Ammonium to Total Nitrogen Ratio Interactive Effects with Salinity Application on Solanum lycopersicum Growth, Physiology, and Fruit Storage in a Closed Hydroponic System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Experimental Set Up
2.3. Plant Growth and Physiological Parameters
2.4. Fruit Harvesting
2.5. Analysis of the Drainage Solution
2.6. Mineral Composition Analysis
2.7. Fruit Macroscopic Examination and Quality Analysis
2.8. Determination of Carotenoids and Total Phenolics
2.9. Organoleptic Control
2.10. Statistical Methods
3. Results
3.1. Plant Growth and Physiological Parameters
3.2. Drainage Solution and Leaf and Fruit Mineral Content
3.3. Quality Parameters at Harvested Fruits
3.4. Organoleptic Test
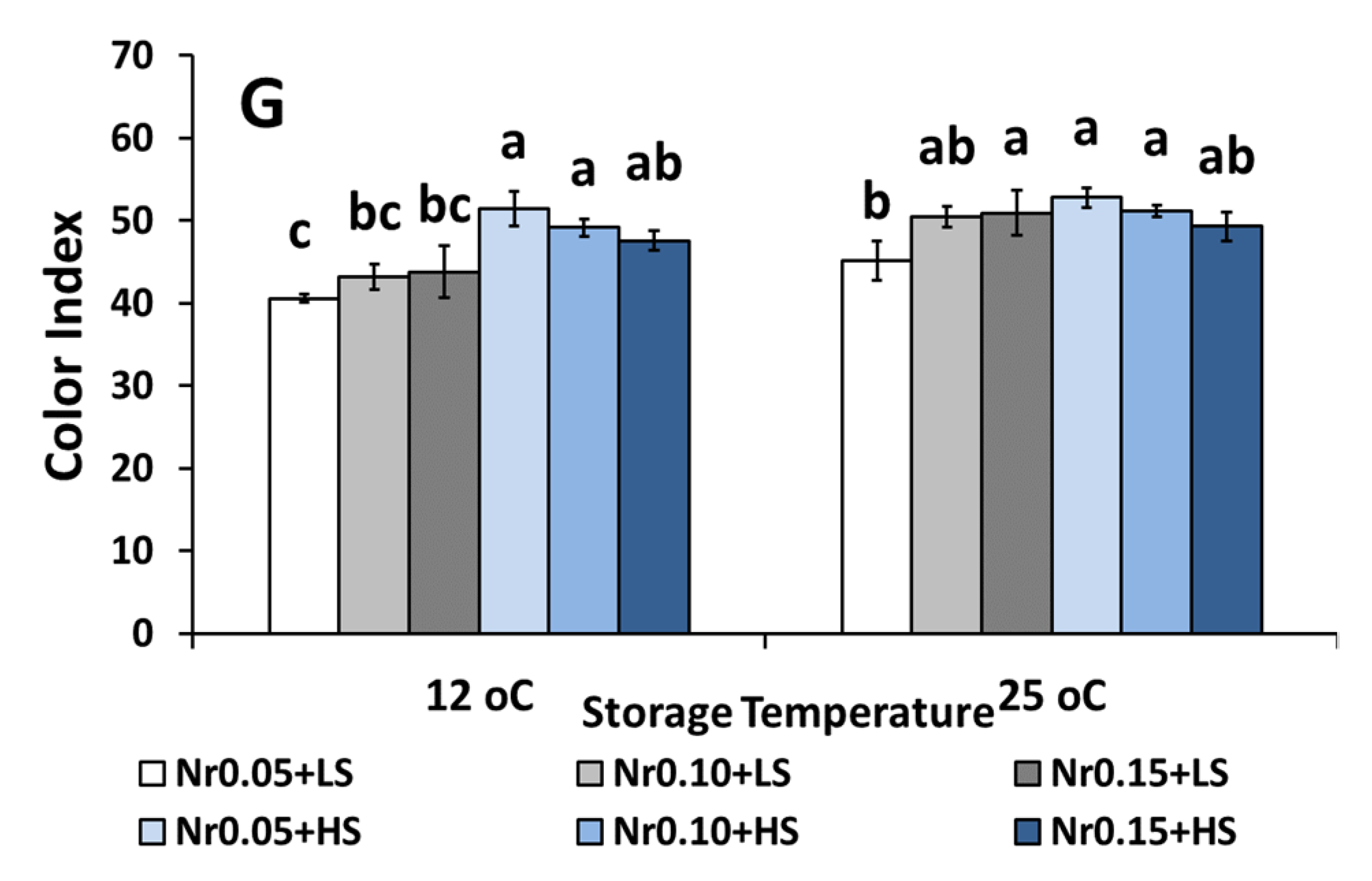
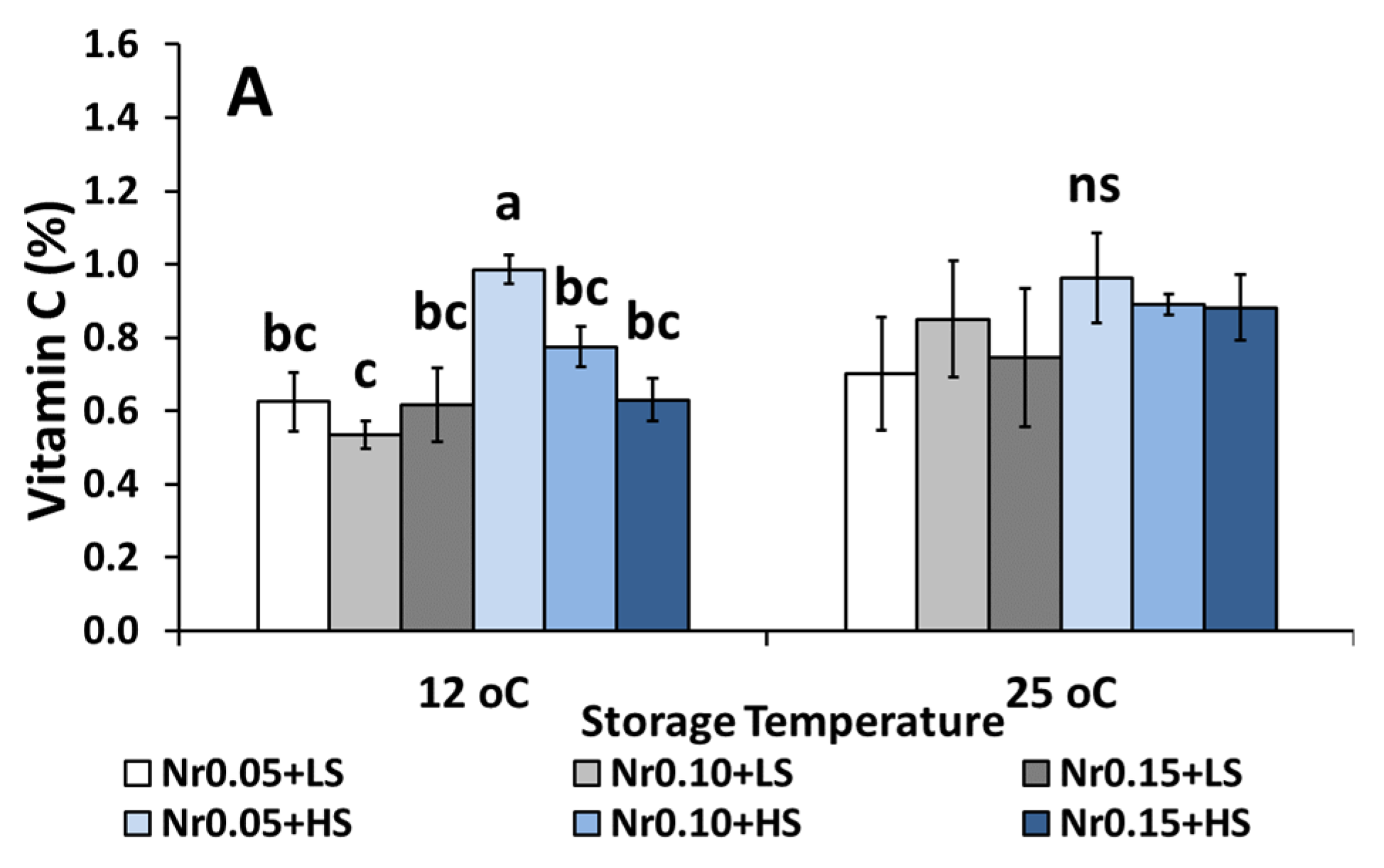
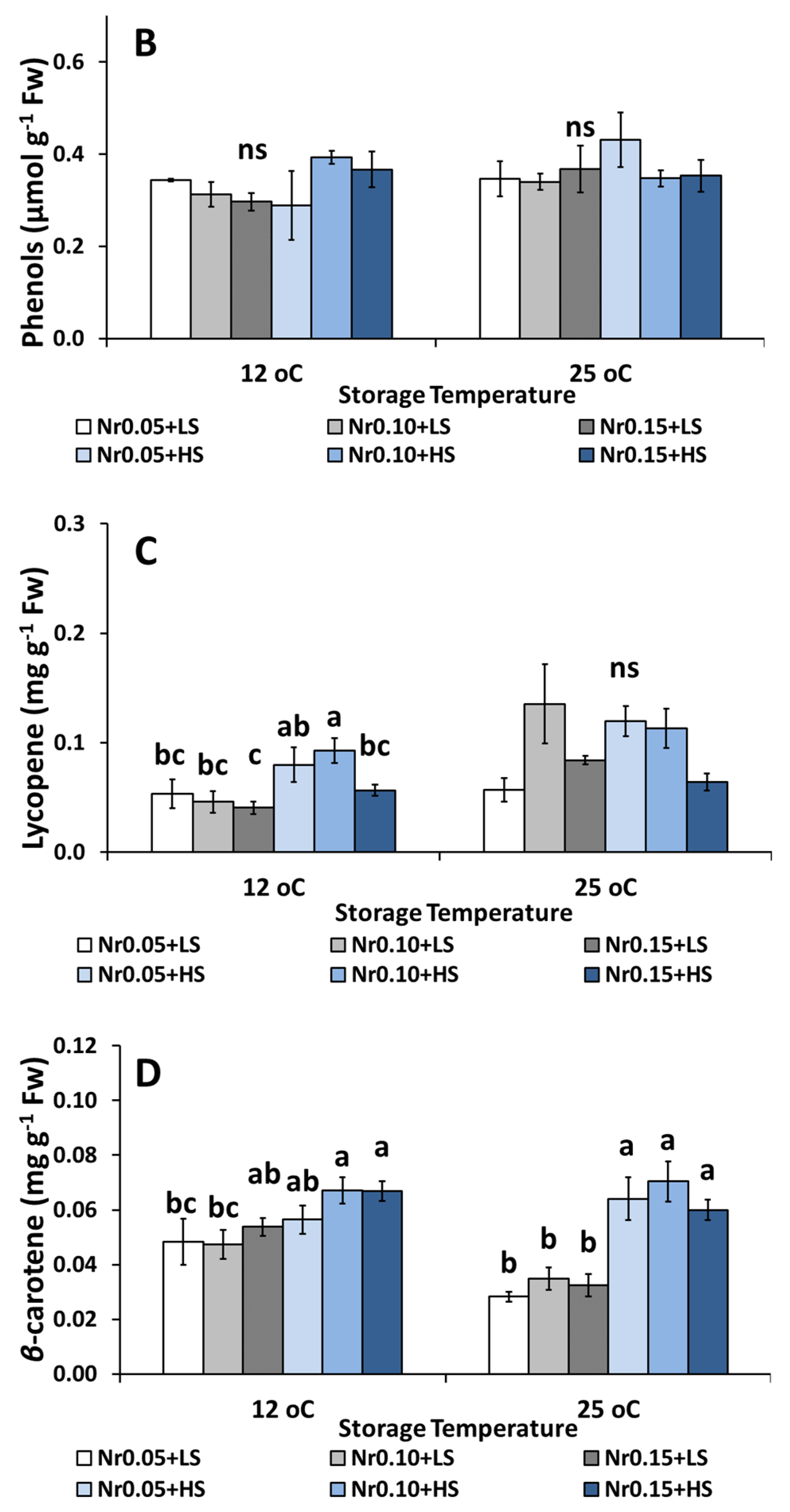
3.5. Postharvest Fruit Quality Attributes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G.; Zhang, C.; Chu, L.Y.; Shao, H.B. Responses of higher plants to abiotic stresses and agricultural sustainable development. J. Plant Interact. 2007, 2, 135–147. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbaz, M.; Ashraf, M. Improving Salinity Tolerance in Cereals. Crit. Rev. Plant Sci. 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildirim, E.; Taylor, A.G. Effect of biological treatments on growth of bean plants under salt stress. Annu. Rep. Bean Improv. Coop. 2005, 48, 176–177. [Google Scholar]
- Hendawy, S.F.; Khalid, K.A. Response of sage (Salvia officinalis L.) plants to zinc application under different salinity levels. J. Appl. Sci. Res. 2005, 1, 147–155. [Google Scholar]
- Gama, P.B.S.S.; Inanaga, S.; Tanaka, K.; Nakazawa, R. Nakazawa Physiological response of common bean (Phaseolus vulgaris L.) seedlings to salinity stress. Afr. J. Biotechnol. 2007, 6, 079–088. [Google Scholar]
- Jamil, M.; Rehman, S.; Rha, E.S. Salinity effect on plant growth, PSII photochemistry and chlorophyll content in sugar beet (Beta vulgaris L.) and cabbage (Brassica oleracea capitata L.). Pak. J. Bot. 2007, 39, 753–760. [Google Scholar]
- Memon, S.A.; Hou, X.; Wang, L.J. Morphlogical analysis of salt stress response of pak choi. Electron. J. Environ. Agric. Food Chem. 2010, 9, 248–254. [Google Scholar]
- Pinheiro, D.T.; Delazari, F.; Nick, C.; Mattiello, E.M.; dos Santos Dias, D.C.F. Emergence and vegetative development of melon in function of the soil salinity. Aust. J. Crop. Sci. 2019, 13, 458–464. [Google Scholar] [CrossRef]
- Petretto, G.L.; Urgeghe, P.P.; Massa, D.; Melito, S. Effect of salinity (NaCl)on plant growth, nutrient content, and glucosinolate hydrolysis products trends in rocket genotypes. Plant Physiol. Biochem. 2019, 141, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Belmecheri-Cherifi, H.; Albacete, A.; Martínez-Andújar, C.; Pérez-Alfocea, F.; Abrous-Belbachir, O. The growth impairment of salinized fenugreek (Trigonella foenum-graecum L.) plants is associated to changes in the hormonal balance. J. Plant Physiol. 2019, 232, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Sotelo, H.; Valdez-Ortiz, Á.; Germán-Báez, L.J.; Fierro-Sañudo, J.F.; León-Cañedo, J.A.; Alarcón-Silvas, S.G.; Reyes-Moreno, C.; Páez-Osuna, F. Quality of lettuce Lactuca sativa (var. Tropicana M1) grown with two low-salinity shrimp effluents. Food Chem. X 2019, 2, 100027. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badeck, F.W.; Citerne, S.; Tangama, M.; David, A.; Ghashghaie, J. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Physiol. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef]
- Kapoor, K.; Srivastava, A. Assessment of salinity tolerance of Vigna mungo var. Pu-19 using ex vitro and in vitro methods. Asian J. Biotechnol. 2010, 2, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Piñero, M.C.; Pérez-Jiménez, M.; López-Marín, J.; del Amor, F.M. Fruit quality of sweet pepper as affected by foliar Ca applications to mitigate the supply of saline water under a climate change scenario. J. Sci. Food Agric. 2018, 98, 1071–1078. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Incerti, A.; Navari-Izzo, F.; Pardossi, A.; Izzo, R. Seasonal variations in polyphenols and lipoic acid in fruits of tomato irrigated with sea water. J. Sci. Food Agric. 2009, 89, 1326–1331. [Google Scholar] [CrossRef]
- Savvas, D.; Passam, H.C.; Olympios, C.; Nasi, E.; Moustaka, E.; Mantzos, N.; Barouchas, P. Effects of ammonium nitrogen on lettuce grown on pumice in a closed hydroponic system. HortScience 2006, 41, 1667–1673. [Google Scholar] [CrossRef] [Green Version]
- Kotsiras, A.; Olympios, C.M.; Passam, H.C. Effects of nitrogen form and concentration on yield and quality of cucumbers grown on rockwool during spring and winter in southern Greece. J. Plant Nutr. 2005, 28, 2027–2035. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A. Producing nitrate-free endive heads: Effect of nitrogen form on growth, yield, and ion composition of endive. J. Am. Soc. Hortic. Sci. 1997, 122, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Chatzigianni, M.; Alkhaled, B.; Livieratos, I.; Stamatakis, A.; Ntatsi, G.; Savvas, D. Impact of nitrogen source and supply level on growth, yield and nutritional value of two contrasting ecotypes of Cichorium spinosum L. grown hydroponically. J. Sci. Food Agric. 2018, 98, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Perner, H.; Schwarz, D.; Krumbein, A.; George, E. Influence of sulfur supply, ammonium nitrate ratio, and arbuscular mycorrhizal colonization on growth and composition of Chinese chive. Sci. Hortic. 2011, 130, 485–490. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Palaniswamy, U.R.; Bible, B.B.; McAvoy, R.J. Oxalic acid concentrations in Purslane (Portulaca oleraceae L.) is altered by the stage of harvest and the nitrate to ammonium ratios in hydroponics. Sci. Hortic. 2004, 102, 267–275. [Google Scholar] [CrossRef]
- Boschiero, B.N.; Mariano, E.; Azevedo, R.A.; Ocheuze Trivelin, P.C. Influence of nitrate-ammonium ratio on the growth, nutrition, and metabolism of sugarcane. Plant Physiol. Biochem. 2019, 139, 246–255. [Google Scholar] [CrossRef]
- Szczerba, M.W.; Britto, D.T.; Balkos, K.D.; Kronzucker, H.J. Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and -insensitive components of NH4+ transport. J. Exp. Bot. 2008, 59, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Maggio, A.; Raimondi, G.; Martino, A.; De Pascale, S. Salt stress response in tomato beyond the salinity tolerance threshold. Environ. Exp. Bot. 2007, 59, 276–282. [Google Scholar] [CrossRef]
- Giannakoula, A.E.; Ilias, I.F. The effect of water stress and salinity on growth and physiology of tomato (Lycopersicon esculentum Mill.). Arch. Biol. Sci. 2013, 65, 611–620. [Google Scholar] [CrossRef]
- Claussen, W. Growth, water use efficiency, and proline content of hydroponically grown tomato plants as affected by nitrogen source and nutrient concentration. Plant Soil 2002, 247, 199–209. [Google Scholar] [CrossRef]
- Akl, I.A.; Savvas, D.; Papadantonakis, N.; Lydakis-Simantiris, N.; Kefalas, P. Influence of Ammonium to Total Nitrogen Supply Ratio on Growth, Yield and Fruit Quality of Tomato Grown in a Closed Hydroponic System. Eur. J. Hortic. Sci. 2003, 68, 204–211. [Google Scholar]
- Chrysargyris, A.; Antoniou, O.; Athinodorou, F.; Vassiliou, R.; Papadaki, A.; Tzortzakis, N. Deployment of olive-stone waste as a substitute growing medium component for Brassica seedling production in nurseries. Environ. Sci. Pollut. Res. 2019, 26, 35461–35472. [Google Scholar] [CrossRef]
- Eaton, A.; Clesceri, L.; Rice, E.; Greenberg, A. Standard Methods for Examination of Water and Wastewater: Centennial Edition, 1368; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Costan, A.; Stamatakis, A.; Chrysargyris, A.; Petropoulos, S.A.; Tzortzakis, N. Interactive effects of salinity and silicon application on Solanum lycopersicum growth, physiology and shelf life of fruit produced hydroponically. J. Sci. Food Agric. 2019, 100, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Rousos, C.; Xylia, P.; Tzortzakis, N. Vapour application of sage essential oil maintain tomato fruit quality in breaker and red ripening stages. Plants 2021, 10, 2645. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International; Horwitz, W., Latimer, G., Eds.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Psarras, G.; Bertaki, M.; Chartzoulakis, K. Response of greenhouse tomato to salt stress and K+ supplement. Plant Biosyst. 2008, 142, 149–153. [Google Scholar] [CrossRef]
- Stamatakis, A.; Papadantonakis, N.; Savvas, D.; Lydakis-Simantiris, N.; Kefalas, P.; Savvas, D.; Lydakis-Simantiris, N.; Kefalas, P. Effects of silicon and salinity on fruit yield and quality of tomato grown hydroponically. Acta Hortic. 2003, 609, 141–147. [Google Scholar] [CrossRef]
- Savvas, D.; Lenz, F. Response of eggplants grown in recirculating nutrient solution to salinity imposed prior to the start of harvesting. J. Hortic. Sci. Biotechnol. 2000, 75, 262–267. [Google Scholar] [CrossRef]
- Zhai, Y.; Yang, Q.; Hou, M. The effects of saline water drip irrigation on tomato yield, quality, and blossom-end rot incidence-A 3a case study in the South of China. PLoS ONE 2015, 10, e0142204. [Google Scholar]
- Kotsiras, A.; Olympios, C.M.; Drosopoulos, J.; Passam, H.C. Effects of nitrogen form and concentration on the distribution of ions within cucumber fruits. Sci. Hortic. 2002, 95, 175–183. [Google Scholar] [CrossRef]
- Balotf, S.; Kavoosi, G. Differential nitrate accumulation, nitrate reduction, nitrate reductase activity, protein production and carbohydrate biosynthesis in response to potassium and sodium nitrate. Afr. J. Biotechnol. 2011, 10, 17973–17980. [Google Scholar]
- Tuna, A.L.; Kaya, C.; Ashraf, M.; Altunlu, H.; Yokas, I.; Yagmur, B. The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ. Exp. Bot. 2007, 59, 173–178. [Google Scholar] [CrossRef]
- Kostic, L.; Nikolic, N.; Bosnic, D.; Samardzic, J.; Nikolic, M. Silicon increases phosphorus (P) uptake by wheat under low P acid soil conditions. Plant Soil 2017, 419, 447–455. [Google Scholar] [CrossRef]
- Agostinho, F.B.; Tubana, B.S.; Martins, M.S.; Datnoff, L.E. Effect of different silicon sources on yield and silicon uptake of rice grown under varying phosphorus rates. Plants 2017, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Grieve, C.; Grattan, S. Mineral Nutrient Acquisition and Response by Plants Grown in Saline Environments. Agric. Ecosyst. Environ. 1999, 32, 203–229. [Google Scholar]
- Del Amor, F.M.; Martinez, V.; Cerdá, A. Salt tolerance of tomato plants as affected by stage of plant development. HortScience 2001, 36, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Altuntas, O.; Dasgan, H.Y.; Akhoundnejad, Y. Silicon nutrition ameliorates salt stress of Capsicum annuum L. by ion regulation. In Proceedings of the XVIth EUCARPIA Capsicum and Eggplant Working Group Meeting in Memoriam Dr. Alain Palloix, Kecskemét, Hungary, 12–14 September 2016; pp. 465–469. [Google Scholar]
- Chrysargyris, A.; Michailidi, E.; Tzortzakis, N. Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front. Plant Sci. 2018, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Ali Turan, M.; Hassan Awad Elkarim, A.; Taban, N.; Taban, S. Effect of salt stress on growth and ion distribution and accumulation in shoot and root of maize plant. Afr. J. Agric. Res. 2010, 5, 584–588. [Google Scholar]
- Adler, P.R.; Wilcox, G.E. Ammonium Increases the Net Rate of Sodium Influx and Partitioning to the Leaf of Muskmelon. J. Plant Nutr. 1995, 18, 1951–1962. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Meisinger, J.J.; Power, J.F.; Suarez, D.L. Root extraction of nutrients associated with long-term soil management. Adv. Soil Sci. 1992, 19, 151–217. [Google Scholar]
- Flores, P.; Carvajal, M.; Cerdá, A.; Martínez, V. Salinity and ammonium/nitrate interactions on tomato plant development, nutrition, and metabolites. J. Plant Nutr. 2001, 24, 1561–1573. [Google Scholar] [CrossRef]
- Rahman, S.; Vance, G.F.; Munn, L.C. Salinity Induced Effects on the Nutrient Status of Soil, Corn Leaves and Kernels. Commun. Soil Sci. Plant Anal. 1993, 24, 2251–2269. [Google Scholar] [CrossRef]
- Izzo, R.; Navari-Izzo, F.; Quartacci, M.F. Growth and mineral absorption in maize seedlings as affected by increasing nacl concentrations. J. Plant Nutr. 1991, 14, 687–699. [Google Scholar] [CrossRef]
- Maas, E.V.; Ogata, G.; Garber, M.J. Influence of salinity on Fe, Mn, and Zn uptake by plants. Agron. J. 1972, 64, 793–795. [Google Scholar] [CrossRef]
- Al-Harbi, A.R. Growth and Nutrient Composition of Tomato and Cucumber Seedlings as Affected by Sodium Chloride Salinity and Supplemental Calcium. J. Plant Nutr. 1995, 18, 1403–1416. [Google Scholar] [CrossRef]
- Krauss, S.; Schnitzler, W.H.; Grassmann, J.; Woitke, M. The influence of different electrical conductivity values in a simplified recirculating soilless system on inner and outer fruit quality characteristics of tomato. J. Agric. Food Chem. 2006, 54, 441–448. [Google Scholar] [CrossRef]
- Moya, C.; Oyanedel, E.; Verdugo, G.; Flores, M.F.; Urrestarazu, M.; Álvaro, J.E. Increased electrical conductivity in nutrient solution management enhances dietary and organoleptic qualities in soilless culture tomato. HortScience 2017, 52, 868–872. [Google Scholar] [CrossRef] [Green Version]
- Zushi, K.; Matsuzoe, N. Metabolic profile of organoleptic and health-promoting qualities in two tomato cultivars subjected to salt stress and their interactions using correlation network analysis. Sci. Hortic. 2015, 184, 8–17. [Google Scholar] [CrossRef]
- Ben, G.O.; Kafkafi, U. Melon fruit quality as affected by timing, duration, and concentration of phosphate and nitrogen sources in recycled hydroponic system. J. Plant Nutr. 2002, 25, 1563–1583. [Google Scholar] [CrossRef]
- Petersen, K.K.; Willumsen, J.; Kaack, K. Composition and taste of tomatoes as affected by increased salinity and different salinity sources. J. Hortic. Sci. Biotechnol. 1998, 73, 205–215. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Fogliano, V.; Ambrosino, P.; Ritieni, A. Irrigation with saline water improves carotenoids content and antioxidant activity of tomato. J. Hortic. Sci. Biotechnol. 2001, 76, 447–453. [Google Scholar] [CrossRef]
- Serio, F.; De Gara, L.; Caretto, S.; Leo, L.; Santamaria, P. Influence of an increased NaCl concentration on yield and quality of cherry tomato grown in posidonia (Posidonia oceanica (L) Delile). J. Sci. Food Agric. 2004, 84, 1885–1890. [Google Scholar] [CrossRef]
- Giuliano, G.; Bartley, G.E.; Scolnik, P.A. Regulation of carotenoids biosynthesis during tomato development. Plant Cell 1993, 5, 379–387. [Google Scholar]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Changes in major antioxidant components of tomatoes during post-harvest storage. Food Chem. 2006, 99, 724–727. [Google Scholar] [CrossRef]
- Iglesias, M.J.; García-López, J.; Collados-Luján, J.F.; López-Ortiz, F.; Díaz, M.; Toresano, F.; Camacho, F. Differential response to environmental and nutritional factors of high-quality tomato varieties. Food Chem. 2015, 176, 278–287. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C.; Ban, Y.; Shoji, K.; Sugiyama, M.; Fukuda, N.; Nishimura, S. Salinity stress affects assimilate metabolism at the gene-expression level during fruit development and improves fruit quality in tomato (Solanum lycopersicum L.). J. Jpn. Soc. Hortic. Sci. 2008, 77, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Manzo, N.; Pizzolongo, F.; Meca, G.; Aiello, A.; Marchetti, N.; Romano, R. Comparative chemical compositions of fresh and stored vesuvian PDO “Pomodorino del Piennolo” tomato and the ciliegino variety. Molecules 2018, 23, 2871. [Google Scholar] [CrossRef] [Green Version]
- Petric, T.; Kiferle, C.; Perata, P.; Gonzali, S. Optimizing shelf life conditions for anthocyanin-rich tomatoes. PLoS ONE 2018, 13, e0205650. [Google Scholar] [CrossRef] [PubMed]
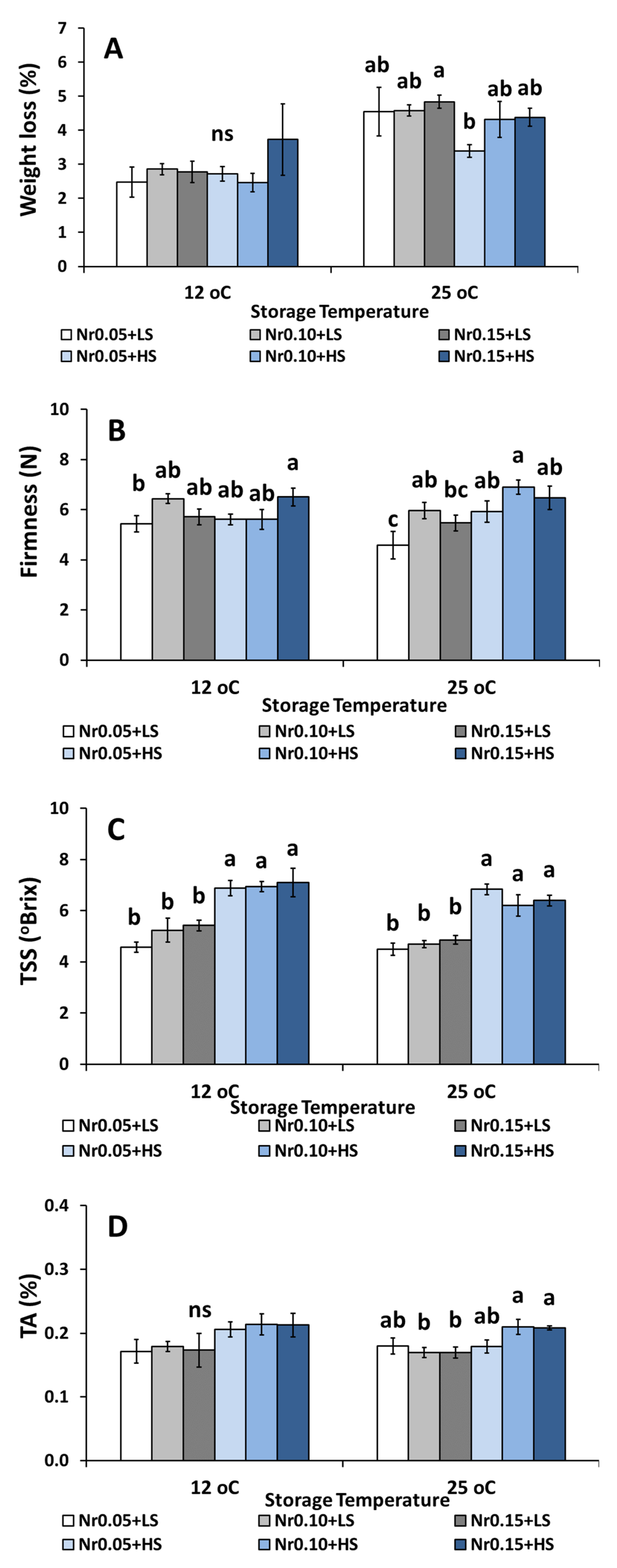
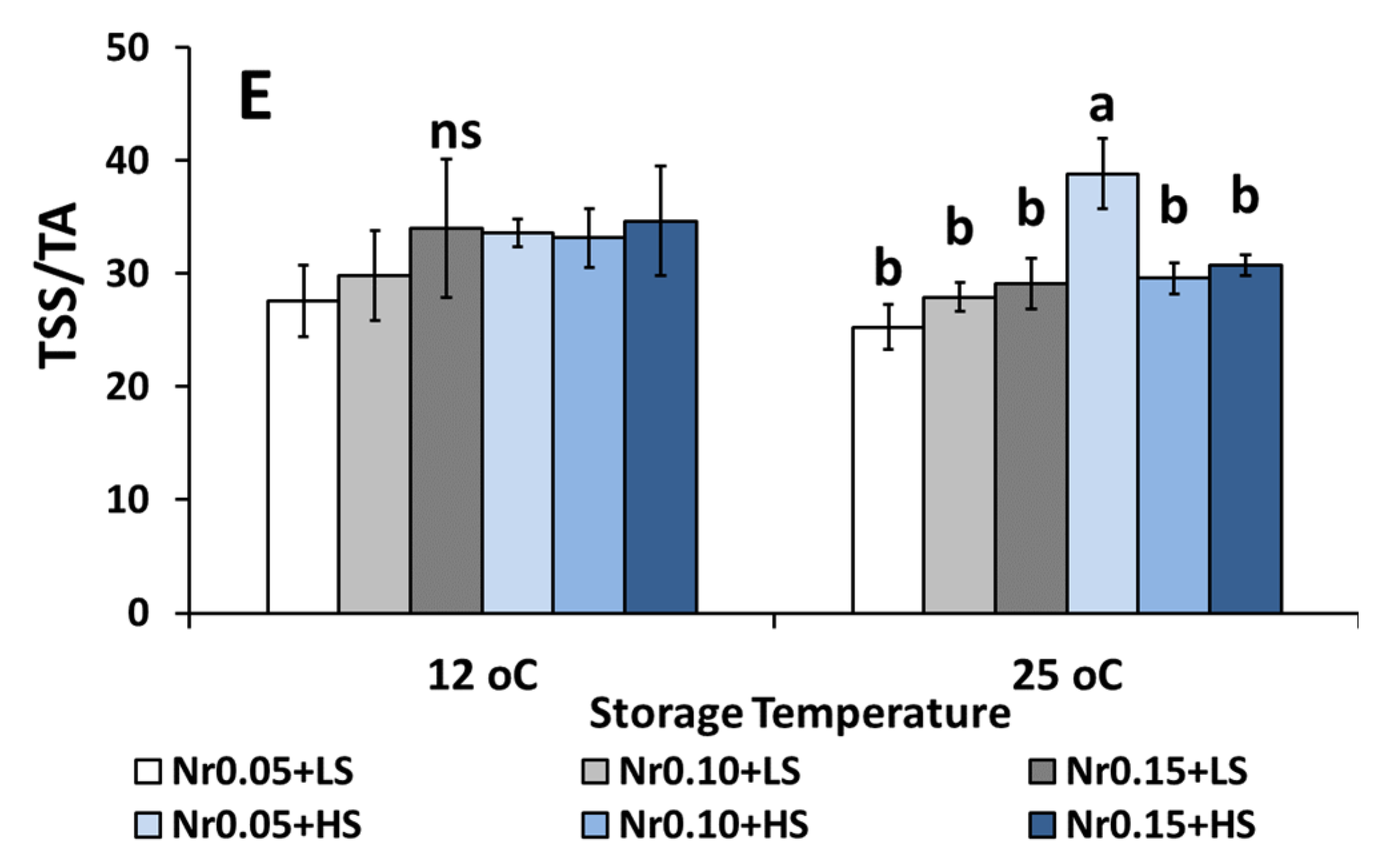
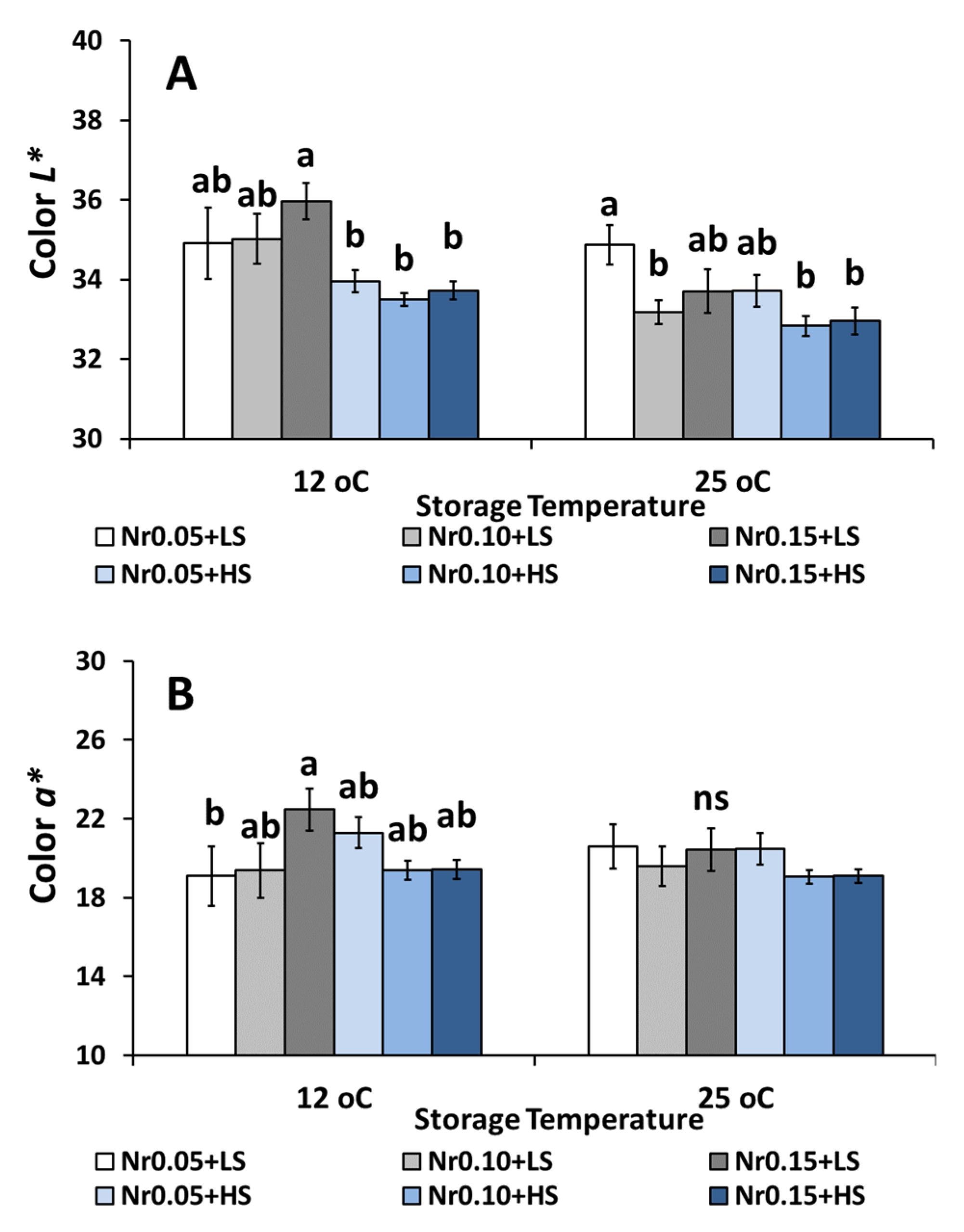
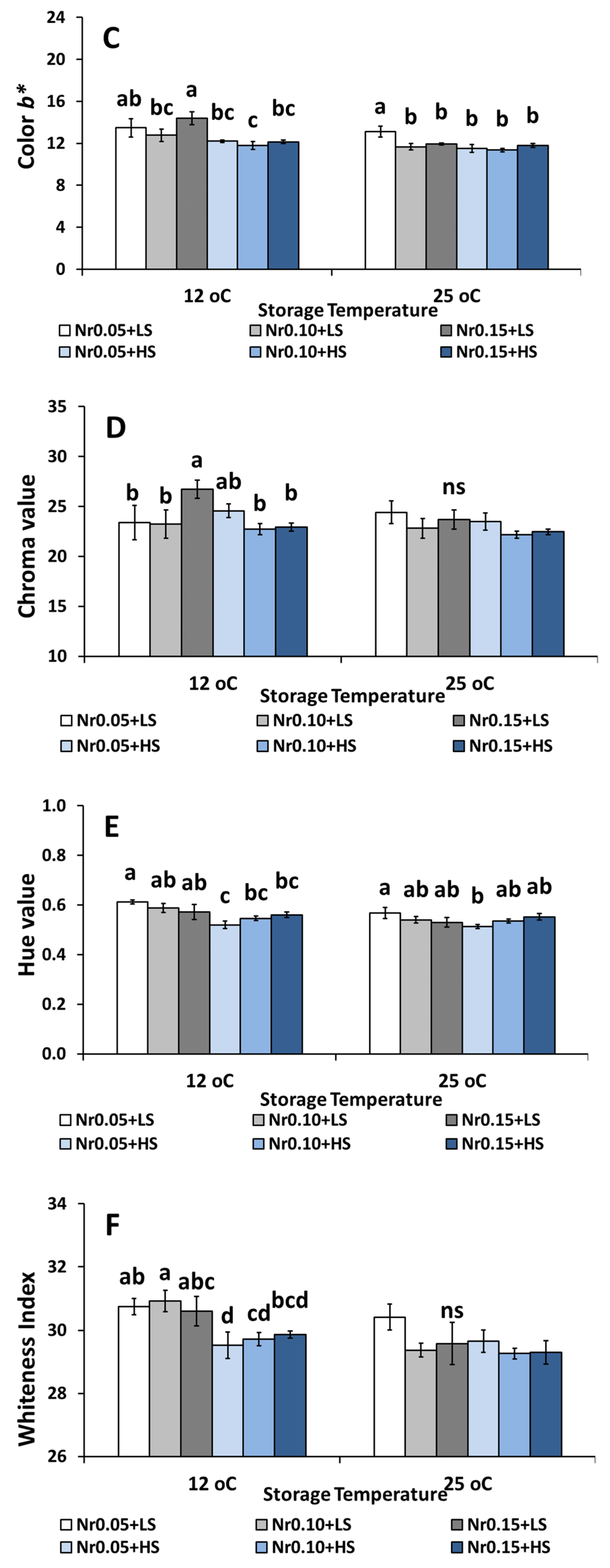
| Low Salinity | High Salinity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nr0.05 + LS | Nr0.10 + LS | Nr0.15 + LS | Nr0.05 + HS | Nr0.10 + HS | Nr0.15 + HS | Nr | Sal | Nr × Sal | |
| Plant height (cm) | 180.00 ± 6.87 a | 183.37 ± 5.16 a | 170.62 ± 5.18 ab | 167.87 ± 6.33 ab | 149.00 ± 7.92 c | 160.85 ± 3.46 bc | ns | ns | ns |
| Number of leaves | 22.50 ± 0.70 a | 21.62 ± 0.59 a | 22.75 ± 0.70 a | 20.87 ± 0.39 a | 20.75 ± 0.90 a | 21.42 ± 0.52 a | ns | ns | ns |
| Stem diameter (mm) | 8.25 ± 0.63 a | 8.31 ± 0.56 a | 7.87 ± 0.78 a | 5.50 ± 0.42 b | 5.12 ± 0.45 b | 5.28 ± 0.24 b | ns | * | ns |
| Fruit number | 11.25 ± 1.35 b | 15.33 ± 1.66 a | 12.14 ± 1.10 ab | 14.12 ± 0.89 ab | 11.71 ± 1.04 ab | 14.00 ± 1.09 ab | ns | ns | *** |
| Fruit fresh weight (g) | 202.38 ± 24.08 a | 180.83 ± 13.16 ab | 188.16 ± 8.74 a | 125.83 ± 2.96 c | 144.64 ± 7.51 bc | 127.92 ± 7.25 c | ns | ns | ns |
| Yield (kg per plant) | 2.30 ± 0.43 ab | 2.71 ± 0.24 a | 2.29 ± 0.26 ab | 1.77 ± 0.11 b | 1.67 ± 0.13 b | 1.77 ± 0.13 b | ns | ns | ns |
| Chlorophyll a (mg g−1) | 14.60 ± 1.86 a | 9.18 ± 1.62 a | 13.91 ± 0.96 a | 13.14 ± 2.27 a | 14.52 ± 2.58 a | 11.79 ± 2.00 a | ns | ns | ns |
| Chlorophyll b (mg g−1) | 6.23 ± 0.80 a | 3.61 ± 0.57 a | 5.52 ± 0.51 a | 6.30 ± 1.10 a | 5.85 ± 0.96 a | 5.39 ± 1.24 a | ns | ns | ns |
| Total Chlorophylls a (mg g−1) | 20.82 ± 2.63 a | 12.80 ± 2.19 a | 19.43 ± 1.39 a | 19.42 ± 3.32 a | 20.37 ± 3.54 a | 17.17 ± 3.24 a | ns | ns | ns |
| Photosynthetic rate (μmol m−2s−1) | 11.46 ± 1.99 b | 14.84 ± 1.11 ab | 17.82 ± 1.14 a | 17.52 ± 2.14 a | 18.17 ± 1.58 a | 15.60 ± 0.85 ab | * | * | ns |
| Stomatal conductance (μmol m−2s−1) | 0.611 ± 0.054 a | 0.687 ± 0.052 a | 0.683 ± 0.122 a | 0.529 ± 0.055 a | 0.660 ± 0.040 a | 0.455 ± 0.157 a | ns | ns | ns |
| Internal CO2 (μmol mol−1) | 280.66 ± 31.59 b | 341.00 ± 18.07 ab | 385.00 ± 30.09 ab | 385.50 ± 42.29 ab | 395.50 ± 31.33 a | 313.00 ± 35.79 ab | * | * | ns |
| Low Salinity | High Salinity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Nr0.05 + LS | Nr0.10 + LS | Nr0.15 + LS | Nr0.05 + HS | Nr0.10 + HS | Nr0.15 + HS | Nr | Sal | Nr × Sal |
| N (g kg−1) | 31.17 ± 0.41 a | 26.82 ± 1.02 b | 27.10 ± 0.49 b | 28.77 ± 0.72 b | 33.35 ± 0.63 a | 33.50 ± 1.03 a | ns | * | *** |
| K(g kg−1) | 33.17 ± 1.45 a | 32.11 ± 1.55 a | 34.74 ± 1.00 a | 24.43 ± 1.51 b | 21.70 ± 0.67 b | 16.32 ± 1.72 c | ns | ** | ns |
| Ca (g kg−1) | 26.46 ± 12.05 a | 23.63 ± 7.21 a | 19.11 ± 2.19 a | 23.71 ± 4.66 a | 29.76 ± 5.81 a | 32.92 ± 10.67 a | ns | ns | ns |
| P (g kg−1) | 3.02 ± 0.25 a | 2.86 ± 0.45 a | 3.90 ± 0.45 a | 5.31 ± 1.09 a | 4.05 ± 0.44 a | 4.91 ± 1.66 a | * | ** | ns |
| Mg(g kg−1) | 3.98 ± 0.33 c | 3.93 ± 0.34 c | 4.25 ± 0.26 c | 8.17 ± 0.62 b | 7.51 ± 0.88 b | 10.85 ± 1.36 a | ns | *** | ns |
| Na(g kg−1) | 2.08 ± 0.18 c | 2.23 ± 0.15 c | 2.41 ± 0.40 c | 10.04 ± 1.10 b | 8.55 ± 1.19 b | 13.95 ± 2.02 a | ns | *** | ns |
| Fe (mg kg−1) | 80.17 ± 6.05 ab | 71.49 ± 3.26 b | 73.36 ± 3.53 ab | 76.98 ± 2.13 ab | 78.38 ± 5.26 ab | 89.22 ± 7.92 a | ns | ns | ns |
| Zn (mg kg−1) | 4.87 ± 0.82 c | 5.73 ± 2.03 c | 6.27 ± 2.67 c | 17.69 ± 1.96 b | 16.89 ± 3.83 b | 37.53 ± 8.25 a | ns | * | ns |
| Cu (mg kg−1) | 1.04 ± 0.24 a | 1.35 ± 0.46 a | 0.55 ± 0.21 a | 2.05 ± 0.54 a | 1.48 ± 0.49 a | 3.98 ± 0.83 a | ns | ns | ns |
| Mn (mg kg−1) | 24.21 ± 2.36 b | 29.77 ± 7.82 ab | 40.97 ± 13.22 ab | 47.28 ± 10.47 ab | 51.37 ± 7.97 ab | 79.98 ± 33.47 a | ns | ns | ns |
| B (mg kg−1) | 72.47 ± 3.62 ab | 63.16 ± 7.69 b | 69.34 ± 3.74 ab | 84.17 ± 5.56 ab | 89.81 ± 5.80 a | 79.52 ± 12.57 ab | ns | ** | ns |
| Fruits | |||||||||
| N (g kg−1) | 29.90 ± 7.01 a | 29.35 ± 5.28 a | 19.12 ± 1.61 ab | 16.87 ± 0.95 b | 16.75 ± 0.72 b | 18.55 ± 1.97 ab | ns | * | ns |
| K(g kg−1) | 27.60 ± 1.56 a | 24.10 ± 2.82 ab | 24.86 ± 1.01 ab | 21.22 ± 1.90 bc | 19.77 ± 1.68 bc | 16.95 ± 1.72 c | ns | * | ns |
| Ca (g kg−1) | 2.65 ± 0.30 a | 1.70 ± 0.11 b | 1.62 ± 0.02 b | 1.47 ± 0.08 b | 1.54 ± 0.09 b | 1.32 ± 0.05 b | ns | ns | ns |
| p(g kg−1) | 1.86 ± 0.18 a | 1.26 ± 0.08 b | 1.56 ± 0.27 ab | 1.13 ± 0.22 b | 1.12 ± 0.15 b | 1.15 ± 0.15 b | ns | ns | ns |
| Mg(g kg−1) | 0.73 ± 0.07 a | 0.55 ± 0.01 ab | 0.70 ± 0.14 a | 0.44 ± 0.06 b | 0.49 ± 0.06 ab | 0.56 ± 0.03 ab | ns | ns | ns |
| Na(g kg−1) | 0.77 ± 0.08 b | 0.45 ± 0.09 b | 0.57 ± 0.09 b | 2.10 ± 0.39 a | 2.17 ± 0.15 a | 1.86 ± 0.29 a | ns | ** | ns |
| Al(g kg−1) | 1.36 ± 0.05 a | 0.95 ± 0.20 ab | 0.71 ± 0.20 b | 1.11 ± 0.09 ab | 1.08 ± 0.10 ab | 0.82 ± 0.12 b | * | ns | ns |
| Fe (mg kg−1) | 47.72 ± 6.66 ab | 48.60 ± 2.35 ab | 67.53 ± 23.42 a | 31.72 ± 3.36 b | 28.10 ± 3.37 b | 29.22 ± 2.19 b | ns | ns | ns |
| Zn (mg kg−1) | 3.85 ± 0.46 a | 7.53 ± 2.77 a | 6.38 ± 3.27 a | 3.10 ± 1.23 a | 1.05 ± 0.38 a | 3.53 ± 1.62 a | ns | ns | ns |
| Cu (mg kg−1) | 1.88 ± 0.36 a | 1.57 ± 0.77 ab | 0.55 ± 0.20 ab | nd | nd | 0.28 ± 0.01 b | ns | ns | ns |
| Mn (mg kg−1) | 14.32 ± 1.74 a | 4.88 ± 0.18 b | 7.57 ± 0.91 b | 4.01 ± 1.61 b | 4.57 ± 3.13 b | 3.23 ± 0.36 b | ns | * | * |
| B (mg kg−1) | 18.20 ± 3.36 a | 14.46 ± 3.83 a | 18.19 ± 9.15 a | 12.07 ± 3.57 a | 13.36 ± 3.51 a | 8.39 ± 1.59 a | ns | ns | ns |
| Low Salinity | High Salinity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nr0.05 + LS | Nr0.10 + LS | Nr0.15 + LS | Nr0.05 + HS | Nr0.10 + HS | Nr0.15 + HS | Nr | Sal | Nr × Sal | |
| Dry matter content (%) | 4.48 ± 0.32 b | 5.02 ± 0.14 b | 4.78 ± 0.12 b | 6.73 ± 0.10 ab | 8.98 ± 2.02 a | 6.92 ± 0.28 ab | ns | *** | ns |
| Firmness (N) | 5.18 ± 0.35 c | 4.77 ± 0.20 c | 5.58 ± 0.31 bc | 5.31 ± 0.36 c | 6.31 ± 0.30 ab | 6.75 ± 0.26 a | ns | * | ns |
| TSS (ºBrix) | 4.00 ± 0.23 b | 3.84 ± 0.16 b | 4.12 ± 0.14 b | 5.46 ± 0.20 a | 5.76 ± 0.21 a | 5.22 ± 0.25 a | ns | *** | ns |
| Citric acid (%) | 0.21 ± 0.01 b | 0.20 ± 0.02 b | 0.21 ± 0.01 b | 0.27 ± 0.03 a | 0.24 ± 0.02 ab | 0.27 ± 0.01 a | ns | ** | ns |
| Ripening (TSS/TA) | 18.95 ± 1.17 a | 20.00 ± 2.53 a | 20.58 ± 2.36 a | 20.89 ± 2.57 a | 24.12 ± 2.07 a | 19.26 ± 1.62 a | ns | ns | ns |
| Color (L* value) | 35.70 ± 0.83 a | 34.30 ± 0.44 ab | 34.77 ± 0.68 ab | 33.79 ± 0.23 b | 33.83 ± 0.31 b | 33.89 ± 0.33 b | ns | ns | ns |
| Color (a* value) | 18.65 ± 1.53 a | 20.08 ± 1.07 a | 20.26 ± 1.26 a | 21.02 ± 0.64 a | 18.39 ± 0.53 a | 20.55 ± 0.99 a | ns | ns | ns |
| Color (b* value) | 12.75 ± 0.66 a | 12.26 ± 0.62 a | 12.61 ± 0.36 a | 11.74 ± 0.33 a | 11.45 ± 0.24 a | 11.89 ± 0.24 a | ns | * | ns |
| Chroma | 22.61 ± 1.60 a | 23.53 ± 1.22 a | 23.89 ± 1.21 a | 24.08 ± 0.64 a | 21.67 ± 0.51 a | 23.75 ± 0.97 a | ns | ns | ns |
| Hue | 0.60 ± 0.01 a | 0.54 ± 0.00 bc | 0.56 ± 0.02 ab | 0.51 ± 0.01 c | 0.55 ± 0.01 abc | 0.53 ± 0.01 bc | ns | * | * |
| Whitening index | 31.74 ± 0.42 a | 30.17 ± 0.32 b | 30.48 ± 0.50 b | 29.53 ± 0.33 b | 30.36 ± 0.40 b | 29.72 ± 0.33 b | ns | ns | * |
| Color index | 40.82 ± 1.19 c | 47.82 ± 1.38 b | 46.21 ± 2.25 b | 53.11 ± 1.72 a | 47.56 ± 1.65 b | 50.94 ± 1.71 b | ns | ** | ** |
| Vitamin C (%) | 0.59 ± 0.08 bc | 0.52 ± 0.05 c | 0.74 ± 0.07 bc | 0.92 ± 0.13 ab | 1.15 ± 0.18 a | 1.14 ± 0.11 a | ns | *** | ns |
| Lycopene (mg g−1 Fw) | 0.054 ± 0.014 a | 0.056 ± 0.009 a | 0.039 ± 0.009 a | 0.073 ± 0.013 a | 0.071 ± 0.013 a | 0.054 ± 0.005 a | ns | ns | ns |
| β-carotene (mg g−1 Fw) | 0.052 ± 0.007 a | 0.044 ± 0.004 a | 0.038 ± 0.010 a | 0.041 ± 0.004 a | 0.049 ± 0.004 a | 0.049 ± 0.005 a | ns | ns | ns |
| Total phenols (μmol GAE g−1 Fw) | 0.35 ± 0.02 ab | 0.29 ± 0.02 ab | 0.28 ± 0.01 b | 0.38 ± 0.04 a | 0.35 ± 0.02 ab | 0.32 ± 0.01 ab | ns | ns | ns |
| Treatment | Appearance | Color | Aroma | Texture | Sweetness | Overall Satisfaction | Marketability | |
|---|---|---|---|---|---|---|---|---|
| NH4/Ntotal ratios | Nr0.05 | 66.00 ± 4.26 a | 64.00 ± 2.66 b | 62.00 ± 6.28 a | 84.00 ± 5.81 a | 58.00 ± 4.66 a | 64.00 ± 4.98 a | 68.00 ± 4.42 a |
| Nr0.10 | 78.00 ± 4.66 a | 76.00 ± 4.00 a | 76.00 ± 4.98 a | 82.00 ± 6.28 a | 70.00 ± 5.37 a | 74.00 ± 3.05 a | 80.00 ± 5.16 a | |
| Nr0.15 | 76.00 ± 4.00 a | 75.00 ± 3.33 a | 70.00 ± 6.14 a | 84.00 ± 4.98 a | 66.00 ± 4.26 a | 69.33 ± 2.99 a | 74.00 ± 5.20 a | |
| Salinity | 0 mM NaCl | 66.00 ± 3.05 a | 78.00 ± 3.59 a | 56.00 ± 4.98 b | 70.00 ± 6.14 a | 74.00 ± 3.05 b | 66.00 ± 4.26 a | 74.00 ± 5.20 a |
| 50 mM NaCl | 66.00 ± 5.02 a | 68.00 ± 5.33 a | 76.00 ± 5.81 a | 82.00 ± 7.57 a | 82.00 ± 2.53 a | 70.00 ± 6.14 a | 76.00 ± 6.53 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzortzakis, N.; Pitsikoulaki, G.; Stamatakis, A.; Chrysargyris, A. Ammonium to Total Nitrogen Ratio Interactive Effects with Salinity Application on Solanum lycopersicum Growth, Physiology, and Fruit Storage in a Closed Hydroponic System. Agronomy 2022, 12, 386. https://doi.org/10.3390/agronomy12020386
Tzortzakis N, Pitsikoulaki G, Stamatakis A, Chrysargyris A. Ammonium to Total Nitrogen Ratio Interactive Effects with Salinity Application on Solanum lycopersicum Growth, Physiology, and Fruit Storage in a Closed Hydroponic System. Agronomy. 2022; 12(2):386. https://doi.org/10.3390/agronomy12020386
Chicago/Turabian StyleTzortzakis, Nikolaos, Georgia Pitsikoulaki, Aristeidis Stamatakis, and Antonios Chrysargyris. 2022. "Ammonium to Total Nitrogen Ratio Interactive Effects with Salinity Application on Solanum lycopersicum Growth, Physiology, and Fruit Storage in a Closed Hydroponic System" Agronomy 12, no. 2: 386. https://doi.org/10.3390/agronomy12020386
APA StyleTzortzakis, N., Pitsikoulaki, G., Stamatakis, A., & Chrysargyris, A. (2022). Ammonium to Total Nitrogen Ratio Interactive Effects with Salinity Application on Solanum lycopersicum Growth, Physiology, and Fruit Storage in a Closed Hydroponic System. Agronomy, 12(2), 386. https://doi.org/10.3390/agronomy12020386








