Morphological and Physiological Characteristics of Rice Cultivars with Higher Yield and Nitrogen Use Efficiency at Various Nitrogen Rates
Abstract
:1. Introduction
2. Materials and Methods
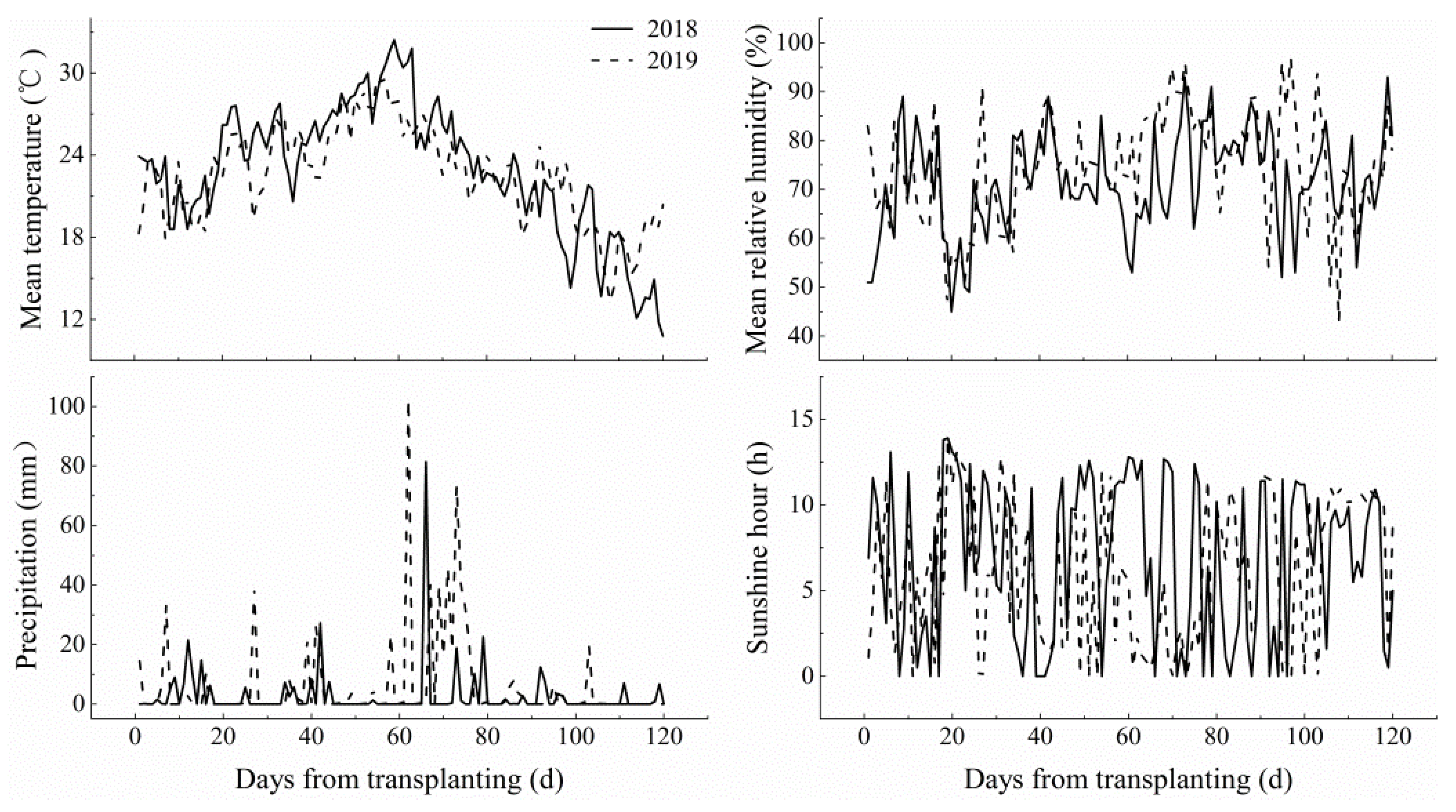
2.1. Plant Materials and Site Description
2.2. Experimental Design
2.3. Sampling and Measurements
2.3.1. Leaf Photosynthesis and Physiological Variables
2.3.2. Root Morphology and Physiological Variables
2.3.3. Grain Yield and Nitrogen Use Efficiency
2.4. Statistical Analysis
3. Results
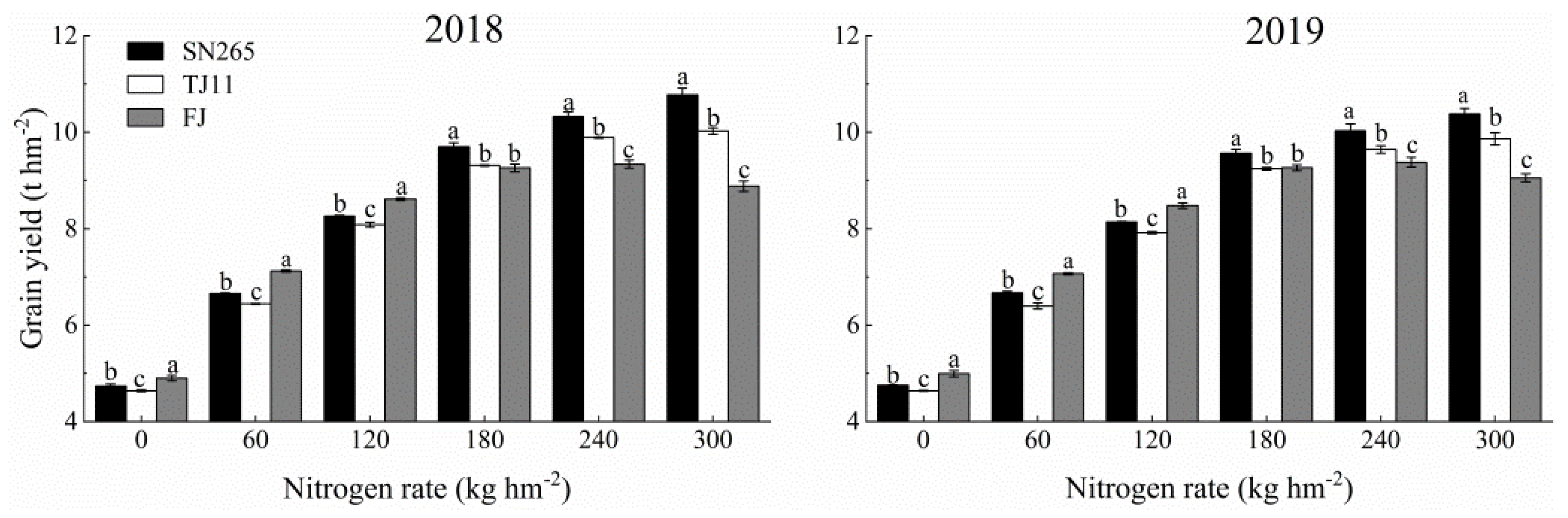
3.1. Grain Yield and Yield Components
3.2. Nitrogen Uptake and Utilization Efficiency
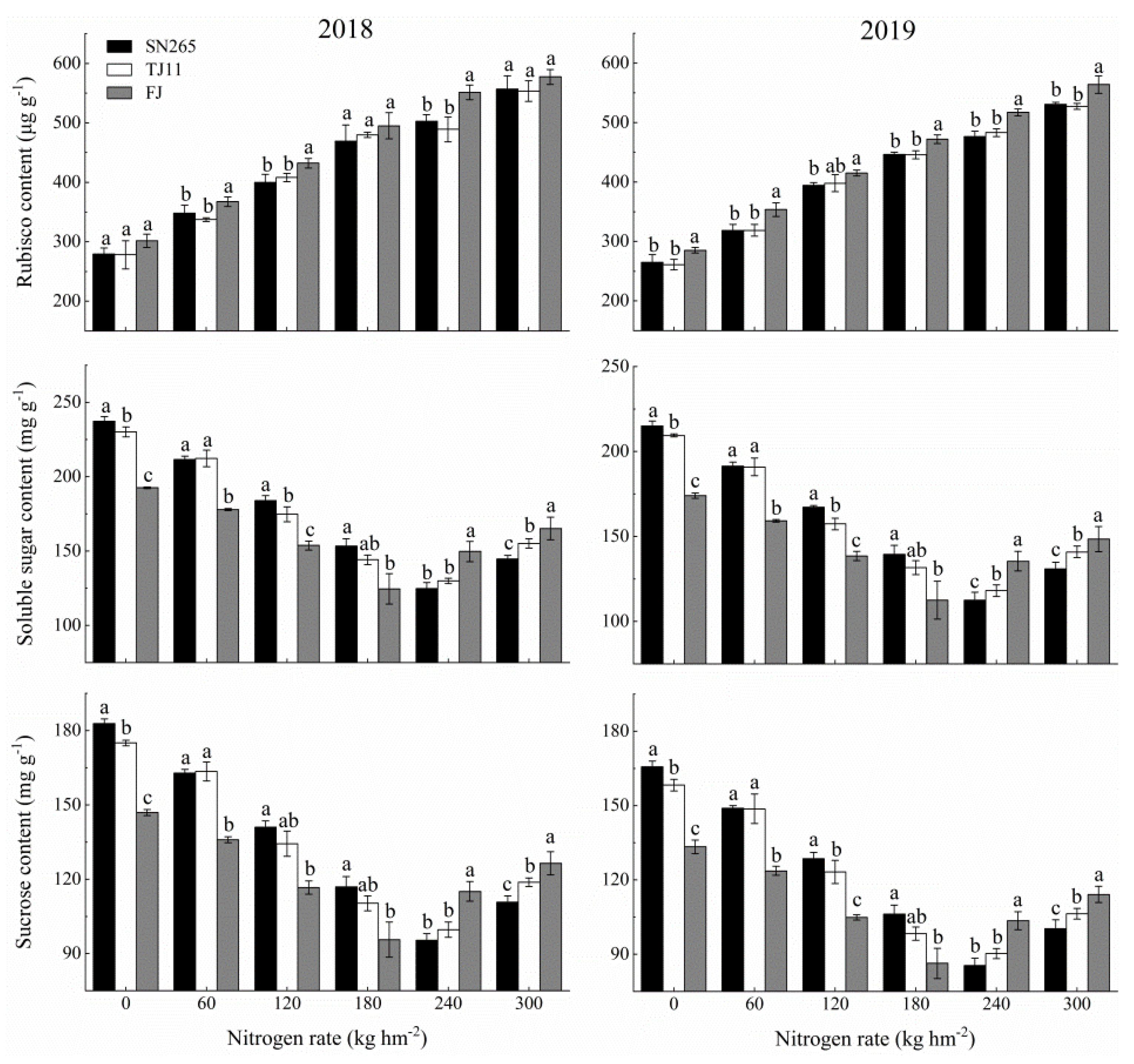
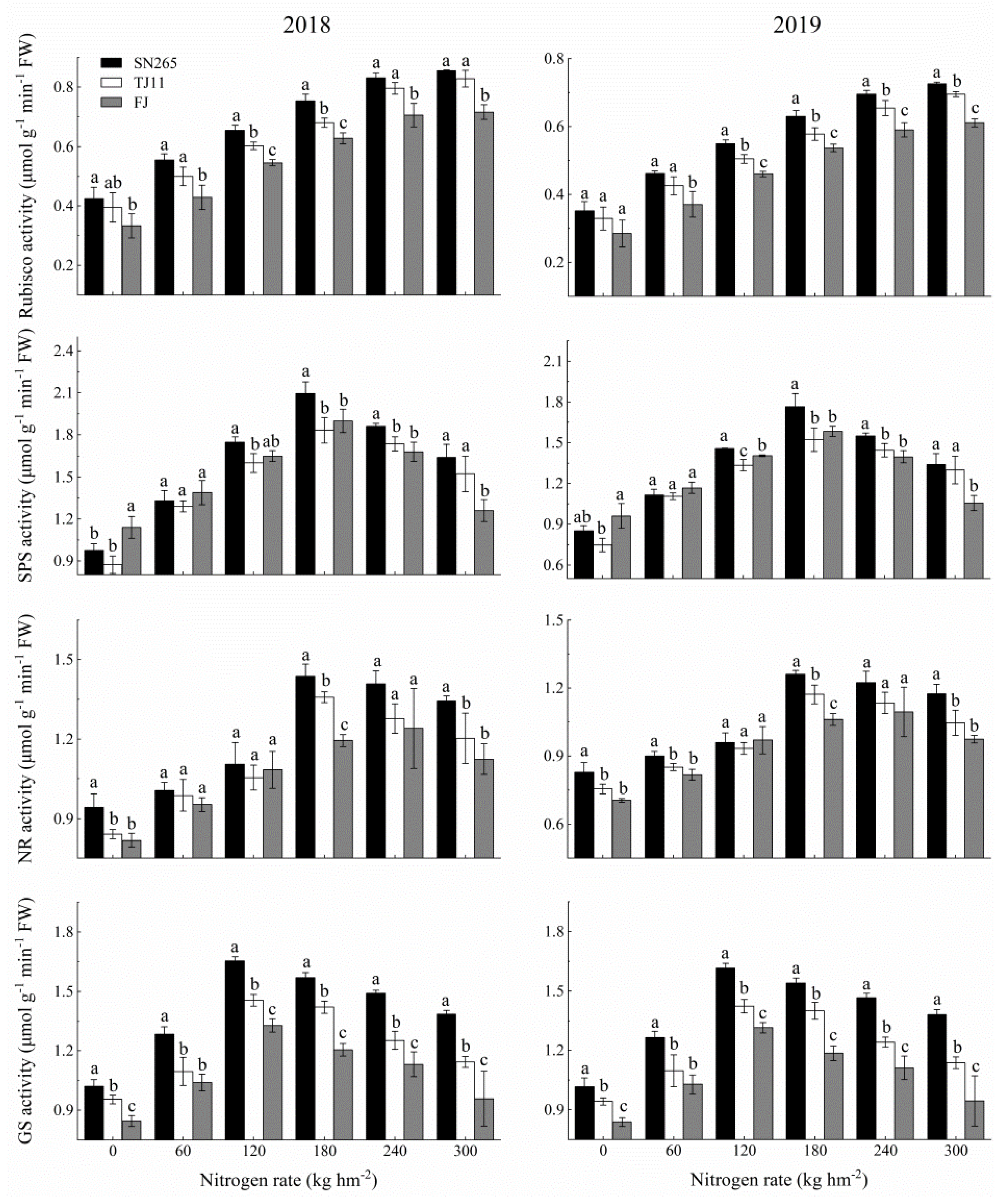
3.3. Leaf Photosynthesis and Physiology
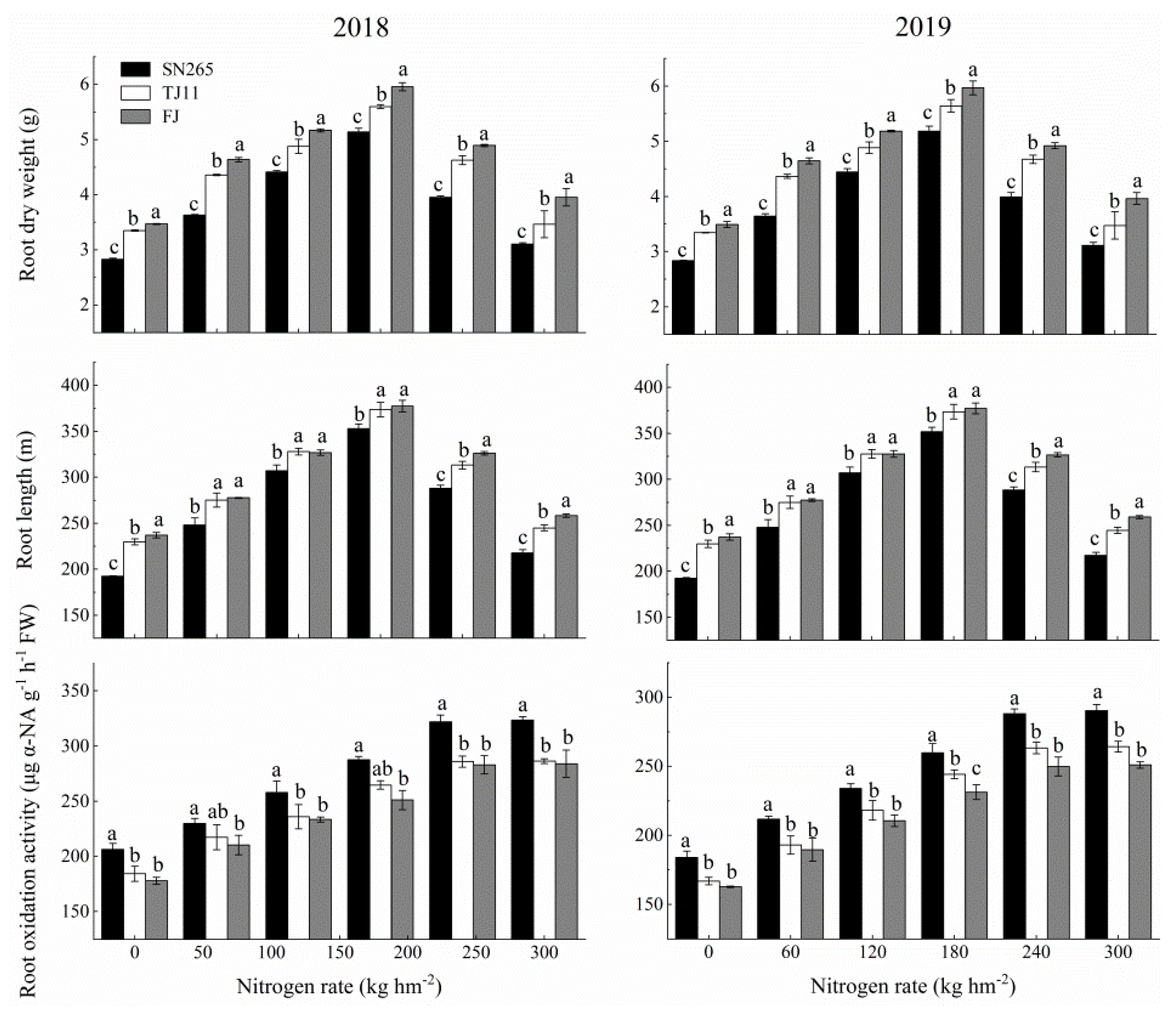
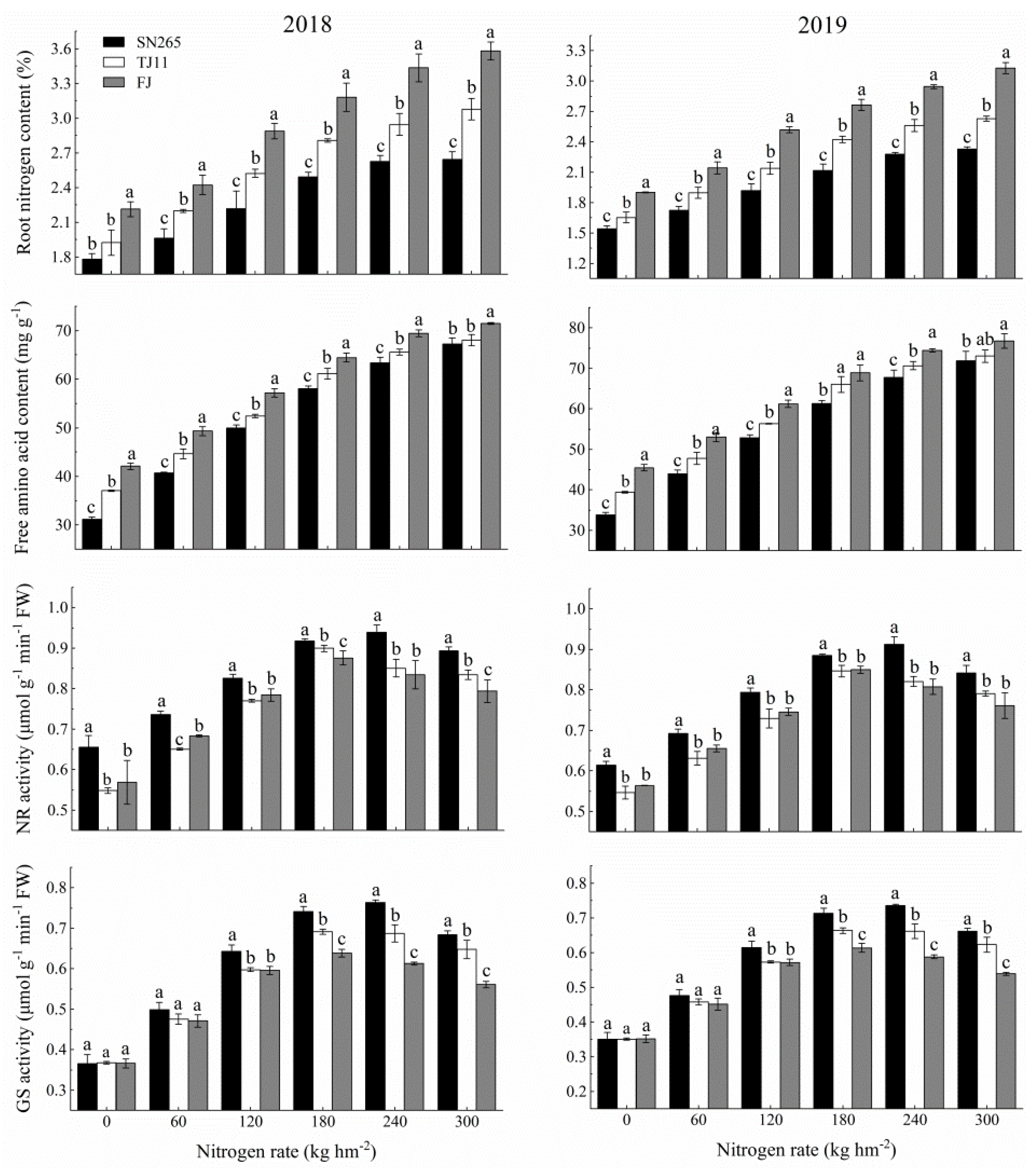
3.4. Root Morphological and Physiological Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sweetlove, L.; Beard, K.; Nunesnesi, A.; Fernie, A.; Ratcliffe, R. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, B.; Chu, C. Nitrogen use efficiency in crops: Lessons from Arabidopsis and rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Coulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Ahmad, A.; Iqbal, M.; Gucel, S.; Ozturk, M. Nitrogen-efficient rice cultivars can reduce nitrate pollution. Environ. Sci. Pollut. Res. 2011, 18, 1184–1193. [Google Scholar] [CrossRef]
- Gutiérrez, R.A. Systems biology for enhanced plant nitrogen nutrition. Science 2012, 336, 1673–1675. [Google Scholar] [CrossRef]
- Garnett, T.; Conn, V.; Kaiser, B.N. Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ. 2009, 32, 1272–1283. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Ju, C.; Buresh, R.J.; Wang, Z.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crops Res. 2015, 175, 47–55. [Google Scholar] [CrossRef]
- Zheng, Z.J. Carbon and nitrogen nutrient balance signaling in plants. Plant Signal. Behav. 2009, 4, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Bao, A.; Zhao, Z.; Ding, G.; Shi, L.; Xu, F.; Cai, H. Accumulated expression level of Cytosolic Glutamine Synthetase 1 Gene (OsGS1;1 or OsGS1;2) alter plant development and the carbon-nitrogen metabolic status in rice. PLoS ONE 2014, 9, e95581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, A.; Zhao, Z.; Ding, G.; Shi, L.; Xu, F.; Cai, H. The stable level of glutamine synthetase 2 plays an important role in rice growth and in carbon-nitrogen metabolic balance. Int. J. Mol. Sci. 2015, 16, 12713–12736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Nian, J.; Xie, Q.; Feng, J.; Zhang, F.; Jing, H.; Zhang, J.; Dong, G.; Liang, Y.; Peng, J. Rice ferredoxin-dependent glutamate synthase regulates nitrogen-carbon metabolomes and is genetically differentiated between japonica and indica subspecies. Mol. Plant 2016, 9, 1520–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, W.; Zhang, L.; Zhang, W.; Gao, J.; Yi, J.; Zhen, X.; Li, Z.; Zhao, Y.; Peng, C.; Zhao, C. An integrated analysis of the rice transcriptome and metabolome reveals differential regulation of carbon and nitrogen metabolism in response to nitrogen availability. Int. J. Mol. Sci. 2019, 20, 2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Xue, Y.; Wang, Z.; Yang, J.; Zhang, J. An alternate wetting and moderate soil drying regime improves root and shoot growth in rice. Crop Sci. 2009, 49, 2246–2260. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, W.; Zhang, L.; Zhang, W.; Gao, J.; Yi, J.; Zhen, X.; Du, M.; Zhao, Y.; Chen, L. An integrated analysis of the rice transcriptome and metabolome reveals root growth regulation mechanisms in response to nitrogen availability. Int. J. Mol. Sci. 2019, 20, 5893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, H.; Chen, J.; Wu, P.; Lai, W.; Yuan, X.; Zhang, S. Ideotype root architecture for efficient nitrogen acquisition by maize in intensive cropping systems. Sci. China Life Sci. 2010, 53, 1369–1373. [Google Scholar] [CrossRef]
- Sun, H.; Qian, Q.; Wu, K.; Luo, J.; Wang, S.; Zhang, C.; Ma, Y.; Liu, Q.; Huang, X.; Yuan, Q.; et al. Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat. Genet. 2014, 46, 652–656. [Google Scholar] [CrossRef]
- Li, Y.; Ren, B.; Yang, X.; Xu, G.; Shen, Q.; Guo, S. Chloroplast downsizing under nitrate nutrition restrained mesophyll conductance and photosynthesis in rice (Oryza sativa L.) under drought conditions. Plant Cell Physiol. 2012, 53, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Maness, N. Extraction and analysis of soluble carbohydrates. Methods Mol. Biol. 2010, 639, 341–370. [Google Scholar] [PubMed]
- Zhang, Z.L.; Qu, W.J. Experimental Guidance in Plant Physiology, 3rd ed.; Higher Education Press: Beijing, China, 2003; pp. 128–129. (In Chinese) [Google Scholar]
- Gibon, Y.; Blaesing, O.E.; Hannemann, J.; Carillo, P.; Höhne, M.; Hendriks, J.H.M.; Palacios, N.; Cross, J.; Selbig, J.; Stitt, M. A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: Comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 2004, 16, 3304–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Lee, P.; Chen, W.; Huang, D.; Su, J. Osmotic stress induced changes of sucrose metabolism in cultured sweet potato cells. J. Exp. Bot. 2000, 51, 1991–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Yuki, K.; Park, S.Y.; Toshihide, O. Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol. 1989, 30, 833–839. [Google Scholar] [CrossRef]
- Cao, H.H.; Zhang, M.; Zhao, H.; Zhang, Y.; Wang, X.X.; Guo, S.S.; Zhang, Z.F.; Liu, T.X. Deciphering the mechanism of β-aminobutyric acid-induced resistance in wheat to the grain aphid, Sitobion avenae. PLoS ONE 2014, 9, e91768. [Google Scholar] [CrossRef]
- Ramasamy, S.; Berge, H.; Purushothaman, S. Yield formation in rice in response to drainage and nitrogen application. Field Crops Res. 1997, 51, 65–82. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Yang, X.; Ge, M.; Ma, Q.; Wei, H.; Dai, Q.; Huo, Z.; Xu, K.; Luo, D. Accumulation and utilization of nitrogen, phosphorus and potassium of irrigated rice cultivars with high productivities and high N use efficiencies. Field Crops Res. 2014, 161, 55–63. [Google Scholar] [CrossRef]
- Peng, S.B.; Buresh, R.J.; Huang, J.L.; Yang, J.C.; Zou, Y.B.; Zhong, X.H.; Wang, G.H.; Zhang, F.S. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crops Res. 2006, 96, 37–47. [Google Scholar] [CrossRef]
- Fan, M.; Shen, J.; Yuan, L.; Jiang, R.; Chen, X.; Davies, W.J.; Zhang, F. Improving crop productivity and resource use efficiency to ensure food security and environmental quality in China. J. Exp. Bot. 2012, 63, 13. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.; Zhang, W.; Sui, Y.; Jin, D.; Xin, W.; Yi, J.; He, D. Biochar prepared at different pyrolysis temperatures affects urea-nitrogen immobilization and N2O emissions in paddy fields. PeerJ 2019, 7, e7027. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Gao, J.; Zhang, W.; Zhao, Y.; Zhao, C.; Zhao, Y.; Ziang Li, Z.; Xin, W. Delayed timing of tillering fertilizer improved grain yield and nitrogen use efficiency in japonica rice. Crop Sci. 2020, 60, 1021–1033. [Google Scholar] [CrossRef]
- Wang, X.B.; Cai, D.X.; Grant, C.; Hoogmoed, W.B.; Oenema, O. Changes in regional grain yield responses to chemical fertilizer use in China over the last 20 years. J. Soil Sci. Plant Nutr. 2018, 18, 312–328. [Google Scholar] [CrossRef] [Green Version]
- Raun, W.R.; Dhillon, J.; Aula, L.; Eickhoff, E.; Weymeyer, G.; Figueirdeo, B.; Lynch, T.; Omara, P.; Nambi, E.; Oyebiyi, F.; et al. Unpredictable nature of the environment on nitrogen supply and demand. Agron. J. 2019, 111, 2786–2791. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, C.; Lange, K.; Parajulee, M.N.; Segarra, E. Dynamic optimization of nitrogen in plateau cotton yield functions with nitrogen carryover considerations. J. Agric. Appl. Econ. 2019, 51, 385–401. [Google Scholar] [CrossRef] [Green Version]
- Coruzzi, G.M.; Zhou, L. Carbon and nitrogen sensing and signaling in plants: Emerging ‘matrix effects’. Curr. Opin. Plant Biol. 2001, 4, 247–253. [Google Scholar] [CrossRef]
- Martin, T.; Oswald, O.; Graham, I.A. Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon: Nitrogen availability. Plant Physiol. 2002, 128, 472–481. [Google Scholar] [CrossRef]
- Krapp, A.; Traong, H.N. Regulation of C/N interaction in model plant species. J. Crop Improv. 2005, 15, 127–173. [Google Scholar] [CrossRef]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Ren, B.; Shen, Q.; Guo, S. Why nitrogen use efficiency decreases under high nitrogen supply in rice (Oryza sativa L.) seedlings. J. Plant Growth Regul. 2012, 31, 47–52. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, X.R.; Sun, S.B.; Xu, G.H.; Hu, J.; Shen, Q.R. Effect of nitrate on activities and transcript levels of nitrate reductase and glutamine synthetase in rice. Pedosphere 2008, 18, 664–673. [Google Scholar] [CrossRef]
- Wang, J.L.; Fu, Z.S.; Chen, G.F.; Zou, G.Y.; Song, X.F.; Liu, F.X. Runoff nitrogen (N) losses and related metabolism enzyme activities in paddy field under different nitrogen fertilizer levels. Environ. Sci. Pollut. Res. 2018, 25, 27583–27593. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.M.; Cheng, S.H. Root genetic research, an opportunity and challenge to rice improvement. Field Crops Res. 2014, 165, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Xiang, D.; Zhu, J.; Li, Y.; Mao, C. Molecular mechanisms of root development in rice. Rice 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Ann. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walch-Liu, P. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.H.; Shane, M.W.; López-Bucio, J. Nutritional regulation of root development. Wiley interdisciplinary reviews. Dev. Biol. 2015, 4, 431–443. [Google Scholar]
- Passioura, J.B. Roots and drought resistance. Agric. Water Manag. 1983, 7, 265–280. [Google Scholar] [CrossRef]

| Year | Treatment | Effective Panicles (Per m−2) | Grain Per Pancile | Seed Setting Rate (%) | 1000-Grain Weight (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SN265 | TJ11 | FJ | SN265 | TJ11 | FJ | SN265 | TJ11 | FJ | SN265 | TJ11 | FJ | ||
| 2018 | 0 | 188.6 fC | 210.2 fB | 305.4 fA | 120.80 eA | 113.99 dB | 83.27 fC | 93.6 aA | 90.8 aB | 92.8 aA | 24.50 aA | 23.58 aB | 23.90 aB |
| 60 | 253.0 eC | 289.2 eB | 405.0 eA | 127.25 dA | 118.55 cB | 90.53 dC | 89.6 bA | 87.6 bA | 89.4 bA | 23.96 bA | 22.12 bC | 22.64 bB | |
| 120 | 321.0 dC | 369.2 dB | 487.0 dA | 133.17 cA | 125.95 bB | 102.34 bC | 88.4 bA | 84.0 cB | 84.6 cA | 23.32 cA | 22.10 bB | 21.84 cB | |
| 180 | 375.0 cC | 425.6 cB | 533.6 cA | 142.13 aA | 129.55 aB | 106.35 aC | 84.6 cA | 84.8 bcA | 82.0 dA | 22.88 dA | 21.16 cB | 21.22 dB | |
| 240 | 428.0 bC | 500.0 bB | 617.0 bA | 144.49 aA | 124.91 bB | 98.17 cC | 80.8 dA | 81.0 dA | 79.4 eA | 22.32 eA | 21.08 cB | 21.32 dB | |
| 300 | 470.8 aC | 549.6 aB | 692.4 aA | 138.23 bA | 120.24 cB | 87.38 eC | 79.4 dA | 76.8 eA | 71.4 fB | 22.10 eA | 21.30 cB | 20.78 eC | |
| 2019 | 0 | 185.0 fC | 208.6 fB | 281.8 fA | 125.00 cA | 116.27 dB | 87.68 dC | 94.8 aA | 92.8 aB | 94.8 aA | 24.36 aA | 23.66 aB | 24.08 aAB |
| 60 | 244.2 eC | 277.2 eB | 375.0 eA | 131.05 bA | 121.87 cB | 92.62 cC | 91.6 bAB | 90.6 bB | 92.2 bA | 23.52 bA | 22.18 bC | 22.96 bB | |
| 120 | 313.2 dC | 344.4 dB | 448.0 dA | 130.36 bA | 126.58 bB | 103.36 aC | 89.4 cA | 89.4 bA | 89.2 cA | 23.30 bA | 21.88 bB | 21.76 cB | |
| 180 | 356.6 cC | 404.4 cB | 512.4 cA | 139.77 aA | 130.20 aB | 103.02 aC | 88.2 cA | 87.4 cA | 85.4 dB | 22.66 cA | 21.08 cB | 21.34 dB | |
| 240 | 420.4 bC | 474.0 bB | 591.0 bA | 138.49 aA | 125.52 bB | 100.01 dC | 83.8 dA | 83.4 dA | 80.8 eB | 22.22 dA | 20.88 cB | 20.84 eB | |
| 300 | 478.6 aC | 530.8 aB | 677.0 aA | 130.51 bA | 114.98 dB | 92.38 cC | 80.8 eA | 79.4 eA | 74.8 fB | 21.60 eA | 21.02 cB | 20.84 eB | |
| F-value | N | 7857.13 ** | 427.83 ** | 426.93 ** | 450.75 ** | ||||||||
| C | 6640.93 ** | 7851.97 ** | 34.8 ** | 378.66 ** | |||||||||
| Y | 207.66 ** | 0.27 ns | 131.46 ** | 7.25 ** | |||||||||
| N × C | 52.88 ** | 32.28 ** | 10.225 ** | 10.36 ** | |||||||||
| N × Y | 4.72 ** | 17.6 ** | 1.47 ns | 1.51 ns | |||||||||
| C × Y | 24.06 ** | 17.5 ** | 1.81 ns | 3.43 * | |||||||||
| □ | N × C × Y | 2.32 * | 2.89 ** | 1.00 ns | 1.49 ns | ||||||||
| Year | Treatment | NRE (%) | NPE (kg kg−1) | NAE (kg kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SN265 | TJ11 | FJ | SN265 | TJ11 | FJ | SN265 | TJ11 | FJ | ||
| 2018 | 0 | |||||||||
| 60 | 62.15 aB | 62.52 aB | 68.83 aA | 51.53 aA | 49.48 aB | 48.98 aC | 32.02 aB | 30.94 aC | 33.71 aA | |
| 120 | 59.15 bB | 59.09 bB | 63.62 bA | 49.68 bA | 48.62 aA | 47.37 bB | 29.39 bAB | 28.73 bB | 30.13 bA | |
| 180 | 58.97 bB | 59.23 bB | 60.74 cA | 46.81 cA | 43.83 bB | 39.87 cC | 27.60 cA | 25.96 cB | 24.22 cC | |
| 240 | 54.98 cB | 55.27 cB | 56.58 dA | 42.38 dA | 39.59 cB | 32.68 dC | 23.30 dA | 21.88 dB | 18.49 dC | |
| 300 | 50.21 dC | 51.20 dB | 54.35 eA | 40.10 eA | 35.07 dB | 24.40 eC | 20.13 eA | 17.96 eB | 13.26 eC | |
| 2019 | 0 | |||||||||
| 60 | 64.73 aB | 64.68 aB | 70.74 aA | 49.47 aA | 47.70 aB | 45.42 aC | 32.02 aA | 30.85 aB | 32.13 aA | |
| 120 | 59.18 bB | 58.85 bB | 64.65 bA | 47.74 bA | 46.47 aB | 43.27 bC | 28.25 bA | 27.34 bB | 27.96 bAB | |
| 180 | 59.61 bB | 59.25 bB | 60.63 cA | 44.87 cA | 43.22 bB | 39.14 cC | 26.75 cA | 25.60 cB | 23.73 cC | |
| 240 | 54.91 cB | 54.86 cB | 57.23 dA | 40.07 dA | 38.03 cA | 31.94 dB | 22.00 dA | 20.86 dA | 18.28 dB | |
| 300 | 50.44 dC | 52.27 dB | 56.27 eA | 37.22 eA | 33.35 dB | 24.07 eC | 18.77 eA | 17.43 eB | 13.55 eC | |
| F-value | N | 2566.32 ** | 1021.73 ** | 2235.77 ** | ||||||
| C | 811.3 ** | 499.53 ** | 156.94 ** | |||||||
| Y | 73.46 ** | 98.25 ** | 50.67 ** | |||||||
| N × C | 51.9 ** | 42.57 ** | 54.72 ** | |||||||
| N × Y | 20.86 ** | 2.55 * | 2.92 * | |||||||
| C × Y | 3.53 * | 1.00 ns | 0.42 ns | |||||||
| □ | N × C × Y | 2.78 * | 2.08 ns | 2.63 * | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, W.; Zhang, L.; Zhang, W.; Gao, J.; Yi, J.; Zhen, X.; Du, M.; Zhao, Y.; Chen, L. Morphological and Physiological Characteristics of Rice Cultivars with Higher Yield and Nitrogen Use Efficiency at Various Nitrogen Rates. Agronomy 2022, 12, 358. https://doi.org/10.3390/agronomy12020358
Xin W, Zhang L, Zhang W, Gao J, Yi J, Zhen X, Du M, Zhao Y, Chen L. Morphological and Physiological Characteristics of Rice Cultivars with Higher Yield and Nitrogen Use Efficiency at Various Nitrogen Rates. Agronomy. 2022; 12(2):358. https://doi.org/10.3390/agronomy12020358
Chicago/Turabian StyleXin, Wei, Lina Zhang, Wenzhong Zhang, Jiping Gao, Jun Yi, Xiaoxi Zhen, Ming Du, Yanze Zhao, and Liqiang Chen. 2022. "Morphological and Physiological Characteristics of Rice Cultivars with Higher Yield and Nitrogen Use Efficiency at Various Nitrogen Rates" Agronomy 12, no. 2: 358. https://doi.org/10.3390/agronomy12020358
APA StyleXin, W., Zhang, L., Zhang, W., Gao, J., Yi, J., Zhen, X., Du, M., Zhao, Y., & Chen, L. (2022). Morphological and Physiological Characteristics of Rice Cultivars with Higher Yield and Nitrogen Use Efficiency at Various Nitrogen Rates. Agronomy, 12(2), 358. https://doi.org/10.3390/agronomy12020358






