Long-Term Decline in Harvester Termites in Madagascar following Multiple Barrier Treatments with Fipronil against Migratory Locust
Abstract
1. Introduction
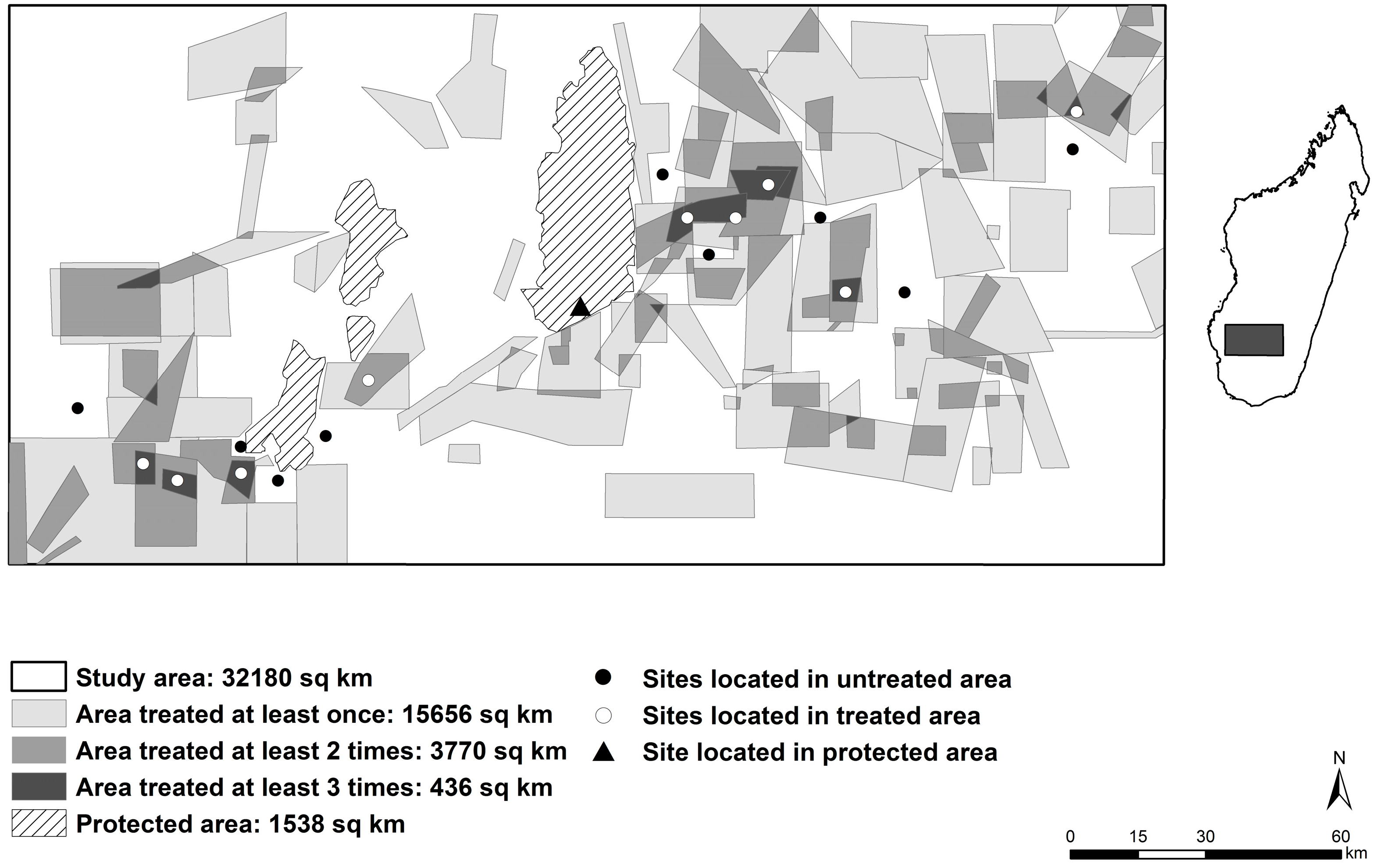
2. Materials and Methods
3. Results
3.1. Density and Occupancy of Termitaria
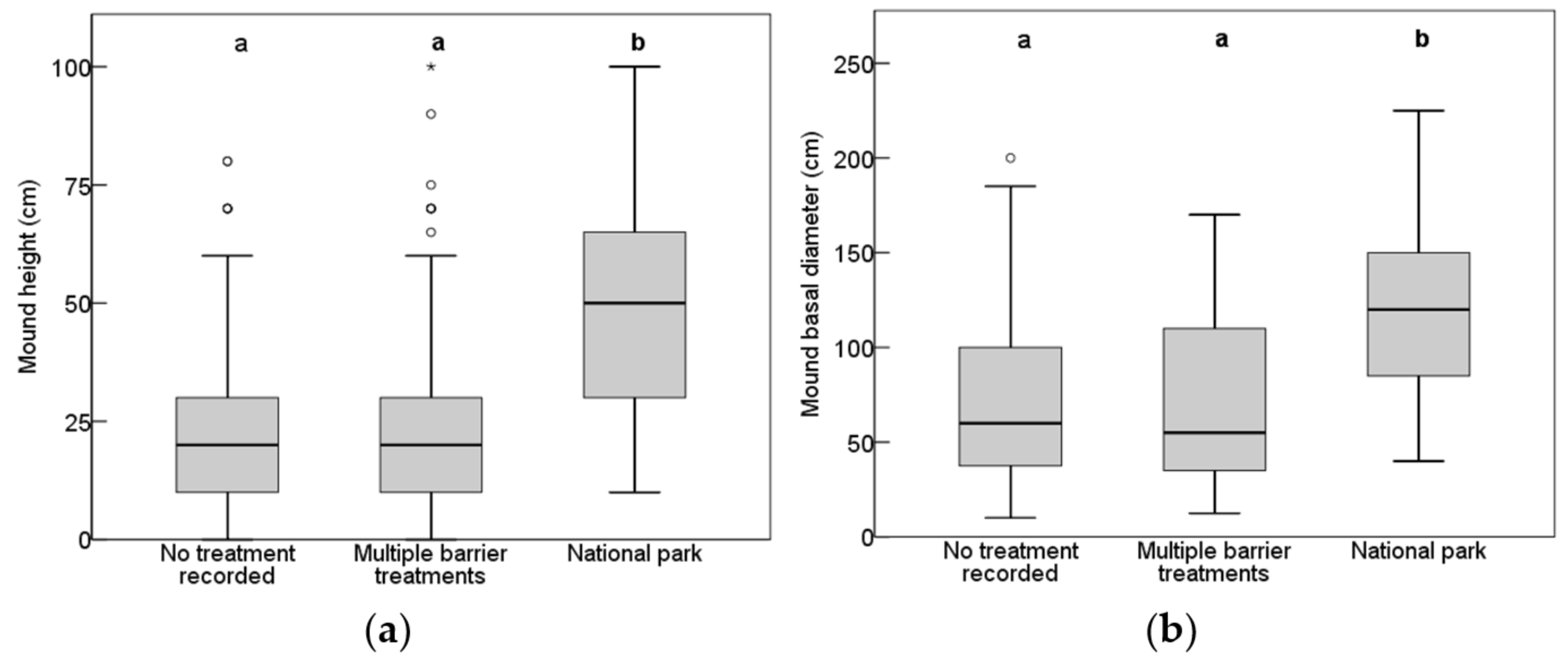
3.2. Mound Morphology
3.3. Human Use of Harvester Termites
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paulian, R. The termites of Madagascar. In Biology of Termites; Krishna, K., Weesner, F.M., Eds.; Academic Press: New York, NY, USA, 1970; Volume 2, pp. 281–294. [Google Scholar]
- Eggleton, P.; Davies, R. Isoptera, termites. In The Natural History of Madagascar; Goodman, S.M., Benstead, J.P., Eds.; The University of Chicago Press: Chicago, IL, USA, 2003; pp. 654–660. [Google Scholar]
- Peveling, R.; McWilliam, A.N.; Nagel, P.; Rasolomanana, H.; Rakotomianina, L.; Ravoninjatovo, A.; Dewhurst, C.F.; Gibson, G.; Rafanomezana, S.; Tingle, C.C.D. Impact of locust control on harvester termites and endemic vertebrate predators in Madagascar. J. Appl. Ecol. 2003, 40, 729–741. [Google Scholar] [CrossRef]
- Jones, D.T.; Eggleton, P. Global biogeography of termites: A compilation of sources. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 477–498. [Google Scholar]
- Traniello, J.F.A.; Leuthold, R.H. Behavior and ecology of foraging termites. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer: Dordrecht, The Netherlands, 2000; pp. 141–168. [Google Scholar]
- Kok, O.B.; Hewitt, P.H. Bird and mammal predators of the harvester termite Hodotermes mossambicus (Hagen) in semi-arid regions of South Africa. S. Afr. J. Sci. 1990, 86, 34–37. [Google Scholar]
- Zehrer, W. Lutte Antiacridienne à Madagascar—Tome III—Ecotoxicologie; DPV/GTZ, Ministère de l’Agriculture: Antananarivo, Madagascar, 2001.
- Van Huis, A. Cultural significance of termites in sub-Saharan Africa. J. Ethnobiol. Ethnomed. 2017, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Magor, J.I.; Lecoq, M.; Hunter, D.M. Preventive control and desert locust plagues. Crop Prot. 2008, 27, 1527–1533. [Google Scholar] [CrossRef]
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and grasshopper management. Annu. Rev. Entomol. 2018, 64, 15–34. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Evaluation of Field Trials Data on the Efficacy and Selectivity of Insecticides on Locusts and Grasshoppers; Report to FAO by the Locust Pesticide Referee Group, 11th Meeting; FAO: Rome, Italy, 2021. [Google Scholar]
- Van der Valk, H. Environmental Impact of Barrier Treatments against Locusts—A Review of Field Studies; Plant Production and Protection Division, Desert Locust Technical Series No. AGP/DL/TS/33; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Mao, L.; Henderson, G.; Scherer, C.W. Toxicity of seven termiticides on the Formosan and Eastern subterranean termites. J. Econ. Entomol. 2011, 104, 1002–1008. [Google Scholar] [CrossRef]
- Rouland-Lefèvre, C. Termites as pests of agriculture. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 499–517. [Google Scholar]
- Lecoq, M. Recent progress in desert and migratory locust management in Africa. Are preventive actions possible? J. Orthoptera Res. 2001, 10, 277–291. [Google Scholar] [CrossRef]
- Tingle, C.C.D.; Rother, J.A.; Dewhurst, C.F.; Lauer, S.; King, W.J. Fipronil: Environmental fate, ecotoxicology, and human health concerns. Rev. Environ. Contam. Toxicol. 2003, 176, 1–66. [Google Scholar] [CrossRef]
- Danfa, A.; Ba, A.L.; Van der Valk, H.; Rouland-Lefèvre, C.; Mullié, W.C.; Everts, J.W. Long-term effects of chlorpyrifos and fipronil on epigeal beetles and soil arthropods in the semi-arid savanna of northern Senegal. In Environmental Side-effects of Locust and Grasshopper Control; Everts, J.W., Mbaye, D., Barry, O., Mullié, W., Eds.; Food and Agriculture Organization of the United Nations, CERES-Locustox Foundation, Ministry of Agriculture–Senegal: Dakar, Senegal, 2002; pp. 184–213. [Google Scholar]
- Mullié, W.C.; Rouland-Lefèvre, C.; Sarr, M.; Danfa, A.; Ba, A.L.; Everts, J.W. Impact of Adonis® 2 UL and 5 UL (fipronil) on Ant and Termite Communities in a Tropical Semi-Arid Savannah—A Two-Year Field Study in Northern SENEGAL; Centre for Ecotoxicological Research in the Sahel, CERES-Locustox Foundation: Dakar, Senegal, 2003. [Google Scholar]
- Steinbauer, M.J.; Peveling, R. The impact of the locust control insecticide fipronil on termites and ants in two contrasting habitats in northern Australia. Crop Prot. 2011, 30, 814–825. [Google Scholar] [CrossRef]
- Maute, K.; French, K.; Story, P.; Bull, C.M.; Hose, G.C. Effects of two locust control methods on wood-eating termites in arid Australia. J. Insect Conserv. 2016, 20, 107–118. [Google Scholar] [CrossRef]
- Rakotoarimanana, V.; Grouzis, M.; Le Floc’h, E. Influence du feu et du pâturage sur l’évolution de la phytomasse d’une savane à Heteropogon contortus de la région de Sakaraha (sud-ouest de Madagascar). Tropicultura 2008, 26, 56–60. [Google Scholar]
- Schneider-Orelli, O. Entomologisches Praktikum: Einführung in Die Land- und Forstwirtschaftliche Insektenkunde; H.R. Sauerländer & Company: Aarau, Switzerland, 1947. [Google Scholar]
- Bordereau, C.; Pasteels, J.M. Pheromones and chemical ecology of dispersal and foraging in termites. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 279–320. [Google Scholar]
- Eggleton, P. An introduction to termites: Biology, taxonomy and functional morphology. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 1–26. [Google Scholar]
- Lavelle, P.; Bignell, D.; Lepage, M.; Wolters, V.; Roger, P.A.; Ineson, P.; Heal, O.W.; Dhillion, S.P. Soil function in a changing world: The role of invertebrate ecosystem engineers. Eur. J. Soil. Biol. 1997, 33, 159–193. [Google Scholar]
- Lobry de Bruyn, L.A.; Conacher, A.J. The role of termites and ants in soil modification: A Review. Aust. J. Soil Res. 1990, 28, 55–93. [Google Scholar]
- Gabet, E.J.; Reichman, O.J.; Seabloom, E.W. The effects of bioturbation on soil processes and sediment transport. Annu. Rev. Earth Planet. Sci. 2003, 31, 249–273. [Google Scholar] [CrossRef]
- Freymann, B.P.; De Visser, S.N.; Olff, H. Spatial and temporal hotspots of termite-driven decomposition in the Serengeti. Ecography 2010, 33, 443–450. [Google Scholar] [CrossRef]
- Moe, S.R.; Mobæk, R.; Narmo, A.K. Mound building termites contribute to savanna vegetation heterogeneity. Plant Ecol. 2009, 202, 31–40. [Google Scholar] [CrossRef]
- Okullo, P.; Moe, S.R. Termite activity, not grazing, is the main determinant of spatial variation in savanna herbaceous vegetation. J. Ecol. 2012, 100, 232–241. [Google Scholar] [CrossRef]
- Palmer, T.M. Spatial habitat heterogeneity influences competition and coexistence in an African acacia ant guild. Ecology 2003, 84, 2843–2855. [Google Scholar] [CrossRef]
- De Visser, S.N.; Freymann, B.P.; Schnyder, H. Trophic interactions among invertebrates in termitaria in the African savanna: A stable isotope approach. Ecol. Entomol. 2008, 33, 758–764. [Google Scholar] [CrossRef]
- Gibbons, D.; Morrissey, C.; Mineau, P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Sileshi, G.W.; Nyeko, P.; Nkunika, P.O.Y.; Sekematte, B.M.; Akinnifesi, F.K.; Ajayi, O.C. Integrating ethno-ecological and scientific knowledge of termites for sustainable termite management and human welfare in Africa. Ecol. Soc. 2009, 14, 48. Available online: http://www.ecologyandsociety.org/vol14/iss1/art48 (accessed on 30 September 2021). [CrossRef]
- Banjo, A.D.; Lawal, O.A.; Songonuga, E.A. The nutritional value of fourteen species of edible insects in southwestern Nigeria. Afr. J. Biotechnol. 2006, 5, 298–301. [Google Scholar]
- Miyagawa, S.; Koyama, Y.; Kokubo, M.; Matsushita, Y.; Adachi, Y.; Sivilay, S.; Kawakubo, N.; Oba, S. Indigenous utilization of termite mounds and their sustainability in a rice growing village of the central plain of Laos. J. Ethnobiol. Ethnomed. 2011, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, C. Interactions between termite mounds, trees, and the Zemba people in the Mopane savanna in Northwestern Namibia. Afr. Stud. Monogr. 2010, 40, 115–128. [Google Scholar]
- Watson, J.A.L.; Barret, R.A.; Green, J.P. Growth of mounds of Australian harvester termite, Drepanotermes perniger (Froggatt) (Termitinae). Sociobiology 1988, 14, 217–244. [Google Scholar]
- Park, H.C.; Majer, J.D.; Hobbs, R.J. Contribution of the Western Australian wheatbelt termite, Drepanotermes tamminensis (Hill), to the soil nutrient budget. Ecol. Res. 1994, 9, 351–356. [Google Scholar] [CrossRef][Green Version]
- Adam, R.A.; Mitchell, J.D. Energetics and development of incipient colonies of the harvester termite, Trinervitermes trinervoides (Sjöstedt) (Termitidae, Nasutitermitinae). Insectes Soc. 2009, 56, 21–27. [Google Scholar] [CrossRef]
- Rasambainarivo, J.H.; Ranaivoarivelo, N. Madagascar Country Pasture/Forage Resource Profiles; Food and Agricultural Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Davies, A.B.; Eggleton, P.; van Rensburg, B.J.; Parr, C.L. The pyrodiversity–biodiversity hypothesis: A test with savanna termite assemblages. J. Appl. Ecol. 2012, 49, 422–430. [Google Scholar] [CrossRef]
- DeSouza, O.; Albuquerque, L.B.; Tonello, V.M.; Pinto, L.P.; Reis Júnior, R. Effects of fire on termite generic richness in a savanna-like ecosystem (‘Cerrado’) of Central Brazil. Sociobiology 2003, 42, 639–649. [Google Scholar]
- Abensperg-Traun, M.; Milewski, A.V. Abundance and diversity of termites (Isoptera) in unburnt versus burnt vegetation at the Barrens in Mediterranean Western Australia. Austr. J. Ecol. 1995, 20, 413–417. [Google Scholar] [CrossRef]
- Pisa, L.; Goulson, D.; Yang, E.; Gibbons, D.; Sánchez-Bayo, F.; Mitchell, E.; Aebi, A.; van der Sluijs, J.; MacQuarrie, C.J.K.; Giorio, C.; et al. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: Impacts on organisms and ecosystems. Environ. Sci. Pollut. Res. 2021, 28, 11749–11797. [Google Scholar] [CrossRef] [PubMed]
- European and Mediterranean Plant Protection Organization. Environmental risk assessment scheme for plant protection products. Chapter 9: Non-target terrestrial arthropods. EPPO Bull. 2003, 33, 131–139. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peveling, R. Long-Term Decline in Harvester Termites in Madagascar following Multiple Barrier Treatments with Fipronil against Migratory Locust. Agronomy 2022, 12, 310. https://doi.org/10.3390/agronomy12020310
Peveling R. Long-Term Decline in Harvester Termites in Madagascar following Multiple Barrier Treatments with Fipronil against Migratory Locust. Agronomy. 2022; 12(2):310. https://doi.org/10.3390/agronomy12020310
Chicago/Turabian StylePeveling, Ralf. 2022. "Long-Term Decline in Harvester Termites in Madagascar following Multiple Barrier Treatments with Fipronil against Migratory Locust" Agronomy 12, no. 2: 310. https://doi.org/10.3390/agronomy12020310
APA StylePeveling, R. (2022). Long-Term Decline in Harvester Termites in Madagascar following Multiple Barrier Treatments with Fipronil against Migratory Locust. Agronomy, 12(2), 310. https://doi.org/10.3390/agronomy12020310




