revitalization management of smallholder land use in Kulon Progo, Java, Indonesia. Int. J. Biol. 2012, 4, 173. [Google Scholar] [CrossRef][Green Version]
- Pramono, A.A.; Fauzi, M.A.; Widyani, N.; Heriansyah, I.; Roshetko, J.M. Pengelolaan Hutan Jati Rakyat-Panduan Lapangan Untuk Petani; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2010; ISBN 978-602-8693-19-6. [Google Scholar]
- Cavalcante, V.S.; Dos Santos, M.L.; Cotta, L.C.; Neves, J.C.L.; Soares, E.M.B. Clonal teak litter in tropical soil: Decomposition, nutrient cycling, and biochemical composition. Rev. Bras. Cienc. Solo 2020, 45. [Google Scholar] [CrossRef]
- Rahman, M.; Majumder, M.; Rahman, G.; Zaman, M.; Mojumder, M. Time of application of teak leaf litter and its effect on boro rice. J. Agrofor. Environ. 2008, 2, 1–6. [Google Scholar]
- Al Mahmud, A.; Rahman, M.M.; Hossain, M.K. The effects of teak monoculture on forest soils: A case study in Bangladesh. J. For. Res. 2018, 29, 1111–1120. [Google Scholar] [CrossRef]
- Triharyanto, E. Budidaya tanaman garut dalam upaya meningkatkan pendapatan petani. Caraka Tani J. Sustain. Agric. 2002, 17, 1–12. [Google Scholar] [CrossRef]
- Fitria, R.; Supriyono, S.; Sudadi, S. Respon pertumbuhan dan hasil garut (Maranta arundinacea) terhadap pembumbunan dan pemupukan kalium. Agrotechnol. Res. J. 2017, 1, 46–50. [Google Scholar] [CrossRef]
- Suhartini, T.; Rais, S.A.; Hadiatmi, L.H.; Dewi, N.; Setyowati, M. Rejuvenasi Dan Karakterisasi Morfologi Plasma Nutfah Tanaman Pangan. In Proceedings of the Prosiding Seminar Hasil Penelitian Rintisan dan Bioteknologi Tanaman, Bogor, Indonesia, 26–27 December 2001. [Google Scholar]
- Sulistyono, E.; Marpaung, J. Studi karakter umbi dan kandungan Nutrisi dioscorea spp. Indones. J. Agron. 2004, 32, 39–43. [Google Scholar]
- Duffy, C.; Toth, G.; Cullinan, J.; Murray, U.; Spillane, C. Climate smart agriculture extension: Gender disparities in agroforestry knowledge acquisition. Clim. Dev. 2021, 13, 21–33. [Google Scholar] [CrossRef]

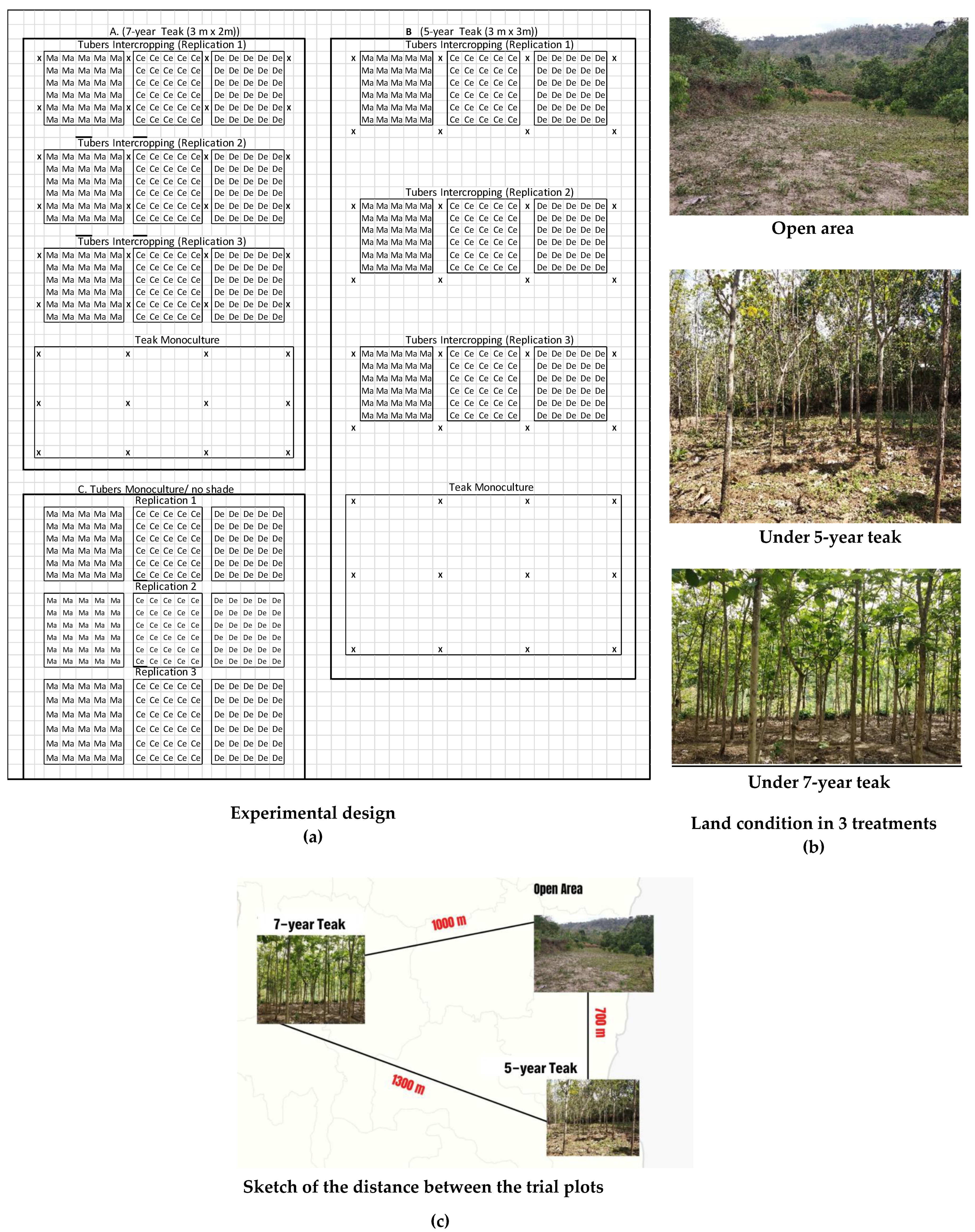

| Variables | Plots | ||||
|---|---|---|---|---|---|
| Open Area | Teak (5 Years) | Teak (7 Years) | |||
| BP | AP | BP | AP | ||
| Microclimate | |||||
| Relative light intensity (%) | 100 | 45.13 | - | 38.76 | - |
| Day temperature (°C) | 32.73 | 31.81 | - | 30.09 | - |
| Day relative humidity (%) | 48.19 | 49.39 | - | 52.67 | - |
| Physical and chemical properties of the soil | |||||
| Texture (%) | |||||
| Sand | 36.50 | 7.78 | - | 50.33 | - |
| Silt | 32.78 | 38.56 | - | 25.83 | - |
| Clay | 30.72 | 53.67 | - | 23.61 | - |
| pH (H2O) | 6.56 (n) | 6.61 (n) | - | 6.25 (n) | - |
| C-organic (%) | 1.53 (l) | 1.62 (l) | - | 1.73 (l) | - |
| N-total (%) | 0.10 (l) | 0.09 (vl) | - | 0.09 (vl) | - |
| K-available (ppm) | 193.72 (vh) | 127.22 (vh) | - | 239.83 (vh) | - |
| P2O5-potential (mg/100 g) | 27.94 (h) | 83.11 (vh) | - | 29.83 (h) | - |
| Dimension of teak | |||||
| Tree height (m) | - | 8.35 | 8.78 | 12.21 | 13.26 |
| Bole height (m) | - | 2.91 | 3.04 | 4.65 | 5.28 |
| Tree diameter (cm) | - | 7.44 | 8.24 | 11.76 | 13.00 |
| Crown diameter (m) | - | 2.29 | 2.65 | 2.71 | 3.4 |
| Teak spacing | - | 3 m × 3 m | 3 m × 3 m | 3 m × 2 m | 3 m × 2 m |
| Crown width (m2) | - | 32.43 | 38.57 | 60.64 | 86.11 |
| Volume (m3/ha) | - | 34.44 | 43.61 | 225.05 | 240.46 |
| Variables and Treatments | Species | |||||
|---|---|---|---|---|---|---|
| Arrowroot | Canna | Yam | ||||
| 1 MAP | 6 MAP | 1 MAP | 6 MAP | 1 MAP | 6 MAP | |
| Plant viability (%) | ||||||
| Open area | 100.00 | 100.00 | 100.00 | 25.56 a | 100.00 | 23.33 a |
| 5-year teak | 100.00 | 100.00 | 100.00 | 43.33 ab | 100.00 | 100.00 b |
| 7-year teak | 100.00 | 100.00 | 100.00 | 100 b | 100.00 | 21.11 a |
| Plant height (cm) | ||||||
| Open area | 27.16 ± 9.26 b | 83.92 ± 26.43 c | 17.00 ± 5.56 ns | 25.22 ± 16.25 ns | 120.33 ± 66.11 ns | 191.33 ± 36.62 a |
| 5-year teak | 22.93 ± 5.41 a | 68.36 ± 13.85 b | 16.96 ± 5.93 | 25.99 ± 19.87 | 92.58 ± 28.06 | 247.52 ± 67.75 b |
| 7-year teak | 30.43± 8.68 c | 34.65 ± 12.02 a | 21.66 ± 6.20 | 39.97 ± 23.52 | 132.70 ± 86.17 | 177.83 ± 71.04 a |
| Diameter (cm) | ||||||
| Open area | 1.23 ± 0.38 b | 10.24 ± 1.84 b | 1.44 ± 0.36 c | 8.98 ± 2.86 ns | 0.34 ± 0.13 ns | 0.25 ± 0.05 ns |
| 5-year teak | 0.78 ± 0.20 a | 5.63 ± 1.45 a | 0.96 ± 0.26 a | 8.07 ± 2.80 | 0.33 ± 0.06 | 0.29 ± 0.08 |
| 7-year teak | 0.82 ± 0.27 a | 5.67 ± 1.57 a | 1.14 ± 0.36 b | 8.55 ± 2.62 | 0.29 ± 0.11 | 0.23 ± 0.04 |
| Number of shoots (pieces) | ||||||
| Open area | 1.88 ± 1.25 ns | 5.85 ± 2.36 ns | 1.65 ± 0.75 ns | 1.85 ± 0.93 ns | 1.40 ± 0.76 ns | 2.06 ± 1.66 ns |
| 5-year teak | 1.53 ± 0.94 | 3.08 ± 1.76 | 1.73 ± 0.75 | 1.75 ± 1.10 | 1.46 ± 0.69 | 1.87 ± 0.78 |
| 7-year teak | 1.61 ± 1.06 | 2.09 ± 1.17 | 1.60 ± 1.01 | 1.64 ± 0.99 | 1.64 ± 0.87 | 1.48 ± 0.69 |
| Number of leaves (sheets) | ||||||
| Open area | 6.40 ± 1.47 c | 8.07 ± 2.64 ns | 5.13 ± 0.82 b | 1.89 ± 1.07 a | 33.27 ± 28.93 b | 105.10 ± 52.74 b |
| 5-year teak | 5.69 ± 1.18 b | 6.83 ± 2.36 | 4.19 ± 0.74 a | 2.83 ± 1.10 b | 15.62 ± 8.56 a | 112.68 ± 54.83 b |
| 7-year teak | 4.97 ± 0.98 a | 6.04 ± 2.91 | 4.32 ± 0.95 a | 3.01 ± 1.07 b | 33.40 ± 28.71 b | 24.93 ± 18.75 a |
| Variables and Treatments | Species of Tuber Plants | ||
|---|---|---|---|
| Arrowroot | Canna | Yam | |
| Tuber weight/clump (g) | |||
| Open area | 665.97 ± 182.45 b | 26.58 ± 28.03 ns | 587.30 ± 185 b |
| 5-year teak | 235.19 ± 73.76 a | 39.97 ± 39.97 | 213.80 ± 87.14 a |
| 7-year teak | 111.36 ± 39.78 a | 59.71 ± 22.86 | 115.91 ± 38.84 a |
| Tuber weight/piece (g) | |||
| Open area | 75.74 ± 12.41 c | - | 103.85 ± 69.10 b |
| 5-year teak | 47.93 ± 13.60 b | - | 31.33 ± 10.97 a |
| 7-year teak | 28.81 ± 6.40 a | - | 28.81 ± 24.74 a |
| Tuber weight/Ha (Mg/ha) | |||
| Open area | 26.64 ± 1.61 b | 1.06 ± 0.25 ns | 23.49 ± 3.45 b |
| 5-year teak | 6.21 ± 1.91 a | 1.06 ± 0.70 | 5.65 ± 1.76 a |
| 7-year teak | 2.94 ± 0.56 a | 1.58 ± 0.51 | 3.06 ± 0.39 a |
| Tuber length (cm) | |||
| Open area | 19.52 ± 1.73 b | 7.22 ± 5.60 ns | 7.38 ± 1.99 b |
| 5-year teak | 14.27 ± 2.40 a | 8.57 ± 8.57 | 4.75 ± 1.08 a |
| 7-year teak | 12.96 ± 1.74 a | 12.24 ± 1.60 | 4.40 ± 0.70 a |
| Diameter of tuber (cm) | |||
| Open area | 2.96 ± 0.15 ns | 1.02 ± 0.64 a | 3.57 ± 0.83 ns |
| 5-year teak | 2.33 ± 1.21 | 1.60 ± 1.60 a | 3.62 ± 2.41 |
| 7-year teak | 2.42 ± 1.57 | 2.98 ± 2.58 b | 3.18 ± 1.41 |
| Number of tubers/clump (pieces) | |||
| Open area | 9.10 ± 2.06 b | - | 7.45 ± 2.46 ns |
| 5-year teak | 5.04 ± 1.73 a | - | 6.79 ± 2.26 |
| 7-year teak | 3.96 ± 1.68 a | - | 4.67 ± 1.43 |
| No. | Treatments | Land Equivalent Ratio | ||
|---|---|---|---|---|
| Arrowroot + Teak | Canna + Teak | Yam + Teak | ||
| 1 | Teak 5 years | 1.65 | 2.65 | 1.27 |
| 2 | Teak 7 years | 0.69 | 2.01 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharani, D.; Sudomo, A.; Swestiani, D.; Murniati; Sabastian, G.E.; Roshetko, J.M.; Fambayun, R.A. Intercropping Tuber Crops with Teak in Gunungkidul Regency, Yogyakarta, Indonesia. Agronomy 2022, 12, 449. https://doi.org/10.3390/agronomy12020449
Maharani D, Sudomo A, Swestiani D, Murniati, Sabastian GE, Roshetko JM, Fambayun RA. Intercropping Tuber Crops with Teak in Gunungkidul Regency, Yogyakarta, Indonesia. Agronomy. 2022; 12(2):449. https://doi.org/10.3390/agronomy12020449
Chicago/Turabian StyleMaharani, Dewi, Aris Sudomo, Dila Swestiani, Murniati, Gerhard E. Sabastian, James M. Roshetko, and Rizki Ary Fambayun. 2022. "Intercropping Tuber Crops with Teak in Gunungkidul Regency, Yogyakarta, Indonesia" Agronomy 12, no. 2: 449. https://doi.org/10.3390/agronomy12020449
APA StyleMaharani, D., Sudomo, A., Swestiani, D., Murniati, Sabastian, G. E., Roshetko, J. M., & Fambayun, R. A. (2022). Intercropping Tuber Crops with Teak in Gunungkidul Regency, Yogyakarta, Indonesia. Agronomy, 12(2), 449. https://doi.org/10.3390/agronomy12020449







