Aroma Volatiles in Tomato Fruits: The Role of Genetic, Preharvest and Postharvest Factors
Abstract
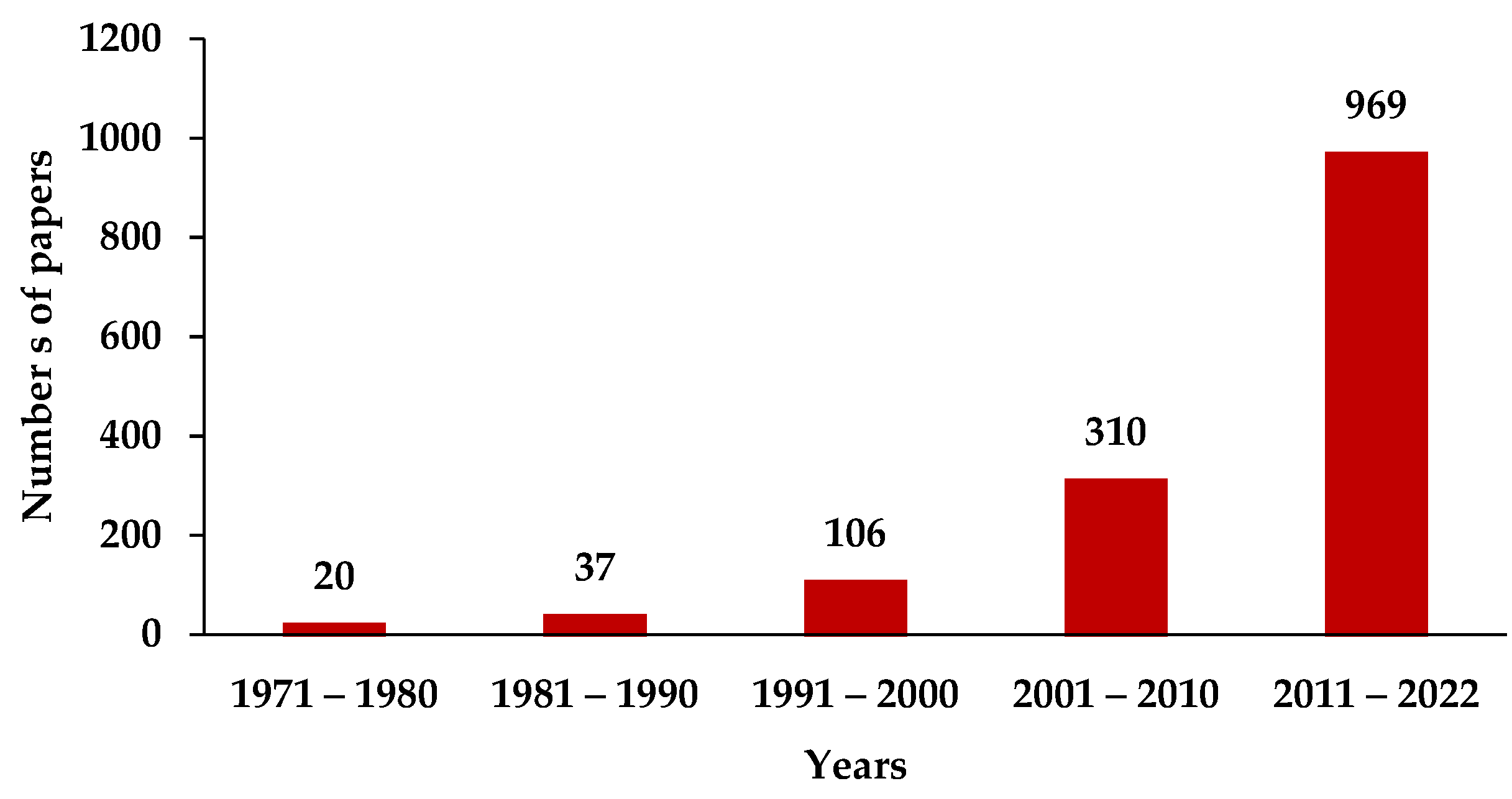
:1. Introduction
2. Tomato Quality: Evolution and Emerging Aspects
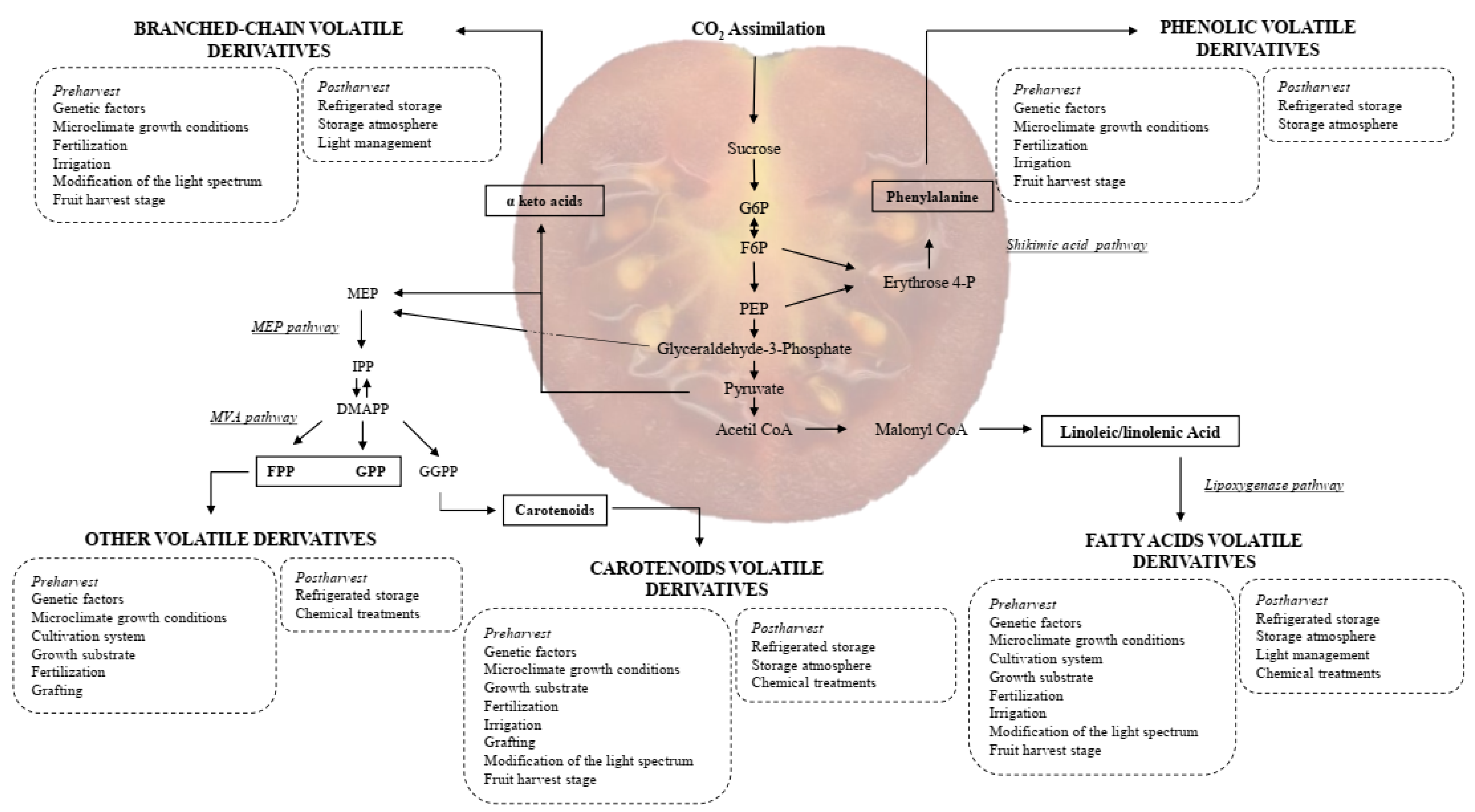
3. VOCs in Tomatoes: Their Role, Classification and Biosynthetic Pathways
3.1. Contribution of VOCs to Tomato Flavour
3.2. Chemical Classification and Biosynthesis of VOCs
3.2.1. Fatty Acids Derivatives
3.2.2. Amino Acid Derivatives
3.2.3. Carotenoid Derivatives
3.2.4. Others
4. Genetic Factors Affecting Tomato Volatiles
5. Preharvest Factors Affecting Tomato Volatiles
5.1. Grafting
5.2. Growth Environment and Conditions
| Volatile Compounds | Grafting | Growth Environment and Conditions | Growing Medium and Fertilisation | Irrigation and Water Quality | Plant Beneficial Micro-Organisms | Harvest Stage |
|---|---|---|---|---|---|---|
| Fatty acid derivates | ||||||
| Hexanol | [23] | [21] | ||||
| Z-3-Hexenal | [87,88] | [21,89] | [90] | [91] | ||
| E-2-Heptenal | [23] | [92] | [21] | [90,93] | ||
| 1-Penten-3-ol | [93] | |||||
| Pentanol | [23] | [93] | ||||
| E-2-Pentenal | ||||||
| Z-3-Hexenol | [23] | [91] | ||||
| E-2-Hexenal | [23] | [92,94] | [87] | [21,89,95] | [90,96] | [91] |
| 1-Penten-3-one | [92,97] | [87,88] | [90,93] | |||
| Hexanal | [23] | [86] | [87,88,98] | [21,89,95] | [90] | [91] |
| Caroteoid derivates | ||||||
| Epoxy-β-ionone | ||||||
| Pseudoionone | ||||||
| β-Damascenone | [90] | |||||
| Neral | [92] | [99] | [21] | |||
| β-Cyclocitral | [77] | [98] | [21] | |||
| β-Ionone | [23,77] | [97] | [99] | [21] | ||
| Geranial | [92] | [99] | [21] | [90] | ||
| Geranylacetone | [77] | [97] | [21] | [90] | ||
| 6-Methyl-5-hepten-2-one | [23] | [87,88,99] | [21] | [90,96] | [100] | |
| Amino acid derivates | ||||||
| 3-Methylbutanenitrile | ||||||
| 2-Phenylacetaldehyde | [94] | [87,88,99] | ||||
| 1-Nitro-2-phenylethane | [90] | |||||
| 3-Methylbutanal | [23] | [101] | [88] | [90] | ||
| 2-Isobutylthiazole | [94] | [21] | [91,102] | |||
| Methyl salicylate | [23,77] | [86,94,101] | [21] | [90,96] | ||
| 3-Methylbutanol | [23] | [97,101] | [95] | |||
| 2-Phenylethanol | [87,88] | [21] | ||||
| Others | ||||||
| Linalool | [98] | [21] | ||||
| 1-Nitro-3-methylbutane |
5.3. Growing Medium and Fertilization
5.4. Irrigation and Water Quality
5.5. Plant-Beneficial Microorganisms
5.6. Harvest Stage
6. Postharvest Factors Affecting Tomato Volatiles
6.1. Microclimate Storage Conditions
6.2. Storage Atmosphere Composition
6.3. Postharvest Chemicals Application
7. Industrial Processing
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Available online: http://www.fao.org/faostat/en/#home (accessed on 26 January 2022).
- Mauro, R.P.; Lo Monaco, A.; Lombardo, S.; Restuccia, A.; Mauromicale, G. Eradication of Orobanche/Phelipanche spp. seedbank by soil solarization and organic supplementation. Sci. Hortic. 2015, 193, 62–68. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Ma, L.; Liao, X.; Hu, X. Non-volatile and volatile metabolic profiling of tomato juice processed by high-hydrostatic-pressure and high-temperature short-time. Food Chem. 2022, 371, 131161. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Shafique, M.W.; Gull, S.; Naveed, W.A.; Javed, T.; Yousef, A.F.; Mauro, R.P. Alleviation of heat stress in tomato by exogenous application of sulfur. Horticulturae 2021, 7, 21. [Google Scholar] [CrossRef]
- Wang, D.; Seymour, G.B. Tomato flavor: Lost and found? Mol. Plant 2017, 10, 782–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Bertin, N.; Génard, M. Tomato quality as influenced by preharvest factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
- Bai, Y.; Lindhout, P. Domestication and breeding of tomatoes: What have we gained and what can we gain in the future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef]
- Klee, H.J. Improving the flavor of fresh fruits: Genomics, biochemistry, and biotechnology. New Phytol. 2010, 187, 44–56. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Shewmaker, C.K.; Schuch, W. Flavor trivia and tomato aroma: Biochemistry and possible mechanisms for control of important aroma components. HortScience 2000, 35, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, E.A.; Goodner, K.; Plotto, A. Interaction of volatiles, sugars, and acids on perception of tomato aroma and flavor descriptors. J. Food Sci. 2008, 73, S294–S307. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Carli, P.; Barone, A.; Fogliano, V.; Frusciante, L.; Ercolano, M.R. Dissection of genetic and environmental factors involved in tomato organoleptic quality. BMC Plant Biol. 2011, 11, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petró-Turza, M. Flavor of tomato and tomato products. Food Rev. Int. 1986, 2, 309–351. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. Genetic challenges of flavor improvement in tomato. Trends Genet. 2013, 29, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Causse, M.; Buret, M.; Robini, K.; Verschave, P. Inheritance of nutritional and sensory quality traits in fresh market tomato and relation to consumer preferences. J. Food Sci. 2003, 68, 2342–2350. [Google Scholar] [CrossRef]
- Farneti, B.; Alarcón, A.A.; Papasotiriou, F.G.; Samudrala, D.; Cristescu, S.M.; Costa, G.; Harren, F.J.M.; Woltering, E.J. Chilling-induced changes in aroma volatile profiles in tomato. Food Bioprocess Technol. 2015, 8, 1442–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- William, L.G.; Stanley, A.B. Genetics in Breeding of Processing Tomatoes. In Tomato Production, Processing and Technology; Elsevier: Amsterdam, The Netherlands, 1992; pp. 83–101. [Google Scholar]
- Krumbein, A.; Peters, P.; Brückner, B. Flavour compounds and a quantitative descriptive analysis of tomatoes (Lycopersicon esculentum Mill.) of different cultivars in short-term storage. Postharvest Biol. Technol. 2004, 32, 15–28. [Google Scholar] [CrossRef]
- Lahoz, I.; Pérez de Castro, A.; Valcárcel, M.; Macua, J.I.; Beltrán, J.; Roselló, S.; Cebolla-Cornejo, J. Effect of water deficit on the agronomical performance and quality of processing tomato. Sci. Hortic. 2016, 200, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Maul, F.; Sargent, S.A.; Sims, C.A.; Baldwin, E.A.; Balaban, M.O.; Huber, D.J. Tomato flavor and aroma quality as affected by storage temperature. J. Food Sci. 2000, 65, 1228–1237. [Google Scholar] [CrossRef]
- Mauro, R.P.; Rizzo, V.; Leonardi, C.; Mazzaglia, A.; Muratore, G.; Distefano, M.; Sabatino, L.; Giuffrida, F. Influence of harvest stage and rootstock genotype on compositional and sensory profile of the elongated tomato cv. “Sir Elyan”. Agriculture 2020, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Powell, A.L.T.; Nguyen, C.V.; Hill, T.; Cheng, K.L.L.; Figueroa-Balderas, R.; Aktas, H.; Ashrafi, H.; Pons, C.; Fernández-Muñoz, R.; Vicente, A.; et al. Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 2012, 336, 1711–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kegge, W.; Pierik, R. Biogenic volatile organic compounds and plant competition. Trends Plant Sci. 2010, 15, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Baldwin, E.A.; Bai, J. Recent advance in aromatic volatile research in tomato fruit: The metabolisms and regulations. Food Bioprocess Technol. 2016, 9, 203–216. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C. Volatile Components of Tomato Fruit and Plant Parts. In Bioactive Volatile Compounds from Plants; Teranishi, R., Buttery, R.G., Sugisawa, H., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 23–34. [Google Scholar]
- Buttery, R.G.; Teranishi, R.; Ling, L.C. Fresh tomato aroma volatiles: A quantitative study. J. Agric. Food Chem. 1987, 35, 540–544. [Google Scholar] [CrossRef]
- Guadagni, D.G.; Buttery, R.G.; Okano, S. Odour thresholds of some organic compounds associated with food flavours. J. Sci. Food Agric. 1963, 14, 761–765. [Google Scholar] [CrossRef]
- Guadagni, D.G.; Buttery, R.G.; Harris, J. Odour intensities of hop oil components. J. Sci. Food Agric. 1966, 17, 142–144. [Google Scholar] [CrossRef]
- Rambla, J.L.; Tikunov, Y.M.; Monforte, A.J.; Bovy, A.G.; Granell, A. The expanded tomato fruit volatile landscape. J. Exp. Bot. 2014, 65, 4613–4623. [Google Scholar] [CrossRef] [Green Version]
- Bezman, Y.; Mayer, F.; Takeoka, G.R.; Buttery, R.G.; Ben-Oliel, G.; Rabinowitch, H.D.; Naim, M. Differential effects of tomato (Lycopersicon esculentum Mill) matrix on the volatility of important aroma compounds. J. Agric. Food Chem. 2003, 51, 722–726. [Google Scholar] [CrossRef]
- Tandon, K.S.; Baldwin, E.A.; Scott, J.W.; Shewfelt, R.L. Linking sensory descriptors to volatile and nonvolatile components of fresh tomato flavor. J. Food Sci. 2003, 68, 2366–2371. [Google Scholar] [CrossRef]
- Vogel, J.T.; Walter, M.H.; Giavalisco, P.; Lytovchenko, A.; Kohlen, W.; Charnikhova, T.; Simkin, A.J.; Goulet, C.; Strack, D.; Bouwmeester, H.J.; et al. SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J. 2010, 61, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Bliss, P.; McIntyre, L.M.; Blandon-Ubeda, A.; Bies, D.; Odabasi, A.Z.; Rodríguez, G.R.; van der Knaap, E.; Taylor, M.G.; Goulet, C.; et al. The chemical interactions underlying tomato flavor preferences. Curr. Biol. 2012, 22, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- Causse, M.; Albert, E.; Sauvage, C. Developing Tomato Varieties with Improved Flavour. In Achieving Sustainable Cultivation of Tomatoes; Mattoo, A., Handa, A., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; pp. 283–313. [Google Scholar]
- Chen, G.; Hackett, R.; Walker, D.; Taylor, A.; Lin, Z.; Grierson, D. Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acid-derived flavor compounds. Plant Physiol. 2004, 136, 2641–2651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidovich-Rikanati, R.; Azulay, Y.; Sitrit, Y.; Tadmor, Y.; Lewinsohn, E. Tomato Aroma: Biochemistry and Biotechnology. In Biotechnology in Flavor Production; Havkin-Frenkel, D., Dudai, N., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2009; pp. 118–129. ISBN 140515649X. [Google Scholar]
- Martina, M.; Tikunov, Y.; Portis, E.; Bovy, A.G. The genetic basis of tomato aroma. Genes 2021, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Tieman, D.; Jones, J.B.; Taylor, M.G.; Schmelz, E.; Huffaker, A.; Bies, D.; Chen, K.; Klee, H.J. A 13-lipoxygenase, TomloxC, is essential for synthesis of C5 flavour volatiles in tomato. J. Exp. Bot. 2014, 65, 419–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tieman, D.M.; Loucas, H.M.; Kim, J.Y.; Clark, D.G.; Klee, H.J. Tomato phenylacetaldehyde reductases catalyze the last step in the synthesis of the aroma volatile 2-phenylethanol. Phytochemistry 2007, 68, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Gonda, I.; Bar, E.; Portnoy, V.; Lev, S.; Burger, J.; Schaffer, A.A.; Tadmor, Y.; Gepstein, S.; Giovannoni, J.J.; Katzir, N.; et al. Branched-chain and aromatic amino acid catabolism into aroma volatiles in Cucumis melo L. fruit. J. Exp. Bot. 2010, 61, 1111–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloney, G.S.; Kochevenko, A.; Tieman, D.M.; Tohge, T.; Krieger, U.; Zamir, D.; Taylor, M.G.; Fernie, A.R.; Klee, H.J. Characterization of the branched-chain amino acid aminotransferase enzyme family in tomato. Plant Physiol. 2010, 153, 925–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochevenko, A.; Araújo, W.L.; Maloney, G.S.; Tieman, D.M.; Do, P.T.; Taylor, M.G.; Klee, H.J.; Fernie, A.R. Catabolism of branched chain amino acids supports respiration but not volatile synthesis in tomato fruits. Mol. Plant 2012, 5, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Flath, R.A.; Stern, D.J. Quantitative studies on origins of fresh tomato aroma volatiles. J. Agric. Food Chem. 1988, 36, 1247–1250. [Google Scholar] [CrossRef]
- Vogel, J.T.; Tieman, D.M.; Sims, C.A.; Odabasi, A.Z.; Clark, D.G.; Klee, H.J. Carotenoid content impacts flavor acceptability in tomato (Solanum lycopersicum). J. Sci. Food Agric. 2010, 90, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J. Carotenoids and apocarotenoids in planta: Their role in plant development, contribution to the flavour and aroma of fruits and flowers, and their nutraceutical benefits. Plants 2021, 10, 2321. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.T.; Tan, B.C.; McCarty, D.R.; Klee, H.J. The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J. Biol. Chem. 2008, 283, 11364–11373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilg, A.; Beyer, P.; Al-Babili, S. Characterization of the rice carotenoid cleavage dioxygenase 1 reveals a novel route for geranial biosynthesis. FEBS J. 2009, 276, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Tandon, K.S.; Baldwin, E.A.; Shewfelt, R.L. Aroma perception of individual volatile compounds in fresh tomatoes (Lycopersicon esculentum, Mill.) as affected by the medium of evaluation. Postharvest Biol. Technol. 2000, 20, 261–268. [Google Scholar] [CrossRef]
- Rottet, S.; Devillers, J.; Glauser, G.; Douet, V.; Besagni, C.; Kessler, F. Identification of plastoglobules as a site of carotenoid cleavage. Front. Plant Sci. 2016, 7, 1855. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone, and geranylacetone. Plant J. 2004, 40, 882–892. [Google Scholar] [CrossRef]
- Ilg, A.; Bruno, M.; Beyer, P.; Al-Babili, S. Tomato carotenoid cleavage dioxygenases 1A and 1B: Relaxed double bond specificity leads to a plenitude of dialdehydes, mono-apocarotenoids and isoprenoid volatiles. FEBS Open Bio 2014, 4, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Tieman, D.; Zeigler, M.; Schmelz, E.; Taylor, M.G.; Rushing, S.; Jones, J.B.; Klee, H.J. Functional analysis of a tomato salicylic acid methyl transferase and its role in synthesis of the flavor volatile methyl salicylate. Plant J. 2010, 62, 113–123. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [Green Version]
- Waché, Y.; Bosser-DeRatuld, A.; Lhuguenot, J.C.; Belin, J.M. Effect of cis/trans isomerism of β-carotene on the ratios of volatile compounds produced during oxidative degradation. J. Agric. Food Chem. 2003, 51, 1984–1987. [Google Scholar] [CrossRef] [PubMed]
- Mageroy, M.H.; Tieman, D.M.; Floystad, A.; Taylor, M.G.; Klee, H.J. A Solanum lycopersicum catechol-O-methyltransferase involved in synthesis of the flavor molecule guaiacol. Plant J. 2012, 69, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Koeduka, T.; Fridman, E.; Gang, D.R.; Vassão, D.G.; Jackson, B.L.; Kish, C.M.; Orlova, I.; Spassova, S.M.; Lewis, N.G.; Noel, J.P.; et al. Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc. Natl. Acad. Sci. USA 2006, 103, 10128–10133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, D.G.; Price, T.S. Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J. Chem. Ecol. 2004, 30, 1613–1628. [Google Scholar] [CrossRef] [PubMed]
- Krumbein, A.; Auerswald, H. Characterization of aroma volatiles in tomatoes by sensory analyses. Nahr. Food 1998, 42, 395–399. [Google Scholar] [CrossRef]
- WIlliams, P.J. Hydrolytic Flavor Release in Fruit and Wines through Hydrolysis of Nonvolatile Precursors. In Flavor Science: Sensible Principles and Techniques; Acree, T.E., Teranishi, R., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 287–308. [Google Scholar]
- Roscher, R.; Bringmann, G.; Schreier, P.; Schwab, W. Radiotracer studies on the formation of 2,5-dimethyl-4-hydroxy-3(2H)-furanone in detached ripening strawberry fruits. J. Agric. Food Chem. 1998, 46, 1488–1493. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Turnbaugh, J.G. Quantitative and sensory studies on tomato paste volatiles. J. Agric. Food Chem. 1990, 38, 336–340. [Google Scholar] [CrossRef]
- Williams, M.P.; Nelson, P.E. Prediction of dimethyl sulfide production in tomato serum. J. Food Sci. 1976, 41, 1241–1242. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bassal, A.; Leonardi, C.; Giuffrida, F.; Colla, G. Vegetable quality as affected by genetic, agronomic and environmental factors. J. Food Agric. Environ. 2012, 10, 680–688. [Google Scholar]
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014, 32, 170–189. [Google Scholar] [CrossRef]
- Picton, S.; Barton, S.L.; Bouzayen, M.; Hamilton, A.J.; Grierson, D. Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene. Plant J. 1993, 3, 469–481. [Google Scholar] [CrossRef]
- McGlasson, W.B.; Last, J.H.; Shaw, K.J.; Meldrum, S.K. Influence of the non-ripening mutants rin and nor on the aroma of tomato fruit. HortScience 1987, 22, 632–634. [Google Scholar]
- Alexander, L.; Grierson, D. Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.J.; Lycett, G.W.; Grierson, D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 1990, 346, 284–287. [Google Scholar] [CrossRef]
- Causse, M. QTL analysis of fruit quality in fresh market tomato: A few chromosome regions control the variation of sensory and instrumental traits. J. Exp. Bot. 2002, 53, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, Y.; Fridman, E.; Gur, A.; Larkov, O.; Lastochkin, E.; Ravid, U.; Zamir, D.; Lewinsohn, E. Identification of malodorous, a wild species allele affecting tomato aroma that was selected against during domestication. J. Agric. Food Chem. 2002, 50, 2005–2009. [Google Scholar] [CrossRef]
- Saliba-Colombani, V.; Causse, M.; Langlois, D.; Philouze, J.; Buret, M. Genetic analysis of organoleptic quality in fresh market tomato. 1. Mapping QTLs for physical and chemical traits. Theor. Appl. Genet. 2001, 102, 259–272. [Google Scholar] [CrossRef]
- Tieman, D.M.; Zeigler, M.; Schmelz, E.A.; Taylor, M.G.; Bliss, P.; Kirst, M.; Klee, H.J. Identification of loci affecting flavour volatile emissions in tomato fruits. J. Exp. Bot. 2006, 57, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, S.; Cin, V.D.; Fei, Z.; Li, H.; Bliss, P.; Taylor, M.G.; Klee, H.J.; Tieman, D.M. Flavour compounds in tomato fruits: Identification of loci and potential pathways affecting volatile composition. J. Exp. Bot. 2009, 60, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Garbowicz, K.; Liu, Z.; Alseekh, S.; Tieman, D.; Taylor, M.; Kuhalskaya, A.; Ofner, I.; Zamir, D.; Klee, H.J.; Fernie, A.R.; et al. Quantitative trait loci analysis identifies a prominent gene involved in the production of fatty acid-derived flavor volatiles in tomato. Mol. Plant 2018, 11, 1147–1165. [Google Scholar] [CrossRef] [Green Version]
- Allevato, E.; Mauro, R.P.; Stazi, S.R.; Marabottini, R.; Leonardi, C.; Ierna, A.; Giuffrida, F. Arsenic accumulation in grafted melon plants: Role of rootstock in modulating root-to-shoot translocation and physiological response. Agronomy 2019, 9, 828. [Google Scholar] [CrossRef] [Green Version]
- Mauro, R.P.; Agnello, M.; Distefano, M.; Sabatino, L.; San Bautista Primo, A.; Leonardi, C.; Giuffrida, F. Chlorophyll fluorescence, photosynthesis and growth of tomato plants as affected by long-term oxygen root zone deprivation and grafting. Agronomy 2020, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Mauro, R.P.; Agnello, M.; Onofri, A.; Leonardi, C.; Giuffrida, F. Scion and rootstock differently influence growth, yield and quality characteristics of cherry tomato. Plants 2020, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Klee, H.J. Better fruits and vegetables through sensory analysis. Curr. Biol. 2013, 23, R374–R378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krumbein, A.; Schwarz, D. Grafting: A possibility to enhance health-promoting and flavour compounds in tomato fruits of shaded plants? Sci. Hortic. 2013, 149, 97–107. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Meir, A.; Zamir, D.; Tadmor, Y. Carotenoid pigmentation affects the volatile composition of tomato and watermelon fruits, as revealed by comparative genetic analyses. J. Agric. Food Chem. 2005, 53, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Casals, J.; Rivera, A.; Sabaté, J.; del Castillo, R.R.; Simó, J. Cherry and fresh market tomatoes: Differences in chemical, morphological, and sensory traits and their implications for consumer acceptance. Agronomy 2019, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.J.; Jayaprakasha, G.K.; Avila, C.A.; Crosby, K.M.; Patil, B.S. Metabolomic studies of volatiles from tomatoes grown in net-house and open-field conditions. Food Chem. 2019, 275, 282–291. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M.J. Seasonal Variations in the Level of Plant Constituents in Greenhouse Production of Cherry Tomatoes. J. Agric. Food Chem. 2005, 53, 3114–3119. [Google Scholar] [CrossRef]
- Cebolla-Cornejo, J.; Roselló, S.; Valcárcel, M.; Serrano, E.; Beltrán, J.; Nuez, F. Evaluation of genotype and environment effects on taste and aroma flavor components of Spanish fresh tomato varieties. J. Agric. Food Chem. 2011, 59, 2440–2450. [Google Scholar] [CrossRef]
- Wang, Y.T.; Huang, S.W.; Liu, R.L.; Jin, J.Y. Effects of nitrogen application on flavor compounds of cherry tomato fruits. J. Plant Nutr. Soil Sci. 2007, 170, 461–468. [Google Scholar] [CrossRef]
- Wang, Y.T.; Liu, R.L.; Huang, S.W.; Jin, J.Y. Effects of potassium application on flavor compounds of cherry tomato fruits. J. Plant Nutr. 2009, 32, 1451–1468. [Google Scholar] [CrossRef]
- Veit-Köhler, U.; Krumbein, A.; Kosegarten, H. Effect of different water supply on plant growth and fruit quality of Lycopersicon esculentum. J. Plant Nutr. Soil Sci. 1999, 162, 583–588. [Google Scholar] [CrossRef]
- Hart, M.; Ehret, D.L.; Krumbein, A.; Leung, C.; Murch, S.; Turi, C.; Franken, P. Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 2015, 25, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.A.; Nisperos-Carriedo, M.O.; Moshonas, M.G. Quantitative analysis of flavor and other volatiles and for certain constituents of two tomato cultivars during ripening. J. Am. Soc. Hortic. Sci. 1991, 116, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Tinyane, P.P.; Sivakumar, D.; Soundy, P. Influence of photo-selective netting on fruit quality parameters and bioactive compounds in selected tomato cultivars. Sci. Hortic. 2013, 161, 340–349. [Google Scholar] [CrossRef]
- Pasković, I.; Soldo, B.; Goreta Ban, S.; Radić, T.; Lukić, M.; Urlić, B.; Mimica, M.; Brkić Bubola, K.; Colla, G.; Rouphael, Y.; et al. Fruit quality and volatile compound composition of processing tomato as affected by fertilisation practices and arbuscular mycorrhizal fungi application. Food Chem. 2021, 359, 129961. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, W.; Huang, J.; Chen, H.; Nie, R.; Li, C.M. Comparison of the nutritional as well as the volatile composition of in-season and off-season Hezuo 903 tomato at red stage. Eur. Food Res. Technol. 2017, 243, 203–214. [Google Scholar] [CrossRef]
- Dorais, M.; Dorval, R.; Demers, D.A.; Micevic, D.; Turcotte, G.; Papadopoulos, P.; Ehret, D.L.; Gosselin, A. Improving tomato fruit quality by increasing salinity: Effects on ion uptake, growth and yield. Acta Hortic. 1998, 511, 185–195. [Google Scholar] [CrossRef]
- Ruiz-Cisneros, M.F.; de Jesús Ornelas-Paz, J.; Olivas-Orozco, G.I.; Acosta-Muñiz, C.H.; Salas-Marina, M.Á.; Molina-Corral, F.J.; Berlanga-Reyes, D.I.; Fernández-Pavía, S.P.; Cambero-Campos, O.J.; Rios-Velasco, C. Effect of rhizosphere inoculation with Bacillus strains and phytopathogens on the contents of volatiles and human health-related compounds in tomato fruits. Food Sci. Technol. 2021, 2061, 1–12. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Bai, J. Sensory and chemical flavor analyses of tomato genotypes grown in Florida during three different growing seasons in multiple years. J. Am. Soc. Hortic. Sci. 2015, 140, 490–503. [Google Scholar] [CrossRef] [Green Version]
- Thybo, A.K.; Edelenbos, M.; Christensen, L.P.; Sørensen, J.N.; Thorup-Kristensen, K. Effect of organic growing systems on sensory quality and chemical composition of tomatoes. LWT-Food Sci. Technol. 2006, 39, 835–843. [Google Scholar] [CrossRef]
- Wright, D.H.; Harris, N.D. Effect of Nitrogen and Potassium Fertilization on Tomato Flavor. J. Agric. Food Chem. 1985, 33, 355–358. [Google Scholar] [CrossRef]
- Klein, D.; Gkisakis, V.; Krumbein, A.; Livieratos, I.; Köpke, U. Old and endangered tomato cultivars under organic greenhouse production: Effect of harvest time on flavour profile and consumer acceptance. Int. J. Food Sci. Technol. 2010, 45, 2250–2257. [Google Scholar] [CrossRef]
- Dalal, K.B.; Olson, L.E.; Yu, M.H.; Salunkhe, D.K. Gas chromatography of the field-, glass-Greenhouse-grown, and artificially ripened tomatoes. Lycopersicon esculentum mill. Phytochemistry 1967, 6, 155–157. [Google Scholar] [CrossRef]
- Du, X.; Song, M.; Baldwin, E.; Rouseff, R. Identification of sulphur volatiles and GC-olfactometry aroma profiling in two fresh tomato cultivars. Food Chem. 2015, 171, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Du, S.; Wang, Y.; Condon, J.; Lin, X.; Zhang, Y. Carbon dioxide enrichment by composting in greenhouses and its effect on vegetable production. J. Plant Nutr. Soil Sci. 2009, 172, 418–424. [Google Scholar] [CrossRef]
- Kläring, H.P.; Hauschild, C.; Heißner, A.; Bar-Yosef, B. Model-based control of CO2 concentration in greenhouses at ambient levels increases cucumber yield. Agric. For. Meteorol. 2007, 143, 208–216. [Google Scholar] [CrossRef]
- Rangaswamy, T.C.; Sridhara, S.; Ramesh, N.; Gopakkali, P.; El-Ansary, D.O.; Mahmoud, E.A.; Abdelmohsen, S.A.M.; Abdelbacki, A.M.M.; Elansary, H.O.; Abdel-Hamid, A.M.E. Assessing the impact of higher levels of CO2 and temperature and their interactions on tomato (Solanum lycopersicum L.). Plants 2021, 10, 256. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Li, D.; Miao, Z. Automatic carbon dioxide enrichment strategies in the greenhouse: A review. Biosyst. Eng. 2018, 171, 101–119. [Google Scholar] [CrossRef]
- Liu, J.; Peng, X.; Abdelhakim, L.O.A.; Fang, L.; Wei, Z.; Liu, F. Carbon dioxide elevation combined with sufficient irrigation and nitrogen fertilization improves fruit quality of tomato grown in glasshouse. Arch. Agron. Soil Sci. 2020, 67, 1134–1149. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Zhang, M.; Zhang, Y.; Wang, Q. Effect of carbon dioxide enrichment on health-promoting compounds and organoleptic properties of tomato fruits grown in greenhouse. Food Chem. 2014, 153, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Loughrin, J.H.; Kasperbauer, M.J. Aroma of fresh strawberries is enhanced by ripening over red versus black mulch. J. Agric. Food Chem. 2002, 50, 161–165. [Google Scholar] [CrossRef]
- Sivakumar, D.; Jifon, J.; Soundy, P. Spectral quality of photo-selective shade nettings improves antioxidants and overall quality in selected fresh produce after postharvest storage. Food Rev. Int. 2018, 34, 290–307. [Google Scholar] [CrossRef]
- Jiménez, E.; Lanza, B.; Antiñolo, M.; Albaladejo, J. Photooxidation of leaf-wound oxygenated compounds, 1-penten-3-ol, (z)-3-hexen-1-ol, and 1-penten-3-one, initiated by OH radicals and sunlight. Environ. Sci. Technol. 2009, 43, 1831–1837. [Google Scholar] [CrossRef]
- Sun, H.; Ni, H.; Yang, Y.; Wu, L.; Cai, H.N.; Xiao, A.F.; Chen, F. Investigation of sunlight-induced deterioration of aroma of pummelo (Citrus maxima) essential oil. J. Agric. Food Chem. 2014, 62, 11818–11830. [Google Scholar] [CrossRef]
- Thybo, A.K.; Bechmann, I.E.; Brandt, K. Integration of sensory and objective measurements of tomato quality: Quantitative assessment of the effect of harvest date as compared with growth medium (soil versus rockwool), electrical conductivity, variety and maturity. J. Sci. Food Agric. 2005, 85, 2289–2296. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Patanè, C.; Tringali, S.; Sortino, O. Effects of deficit irrigation on biomass, yield, water productivity and fruit quality of processing tomato under semi-arid Mediterranean climate conditions. Sci. Hortic. 2011, 129, 590–596. [Google Scholar] [CrossRef]
- Zegbe-Domínguez, J.A.; Behboudian, M.H.; Lang, A.; Clothier, B.E. Deficit irrigation and partial rootzone drying maintain fruit dry mass and enhance fruit quality in “Petopride” processing tomato (Lycopersicon esculentum, Mill.). Sci. Hortic. 2003, 98, 505–510. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Zhang, X.; Tian, P.; Li, J.; Tian, Y. Comprehensive comparison of different saline water irrigation strategies for tomato production: Soil properties, plant growth, fruit yield and fruit quality. Agric. Water Manag. 2019, 213, 521–533. [Google Scholar] [CrossRef]
- Tomescu, D.; Şumǎlan, R.; Copolovici, L.; Copolovici, D. The influence of soil salinity on volatile organic compounds emission and photosynthetic parameters of Solanum lycopersicum L. varieties. Open Life Sci. 2017, 12, 135–142. [Google Scholar] [CrossRef]
- Cliff, M.A.; Li, J.B.; Toivonen, P.M.A.; Ehret, D.L. Effects of nutrient solution electrical conductivity on the compositional and sensory characteristics of greenhouse tomato fruit. Postharvest Biol. Technol. 2012, 74, 132–140. [Google Scholar] [CrossRef]
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do pesticides promote or hinder sustainability in agriculture? The challenge of sustainable use of pesticides in modern agriculture. Sci. Total Environ. 2021, 795, 148625. [Google Scholar] [CrossRef]
- Mauro, R.P.; Sortino, O.; Dipasquale, M.; Mauromicale, G. Phenological and growth response of legume cover crops to shading. J. Agric. Sci. 2014, 152, 917–931. [Google Scholar] [CrossRef]
- Castello, I.; D’Emilio, A.; Raviv, M.; Vitale, A. Soil solarization as a sustainable solution to control tomato Pseudomonads infections in greenhouses. Agron. Sustain. Dev. 2017, 37, 59. [Google Scholar] [CrossRef] [Green Version]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Yousef, A.F.; Youssef, M.A.; Ali, M.M.; Ibrahim, M.M.; Xu, Y.; Mauro, R.P. Improved growth and yield response of Jew’s mallow (Corchorus olitorius L.) plants through biofertilization under semi-arid climate conditions in Egypt. Agronomy 2020, 10, 14. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohge, T.; Alseekh, S.; Fernie, A.R. On the regulation and function of secondary metabolism during fruit development and ripening. J. Exp. Bot. 2014, 65, 4599–4611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brezmes, J.; Luisa, M.; Fructuoso, L.; Llobet, E.; Vilanova, X.; Recasens, I.; Orts, J.; Saiz, G.; Correig, X. Evaluation of an electronic nose to assess fruit ripeness. IEEE Sens. J. 2005, 5, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Galliard, T.; Matthew, J.A.; Wright, A.J.; Fishwick, M.J. The enzymic breakdown of lipids to volatile and non-volatile carbonyl fragments in disrupted tomato fruits. J. Sci. Food Agric. 1977, 28, 863–868. [Google Scholar] [CrossRef]
- Kader, A.A. Effects of postharvest handling procedures on tomato quality. Acta Hortic. 1986, 190, 209–221. [Google Scholar] [CrossRef]
- Saltveit, M.E. Fruit ripening and fruit quality. In Tomatoes; CABI Publishing: Wallingford, UK, 2005; pp. 145–170. ISBN 0851993966. [Google Scholar]
- Zhang, Z.M.; Zeng, D.D.; Li, G.K. Study of the volatile composition of tomato during storage by a combination sampling method coupled with gas chromatography/mass spectrometry. J. Sci. Food Agric. 2008, 88, 116–124. [Google Scholar] [CrossRef]
- Distefano, M.; Arena, E.; Mauro, R.P.; Brighina, S.; Leonardi, C.; Fallico, B.; Giuffrida, F. Effects of Genotype, Storage Temperature and Time on Quality and Compositional Traits of Cherry Tomato. Foods 2020, 9, 1729. [Google Scholar] [CrossRef]
- Ponce-Valadez, M.; Escalona-Buendía, H.B.; Villa-Hernández, J.M.; de León-Sánchez, F.D.; Rivera-Cabrera, F.; Alia-Tejacal, I.; Pérez-Flores, L.J. Effect of refrigerated storage (12.5 °C) on tomato (Solanum lycopersicum) fruit flavor: A biochemical and sensory analysis. Postharvest Biol. Technol. 2016, 111, 6–14. [Google Scholar] [CrossRef]
- Wu, Q.; Tao, X.; Ai, X.; Luo, Z.; Mao, L.; Ying, T.; Li, L. Contribution of abscisic acid to aromatic volatiles in cherry tomato (Solanum lycopersicum L.) fruit during postharvest ripening. Plant Physiol. Biochem. 2018, 130, 205–214. [Google Scholar] [CrossRef]
- Wu, Q.; Tao, X.; Ai, X.; Luo, Z.; Mao, L.; Ying, T.; Li, L. Effect of exogenous auxin on aroma volatiles of cherry tomato (Solanum lycopersicum L.) fruit during postharvest ripening. Postharvest Biol. Technol. 2018, 146, 108–116. [Google Scholar] [CrossRef]
- Kelebek, H.; Kesen, S.; Sonmezdag, A.S.; Cetiner, B.; Kola, O.; Selli, S. Characterization of the key aroma compounds in tomato pastes as affected by hot and cold break process. J. Food Meas. Charact. 2018, 12, 2461–2474. [Google Scholar] [CrossRef]
- Viljanen, K.; Lille, M.; Heiniö, R.L.; Buchert, J. Effect of high-pressure processing on volatile composition and odour of cherry tomato purée. Food Chem. 2011, 129, 1759–1765. [Google Scholar] [CrossRef]
- Colquhoun, T.A.; Schwieterman, M.L.; Gilbert, J.L.; Jaworski, E.A.; Langer, K.M.; Jones, C.R.; Rushing, G.V.; Hunter, T.M.; Olmstead, J.; Clark, D.G.; et al. Light modulation of volatile organic compounds from petunia flowers and select fruits. Postharvest Biol. Technol. 2013, 86, 37–44. [Google Scholar] [CrossRef]
- Raffo, A.; Baiamonte, I.; Nardo, N.; Nicoli, S.; Moneta, E.; Peparaio, M.; Sinesio, F.; Paoletti, F. Impact of early harvesting and two cold storage technologies on eating quality of red ripe tomatoes. Eur. Food Res. Technol. 2018, 244, 805–818. [Google Scholar] [CrossRef]
- Wang, L.; Baldwin, E.A.; Plotto, A.; Luo, W.; Raithore, S.; Yu, Z.; Bai, J. Effect of methyl salicylate and methyl jasmonate pre-treatment on the volatile profile in tomato fruit subjected to chilling temperature. Postharvest Biol. Technol. 2015, 108, 28–38. [Google Scholar] [CrossRef]
- Boukobza, F.; Taylor, A.J. Effect of postharvest treatment on flavour volatiles of tomatoes. Postharvest Biol. Technol. 2002, 25, 321–331. [Google Scholar] [CrossRef]
- McDonald, R.E.; McCollum, T.G.; Baldwin, E.A. Prestorage heat treatments influence free sterols and flavor volatiles of tomatoes stored at chilling temperature. J. Am. Soc. Hortic. Sci. 1996, 121, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Deltsidis, A.I.; Pliakoni, E.D.; Baldwin, E.A.; Bai, J.; Plotto, A.; Brecht, J.K. Tomato flavor changes at chilling and non-chilling temperatures as influenced by controlled atmospheres. Acta Hortic. 2015, 1071, 703–710. [Google Scholar] [CrossRef]
- Arah, I.; Arah, I.K.; Amaglo, H.; Kumah, E.K.; Ofori, H. Preharvest and Postharvest Factors Affecting the Quality and Shelf Life of Harvested Tomatoes: A Mini Review. Int. J. Agron. 2015, 2015, 478041. [Google Scholar] [CrossRef] [Green Version]
- Salveit, M.E. Physical and Physiological Changes in Minimally Processed Fruits and Vegetable. In Phytochemistry of Fruit and Vegetables; Tomás-Barberán, A., Ed.; Oxford University Press: New York, NY, USA, 1997; pp. 205–220. [Google Scholar]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Zou, J.; Chen, J.; Tang, N.; Gao, Y.; Hong, M.; Wei, W.; Cao, H.; Jian, W.; Li, N.; Deng, W.; et al. Transcriptome analysis of aroma volatile metabolism change in tomato (Solanum lycopersicum) fruit under different storage temperatures and 1-MCP treatment. Postharvest Biol. Technol. 2018, 135, 57–67. [Google Scholar] [CrossRef]
- Saltveit, M.E. The rate of ion leakage from chilling-sensitive tissue does not immediately increase upon exposure to chilling temperatures. Postharvest Biol. Technol. 2002, 26, 295–304. [Google Scholar] [CrossRef]
- Folta, K.M.; Childers, K.S. Light as a growth regulator: Controlling plant biology with narrow-bandwidth solid-state lighting systems. HortScience 2008, 43, 1957–1964. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.I.; Conesa, M.A.; Artés, F. Quality changes in fresh cut tomato as affected by modified atmosphere packaging. Postharvest Biol. Technol. 2002, 25, 199–207. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Al-Said, F.A.J.; Opara, U.L. Modified atmosphere packaging technology of fresh and fresh-cut produce and the microbial consequences-A review. Food Bioprocess Technol. 2013, 6, 303–329. [Google Scholar] [CrossRef]
- Stern, D.J.; Buttery, R.G.; Teranishi, R.; Ling, L.; Scott, K.; Cantwell, M. Effect of storage and ripening on fresh tomato quality, Part I. Food Chem. 1994, 49, 225–231. [Google Scholar] [CrossRef]
- Auerswald, H.; Peters, P.; Brückner, B.; Krumbein, A.; Kuchenbuch, R. Sensory analysis and instrumental measurements of short-term stored tomatoes (Lycopersicon esculentum Mill.). Postharvest Biol. Technol. 1999, 15, 323–334. [Google Scholar] [CrossRef]
- Ratanachinakorn, B.; Klieber, A.; Simons, D.H. Effect of short-term controlled atmospheres and maturity on ripening and eating quality of tomatoes. Postharvest Biol. Technol. 1997, 11, 149–154. [Google Scholar] [CrossRef]
- Bailén, G.; Guillén, F.; Castillo, S.; Serrano, M.; Valero, D.; Martínez-Romero, D. Use of Activated Carbon inside Modified Atmosphere Packages To Maintain Tomato Fruit Quality during Cold Storage. Food Chem. 2006, 54, 2229–2235. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Ku, V.V.V. Use of 1-MCP to extend the time to ripen of green tomatoes and postharvest life of ripe tomatoes. Postharvest Biol. Technol. 2002, 26, 85–90. [Google Scholar] [CrossRef]
- Mir, N.; Canoles, M.; Beaudry, R.; Baldwin, E.; Mehla, C.P. Inhibiting tomato ripening with 1-Methylcyclopropene. J. Am. Soc. Hortic. Sci. 2004, 129, 112–120. [Google Scholar] [CrossRef]
- Reymond, P.; Farmer, E.E. Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1998, 1, 404–411. [Google Scholar] [CrossRef]
- Gould, W.A. Tomato Production, Processing and Technology, 3rd ed.; CTI Publications Inc.: Baltimore, MD, USA, 1992; ISBN 9781845696146 184569614X. [Google Scholar]
- Birtić, S.; Ginies, C.; Causse, M.; Renard, C.M.G.C.; Page, D. Changes in volatiles and glycosides during fruit maturation of two contrasted tomato (Solanum lycopersicum) lines. J. Agric. Food Chem. 2009, 57, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, D.G.; Miers, J.C.; Venstrom, D.W. Concentration effect on odor addition or synergism in mixtures of methyl sulfide and tomato juice. J. Food Sci. 1969, 34, 630–632. [Google Scholar] [CrossRef]
- Jeyaprakash, S.; Heffernan, J.E.; Driscoll, R.H.; Frank, D.C. Impact of drying technologies on tomato flavor composition and sensory quality. LWT 2020, 120, 108888. [Google Scholar] [CrossRef]
- Rambla, J.L.; Alfaro, C.; Medina, A.; Zarzo, M.; Primo, J.; Granell, A. Tomato fruit volatile profiles are highly dependent on sample processing and capturing methods. Metabolomics 2015, 11, 1708–1720. [Google Scholar] [CrossRef]
- Baenas, N.; Bravo, S.; García-Alonso, F.J.; Gil, J.V.; Periago, M.J. Changes in volatile compounds, flavour-related enzymes and lycopene in a refrigerated tomato juice during processing and storage. Eur. Food Res. Technol. 2021, 247, 975–984. [Google Scholar] [CrossRef]
- Rodrigo, D.; Jolie, R.; Van Loey, A.; Hendrickx, M. Thermal and high pressure stability of tomato lipoxygenase and hydroperoxide lyase. J. Food Eng. 2007, 79, 423–429. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Effects of high-intensity pulsed electric fields on lipoxygenase and hydroperoxide lyase activities in tomato juice. J. Food Sci. 2009, 74, C595–C601. [Google Scholar] [CrossRef]
| Volatile Compounds | Classification | Average Concentration(ng L−1) | Odour Threshold(ng L−1) | Log Odour Unit | Odor Descriptor |
|---|---|---|---|---|---|
| Fatty acid derivatives | |||||
| hexanol | Alcohol | 7 | 5000 | −1.9 | Resin, flower, green |
| Z-3-hexenal | Aldehyde | 12 | 0.25 | 3.7 | Tomato, green |
| E-2-heptenal | Aldehyde | 60 | 13 | 0.7 | Green |
| 1-penten-3-ol | Alcohol | 110 | 400 | −0.6 | Sweet, fruity, grassy |
| pentanol | Alcohol | 120 | 4000 | −1.5 | Balsamic |
| E-2-pentenal | Aldehyde | 140 | 1500 | −1 | Strawberry, fruity, tomato |
| Z-3-hexenol | Alcohol | 150 | 70 | 0.3 | Green |
| E-2-hexenal | Aldehyde | 270 | 17 | 1.2 | Green |
| 1-penten-3-one | Ketone | 520 | 1 | 2.7 | Fruity, floral, green |
| hexanal | Aldehyde | 3100 | 4.5 | 2.8 | Green, grassy |
| Carotenoid derivatives | |||||
| epoxy-β-ionone | Ketone | 1 | 100 | −2 | Fruity, sweet, wood |
| pseudoionone | Ketone | 1 | 800 | −1.9 | Balsamic |
| β-damascenone | Ketone | 1 | 0.002 | 2.7 | Fruity |
| neral | Aldehyde | 2 | 30 | −1.2 | Lemon |
| β-cyclocitral | Aldehyde | 3 | 5 | −0.2 | Mint |
| β-ionone | Ketone | 4 | 0.007 | 2.8 | Fruity, floral |
| geranial | Aldehyde | 12 | 32 | −0.4 | Citrus |
| geranylacetone | Ketone | 57 | 60 | −0.02 | Sweet, floral, estery |
| 6-methyl-5-hepten-2-one | Ketone | 130 | 50 | 0.4 | Fruity, floral |
| Amino acid derivatives | |||||
| 3-methylbutanenitrile | N-compound | 13 | 1000 | −1.9 | Pungent |
| 2-phenylacetaldehyde | Aldehyde | 15 | 4 | 0.6 | Floral, alcohol |
| 1-nitro-2-phenylethane | N-compound | 17 | 2 | 0.9 | Musty, earthy |
| 3-methylbutanal | Aldehyde | 27 | 0.2 | 2.1 | Musty |
| 2-isobutylthiazole | S- and N-compound | 36 | 3.5 | 1 | Tomato vine, green |
| methyl salicylate | Ester | 48 | 40 | 0.008 | Wintergreen |
| 3-methylbutanol | Alcohol | 380 | 250 | 0.2 | Earthy, musty |
| 2-phenylethanol | Alcohol | 1900 | 1000 | 0.3 | Nutty, fruity |
| Others | |||||
| linalool | Alcohol | 2 | 6 | −0.5 | Citrus, fruity, sweet |
| 1-nitro-3-methylbutane | N- and O-compound | 59 | 150 | −0.4 | - |
| Locus | Associated Volatile/s | C number | Precursors | Identification | Reference |
|---|---|---|---|---|---|
| ADH | Hexanal:hexanol ratio | C6 | Fatty acids | BP/QTL | Speirs et al., 1998 |
| AADC | Phenylacetaldehyde, 2-phenylethanol,1-nitro-2-phenethane, 2-phenylacetonitrile | C8 | Phenylalanine | BP/QTL | Tieman et al., 2006 |
| PAR | 2-Phenylethanol | C8 | Phenylalanine | BP | Tieman et al., 2007 |
| LoxC | Z-3-Hexenal, Z-3-hexenol, hexanal, hexanol | C6 | Fatty acids | CG | Chen et al., 2004 |
| SAMT | Methylsalicylate | C8 | Esters | BP | Tieman et al., 2010 |
| CTOMT | 2-Methoxyphenol (Guaiacol) | C7 | Phenols | BP | Mageroy et al., 2012 |
| CXE1 | Multiple alcohols | Cn | Fatty acids, amino acids | QTL | Goulet et al., 2012 |
| CCD1 | Multiple apocarotenoids | Cn | Carotenoids | CG | Simkin et al., 2004 |
| GT1 | Multiple phenylpropanoids | Cn | Amino acids | QTL | Tikunov et al., 2013 |
| LIP1, LIP2 | Z-4-Decenal | C10 | Fatty acids | QTL | Garbowicz et al., 2018 |
| Volatile Compounds | Microclimate Storage Conditions | Storage Atmosphere Composition | Postharvest Chemicals Application | Industrial Processing |
|---|---|---|---|---|
| Fatty acid derivates | ||||
| Hexanol | [135] | [136,137] | [138,139] | |
| Z-3-Hexenal | [140] | [64,138,139] | ||
| E-2-Heptenal | [64] | |||
| 1-Penten-3-ol | [141] | [136] | [138] | |
| Pentanol | [137] | [138] | ||
| E-2-Pentenal | [142] | |||
| Z-3-Hexenol | [139] | |||
| E-2-Hexenal | [17,22,135] | [136,137] | [64,139] | |
| 1-Penten-3-one | [141] | [142] | [64] | |
| Hexanal | [17,22,135] | [143] | [144] | [64,139] |
| Caroteoid derivates | ||||
| Epoxy-β-ionone | ||||
| Pseudoionone | [136,137] | |||
| β-Damascenone | [64] | |||
| Neral | [145] | [138] | ||
| β-Cyclocitral | ||||
| β-Ionone | [141] | [145] | [136,137] | [138] |
| Geranial | [145] | [142] | ||
| Geranylacetone | [22] | [136,137,142,144] | ||
| 6-Methyl-5-hepten-2-one | [22] | [145] | [136,142,144] | [64,138,139] |
| Amino acid derivates | ||||
| 3-Methylbutanenitrile | ||||
| 2-Phenylacetaldehyde | [142] | [64] | ||
| 1-Nitro-2-phenylethane | [22] | [144] | ||
| 3-Methylbutanal | [143] | [137,142] | ||
| 2-Isobutylthiazole | [17,22,141] | [143,144] | [64] | |
| Methyl salicylate | [141] | [137,142] | [138] | |
| 3-Methylbutanol | [135,140] | [142] | [64] | |
| 2-Phenylethanol | [141] | [137] | [138] | |
| Others | ||||
| Linalool | [64,138] | |||
| 1-Nitro-3-methylbutane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Distefano, M.; Mauro, R.P.; Page, D.; Giuffrida, F.; Bertin, N.; Leonardi, C. Aroma Volatiles in Tomato Fruits: The Role of Genetic, Preharvest and Postharvest Factors. Agronomy 2022, 12, 376. https://doi.org/10.3390/agronomy12020376
Distefano M, Mauro RP, Page D, Giuffrida F, Bertin N, Leonardi C. Aroma Volatiles in Tomato Fruits: The Role of Genetic, Preharvest and Postharvest Factors. Agronomy. 2022; 12(2):376. https://doi.org/10.3390/agronomy12020376
Chicago/Turabian StyleDistefano, Miriam, Rosario Paolo Mauro, David Page, Francesco Giuffrida, Nadia Bertin, and Cherubino Leonardi. 2022. "Aroma Volatiles in Tomato Fruits: The Role of Genetic, Preharvest and Postharvest Factors" Agronomy 12, no. 2: 376. https://doi.org/10.3390/agronomy12020376
APA StyleDistefano, M., Mauro, R. P., Page, D., Giuffrida, F., Bertin, N., & Leonardi, C. (2022). Aroma Volatiles in Tomato Fruits: The Role of Genetic, Preharvest and Postharvest Factors. Agronomy, 12(2), 376. https://doi.org/10.3390/agronomy12020376









