Microbacterium oxydans Regulates Physio-Hormonal and Molecular Attributes of Solanum lycopersicum under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and PGPR Isolation
2.2. Growth Promotion of Waito-C Rice under Drought Stress
2.3. Effect of Selected Isolate on Tomato Growth under PEG Stress
2.4. Levels of Endogenous Phytohormones in Tomato under PEG Stress
2.5. Molecular Identification of the Bacterial Isolate AGH3
2.6. RNA Extraction, cDNA Synthesis, and qRT-PCR Analysis for Gene Expression
2.7. Statistical Analysis
3. Results
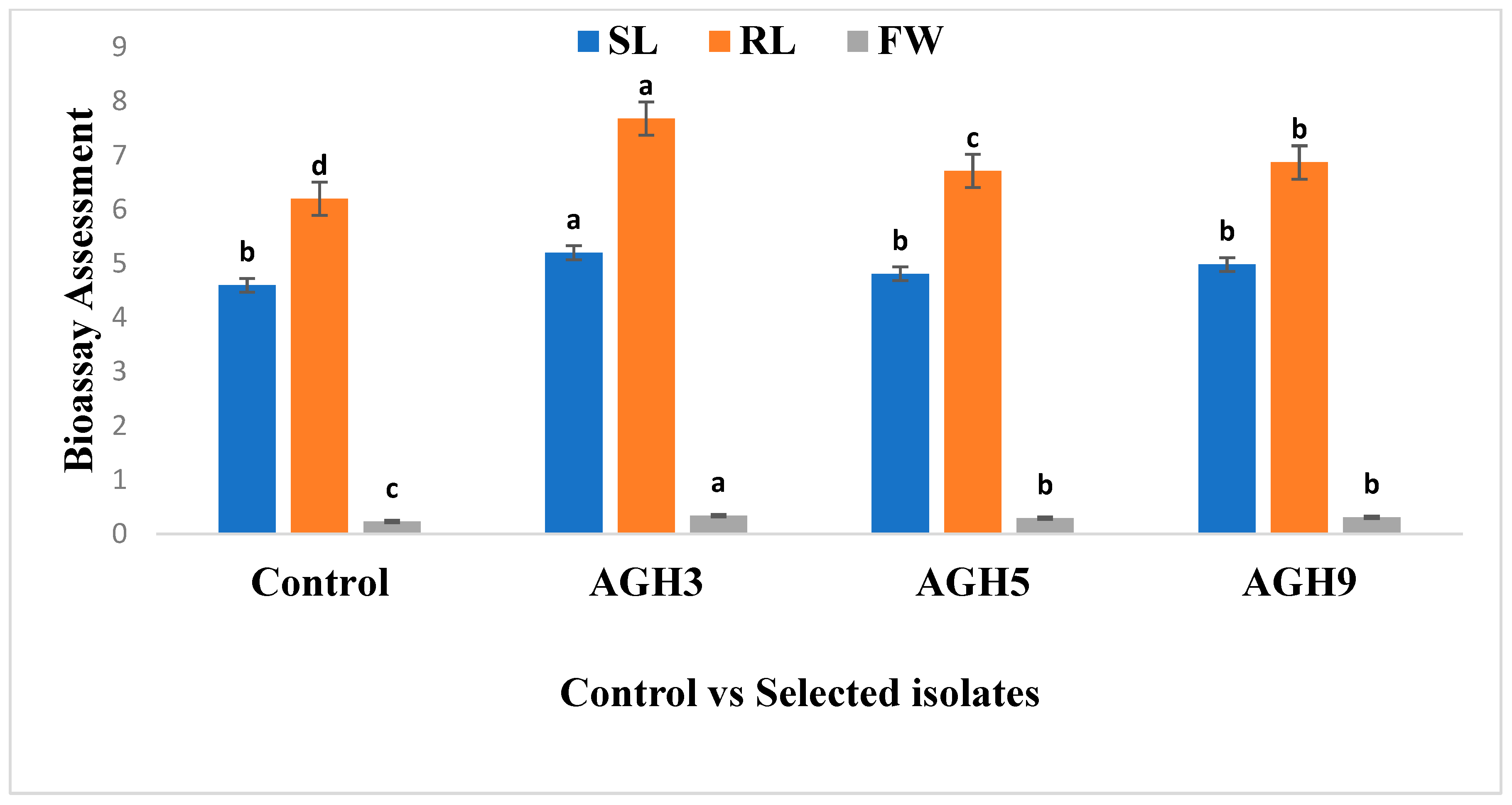
3.1. Screening of Selected PGPR on Waito-C Rice (GA Deficient Mutant)
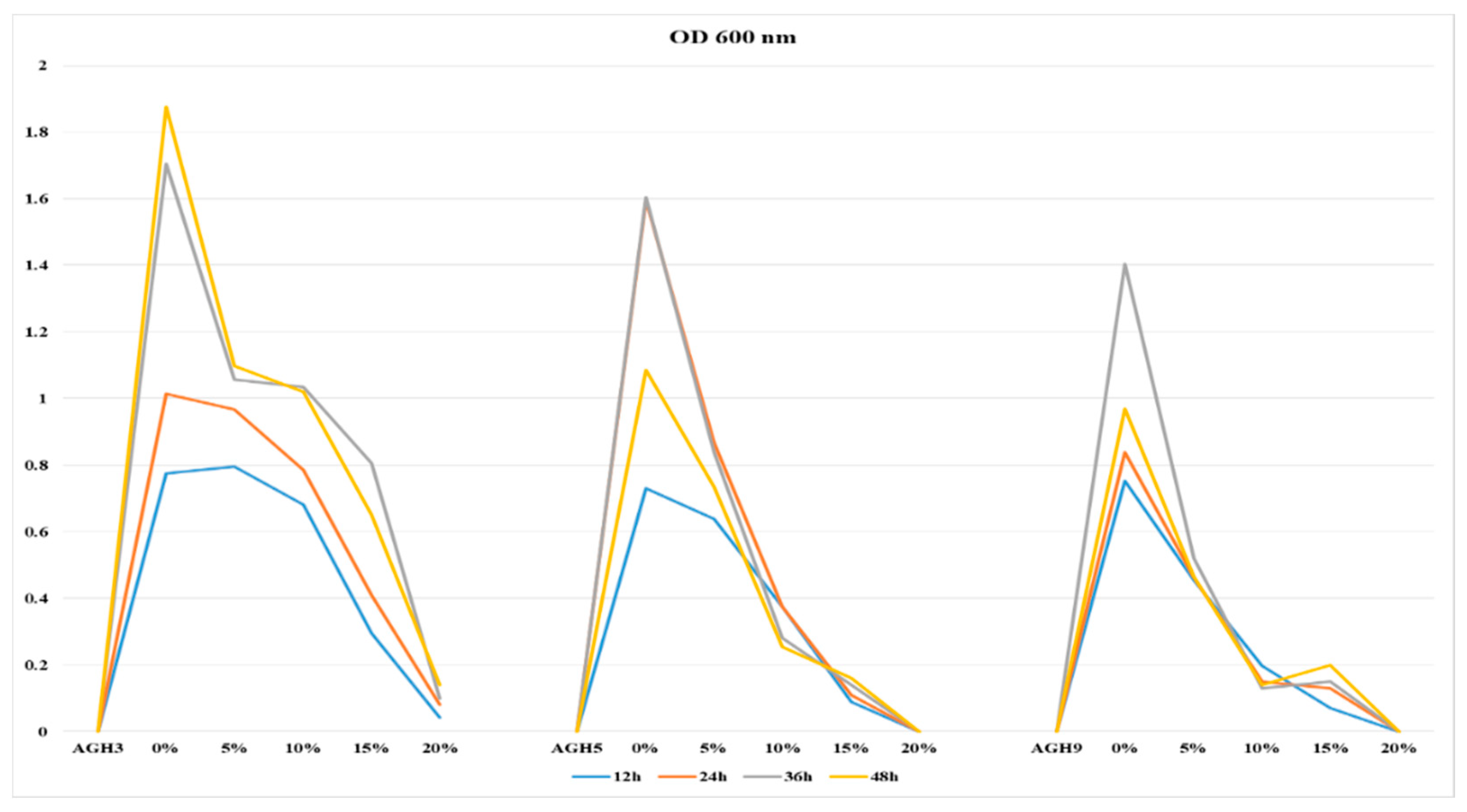
3.2. Screening of Selected Isolates for PEG Tolerance
3.3. PGP Effect of AGH3 on Solanum lycopersicum L. under PEG Stress
3.4. Effect of AGH3 on Plant Endogenous Abscisic Acid (ABA) and Jasmonic Acid (JA)
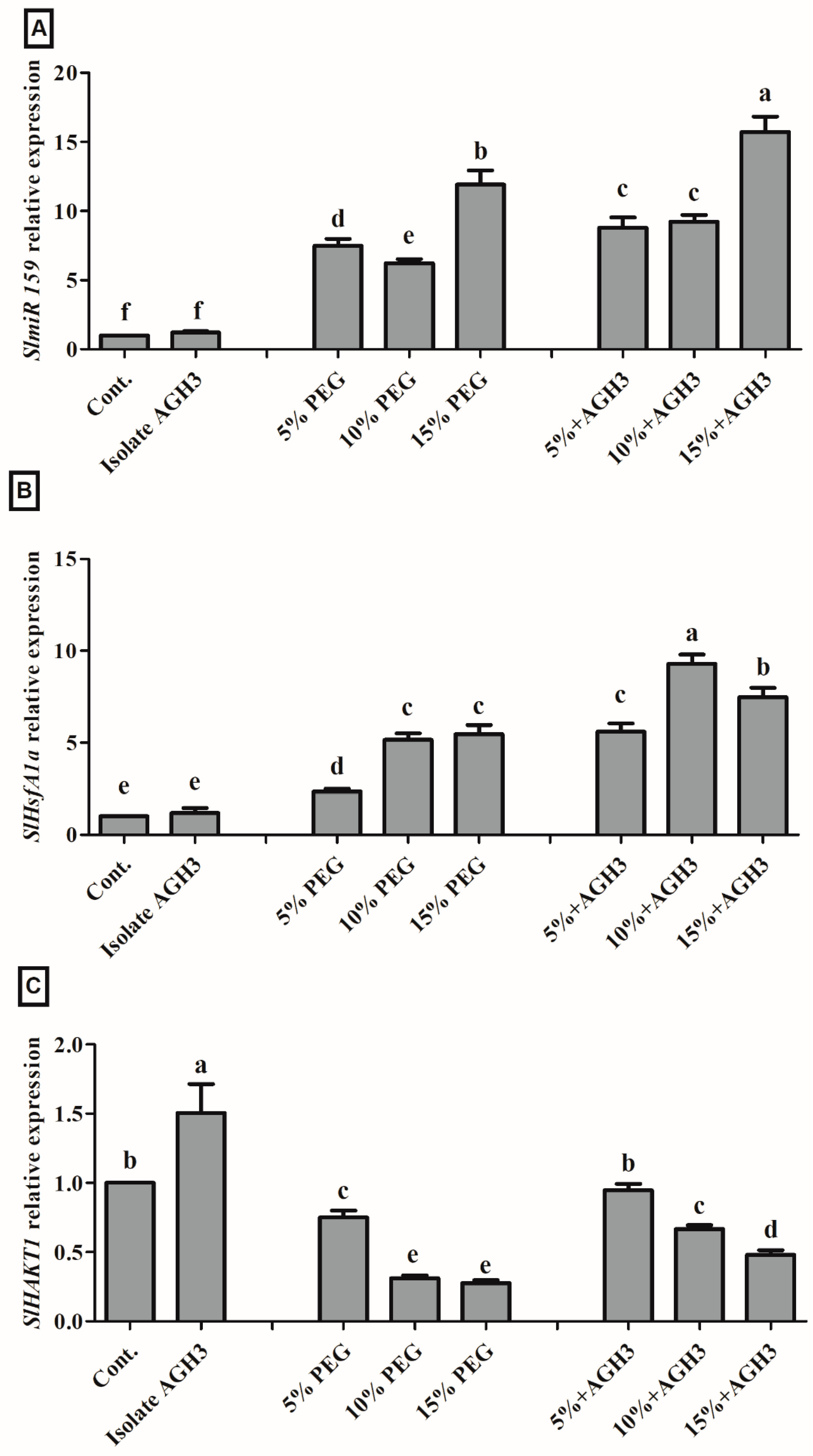
3.5. Gene Regulation under Drought Stress
3.6. Molecular Identification of Multi-Trait PGP AGH3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bera, K.; Dutta, P.; Sadhukhan, S. Plant Responses Under Abiotic Stress and Mitigation Options Towards Agricultural Sustainability. In Plant Stress: Challenges and Management in the New Decade; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–28. [Google Scholar]
- Khan, A.L.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Jung, H.-Y.; Lee, J.-H.; Lee, I.-J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 2012, 12, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.; Kim, W.-C. Plant growth promotion under water: Decrease of waterlogging-induced ACC and ethylene levels by ACC deaminase-producing bacteria. Front. Microbiol. 2018, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Hakim, U.M.Q.; Quraishi, U.M.; Chaudhary, H.J.; Munis, M.F.H. Indole-3-acetic acid induces biochemical and physiological changes in wheat under drought stress conditions. Former. Philipp. Agric. 2016, 99, 19–24. [Google Scholar]
- Ali, S.; Moon, Y.-S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.-J. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.-J.J.B.R.I. Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, H.; Xing, H.; Cui, N.; Liu, X.; Meng, X.; Wang, X.; Fan, L.; Fan, H. Regulation of Growth and Salt Resistance in Cucumber Seedlings by Hydrogen-Rich Water. J. Plant Growth Regul. 2021, 1–20. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, Z.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Wang, J. Rhizobacterial strain Bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought resistance. Int. J. Mol. Sci. 2016, 17, 976. [Google Scholar] [CrossRef]
- Ali, Q.; Ayaz, M.; Mu, G.; Hussain, A.; Yuanyuan, Q.; Yu, C.; Xu, Y.; Manghwar, H.; Gu, Q.; Wu, H.J. Revealing plant growth-promoting mechanisms of Bacillus strains in elevating rice growth and its interaction with salt stress. Front. Plant Sci. 2022, 13, 994902. [Google Scholar] [CrossRef]
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.M.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D. Radically rethinking agriculture for the 21st century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Mushtaq, H.; Fahad, S.; Shah, A.; Chaudhary, H.J. Plant growth promoting potential of bacterial endophytes in novel association with Olea ferruginea and Withania coagulans. Microbiology 2017, 86, 119–127. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Ali, S. Isolation and identification of multi-trait plant growth–promoting rhizobacteria from coastal sand dune plant species of Pohang beach. Folia Microbiol. 2022, 67, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-N.; Khan, M.A.; Kang, S.-M.; Hamayun, M.; Lee, I.-J. Enhancement of Drought-Stress Tolerance of Brassica Oleracea var. Italica L. by Newly Isolated Variovorax sp. YNA59. JMB 2020, 30, 1500–1509. [Google Scholar]
- Zhang, X.; Lei, L.; Lai, J.; Zhao, H.; Song, W. Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol. 2018, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Defez, R.; Andreozzi, A.; Dickinson, M.; Charlton, A.; Tadini, L.; Pesaresi, P.; Bianco, C. Improved drought stress response in alfalfa plants nodulated by an IAA over-producing Rhizobium strain. Front. Microbiol. 2017, 8, 2466. [Google Scholar] [CrossRef] [Green Version]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-M.; Khan, M.-A.; Hamayun, M.; Kim, L.-R.; Kwon, E.-H.; Kang, Y.-S.; Kim, K.-Y.; Park, J.-J.; Lee, I.-J. Phosphate-solubilizing Enterobacter ludwigii AFFR02 and Bacillus megaterium Mj1212 rescues alfalfa’s growth under post-drought stress. Agriculture 2021, 11, 485. [Google Scholar] [CrossRef]
- Mehrotra, R.; Bhalothia, P.; Bansal, P.; Basantani, M.K.; Bharti, V.; Mehrotra, S. Abscisic acid and abiotic stress tolerance–Different tiers of regulation. J. Plant Physiol. 2014, 171, 486–496. [Google Scholar] [CrossRef]
- Åkerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Khan, I.U.; Jan, M.; Khan, H.A.; Hussain, S.; Nisar, M.; Chung, W.S.; Yun, D.-J. The high-affinity potassium transporter EpHKT1; 2 from the extremophile Eutrema parvula mediates salt tolerance. Front. Plant Sci. 2018, 9, 1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairbairn, D.J.; Liu, W.; Schachtman, D.P.; Gomez-Gallego, S.; Day, S.R.; Teasdale, R.D. Characterisation of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis. Plant Mol. Biol. 2000, 43, 515–525. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R.J.R. Environment; Sustainability. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought stress in plants: Causes, consequences, and tolerance. In Drought Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1, pp. 1–16. [Google Scholar]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Ullah, I.; Ali, S.; Kang, S.-M.; Lee, I.-J. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019, 69, 797–808. [Google Scholar] [CrossRef]
- Ademola, T.O. Assessment of Rain-Fed and Irrigated Farming Systems of Sugarcane Production in Bauchi State. Master’s thesis, Federal University of Technology, Minna, Nigeria, 2021. [Google Scholar]
- García-Caparrós, P.; Romero, M.J.; Llanderal, A.; Cermeño, P.; Lao, M.T.; Segura, M.L. Effects of drought stress on biomass, essential oil content, nutritional parameters, and costs of production in six Lamiaceae species. Water 2019, 11, 573. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef] [Green Version]
- Morisaku, T.; Yui, H.J.A. Laser-induced surface deformation microscope for the study of the dynamic viscoelasticity of plasma membrane in a living cell. Analyst 2018, 143, 2397–2404. [Google Scholar] [CrossRef]
- dos Santos, A.R.; Melo, Y.L.; de Oliveira, L.F.; Cavalcante, I.E.; de Souza Ferraz, R.L.; da Silva Sá, F.V.; de Lacerda, C.F.; de Melo, A.S.; Nutrition, P. Exogenous Silicon and Proline Modulate Osmoprotection and Antioxidant Activity in Cowpea Under Drought Stress. Soil Sci. Plant Nutr. 2022, 1–8. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N.J.N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Jin, N.; Ren, W.; Tao, B.; He, L.; Ren, Q.; Li, S.; Yu, Q.J. Effects of water stress on water use efficiency of irrigated and rainfed wheat in the Loess Plateau, China. Sci. Total Environ. 2018, 642, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global synthesis of drought effects on cereal, legume, tuber and root crops production: A review. Agric. Water Manag. 2017, 179, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Hussain, M.; Nawaz, A.; Lee, D.-J.; Alghamdi, S.S.; Siddique, K.H. Seed priming improves chilling tolerance in chickpea by modulating germination metabolism, trehalose accumulation and carbon assimilation. Plant Physiol. Biochem. 2017, 111, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Bano, A.; Fazal, A.J. Recent methods of drought stress tolerance in plants. Plant Growth Regul. 2017, 82, 363–375. [Google Scholar] [CrossRef]
- Moon, Y.S.; Ali, S.J. Possible mechanisms for the equilibrium of ACC and role of ACC deaminase-producing bacteria. Appl. Microbiol. Biotechnol. 2022, 106, 877–887. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Ali, S.J.T.; Physiology, E.P. A fruitful decade of bacterial ACC deaminase biotechnology: A pragmatic approach towards abiotic stress relief in plants. Theor. Exp. Plant Physiol. 2022, 1–21. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, C.; Xu, Y.; Wei, Q.; Imtiaz, M.; Lan, H.; Gao, S.; Cheng, L.; Wang, M.; Fei, Z. A zinc finger protein regulates flowering time and abiotic stress tolerance in Chrysanthemum by modulating gibberellin biosynthesis. Plant Cell 2014, 26, 2038–2054. [Google Scholar] [CrossRef] [Green Version]
- Miceli, A.; Moncada, A.; Vetrano, F. Use of microbial biostimulants to increase the salinity tolerance of vegetable transplants. Agronomy 2021, 11, 1143. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. New Perspect. Approaches Plant Growth-Promot. Rhizobacteria Res. 2007, 329–339. [Google Scholar]
- Ullah, A.; Akbar, A.; Luo, Q.; Khan, A.H.; Manghwar, H.; Shaban, M.; Yang, X.J. Microbiome diversity in cotton rhizosphere under normal and drought conditions. Microb. Ecol. 2019, 77, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Cho, Y.-G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Johri, B.N.; Sharma, A.; Virdi, J.J. Rhizobacterial diversity in India and its influence on soil and plant health. Adv. Biochem. Eng. Biotechnol. 2003, 84, 49–89. [Google Scholar]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.-M.; Lee, I.-J. Halotolerant rhizobacterial strains mitigate the adverse effects of NaCl stress in soybean seedlings. BioMed Res. Int. 2019, 2019, 9530963. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S.J.E.S.; Research, P. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Jan, F.G.; Hamayun, M.; Hussain, A.; Jan, G.; Ali, S.; Khan, S.A.; Lee, I.-J. Endophytic Candida membranifaciens from Euphorbia milii L. Alleviate Salt Stress Damages in Maize. Agronomy 2022, 12, 2263. [Google Scholar] [CrossRef]
- Yazdani, M.; Bahmanyar, M.A.; Pirdashti, H.; Esmaili, M.A. Engineering; Technology. Effect of phosphate solubilization microorganisms (PSM) and plant growth promoting rhizobacteria (PGPR) on yield and yield components of corn (Zea mays L.). Int. J. Agric. Biosyst. Eng. 2009, 49, 90–92. [Google Scholar]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Drought-tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusine coracana (L.) Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere 2018, 28, 227–240. [Google Scholar] [CrossRef]
- Eun, H.-D.; Ali, S.; Jung, H.; Kim, K.; Kim, W.-C. Profiling of ACC synthase gene (ACS11) expression in Arabidopsis induced by abiotic stresses. Appl. Biol. Chem. 2019, 62, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gowtham, H.; Singh, B.; Murali, M.; Shilpa, N.; Prasad, M.; Aiyaz, M.; Amruthesh, K.; Niranjana, S. Induction of drought tolerance in tomato upon the application of ACC deaminase producing plant growth promoting rhizobacterium Bacillus subtilis Rhizo SF 48. Microbiol. Res. 2020, 234, 126422. [Google Scholar]
- Showemimo, F.; Olarewaju, J. Drought tolerance indices in sweet pepper (Capsicum annuum L.). Int. J. Plant Breed. Genet. 2007, 1, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Earl, H.J.; Davis, R.F. Effect of drought stress on leaf and whole canopy radiation use efficiency and yield of maize. Agron. J. 2003, 95, 688–696. [Google Scholar] [CrossRef]
- Mushtaq, N.; Iqbal, S.; Hayat, F.; Raziq, A.; Ayaz, A.; Zaman, W. Melatonin in Micro-Tom Tomato: Improved Drought Tolerance via the Regulation of the Photosynthetic Apparatus, Membrane Stability, Osmoprotectants, and Root System. Life 2022, 12, 1922. [Google Scholar] [CrossRef]
- Hamayun, M.; Sohn, E.-Y.; Khan, S.A.; Shinwari, Z.K.; Khan, A.L.; Lee, I.-J.J.P.J.B. Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Pak. J. Bot. 2010, 42, 1713–1722. [Google Scholar]
- Calvo-Polanco, M.; Sánchez-Romera, B.; Aroca, R.; Asins, M.J.; Declerck, S.; Dodd, I.C.; Martínez-Andújar, C.; Albacete, A.; Ruiz-Lozano, J.M.J.E.; Botany, E. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Envi. Expe. Bot. 2016, 131, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Amruthesh, K.N.; Murali, M.; Poczai, P.; Gafur, A. Insight into Recent Progress and Perspectives in Improvement of Antioxidant Machinery upon PGPR Augmentation in Plants under Drought Stress: A Review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef]
- Bibi, N.; Hamayun, M.; Khan, S.A.; Iqbal, A.; Islam, B.; Shah, F.; Lee, I.J. Anthracene biodegradation capacity of newly isolated rhizospheric bacteria Bacillus cereus S13. PLoS ONE 2018, 13, e0201620. [Google Scholar] [CrossRef] [PubMed]
- Uzma, M.; Iqbal, A.; Hasnain, S. Drought tolerance induction and growth promotion by indole acetic acid producing Pseudomonas aeruginosa in Vigna radiata. PloS ONE 2022, 17, e0262932. [Google Scholar] [CrossRef]
- Javed, T.; Zhou, J.-R.; Li, J.; Hu, Z.-T.; Wang, Q.-N.; Gao, S.-J. Identification and expression profiling of WRKY family genes in sugarcane in response to bacterial pathogen infection and nitrogen implantation dosage. Front. Plant Sci. 2022, 13, 917953. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Manghwar, H.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. Melatonin function and crosstalk with other phytohormones under normal and stressful conditions. Genes 2022, 13, 1699. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Park, S.-K.; Kim, W.-C. The pragmatic introduction and expression of microbial transgenes in plants. J. Microbiol. Biotechnol. 2018, 28, 1955–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.; Ali, M.; Zaman, W.; Brohi, R.D. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front. Plant Sci. 2022, 13, 1039329. [Google Scholar] [CrossRef] [PubMed]


| Machine | Mass Selective Detector (Hewlett-Packard 6890, 5973N) |
|---|---|
| Column | HP-1 capillary column (30 m × 0.25 mm i.d. 0.25 µm film thickness) (J & W Scientific Co., Folsom, CA, USA) |
| Carrier gas Head pressure | He 40 mL/min 30 kPa |
| Source temperature | 250 °C |
| Oven conditions | ABA: 60 °C (1 min) → 15 °C/min → 200 °C →5 °C/min → 250 °C →10 °C/min → 280 °C JA: 60 °C (2 min) → 10 °C/min → 140 °C (3 min) →3 °C/min 170 °C → 15 °C/min → 285 °C (8 min) |
| Injector temperature | 200 °C |
| Ionizing voltage | 70 ev |
| Rhizobacteria | Phosphate Solubilization | IAA Production | GAs Production | Siderophore Production | |
|---|---|---|---|---|---|
| Positive/Negative with L. Tryptophan | Positive/Negative without L. Tryptophan | ||||
| AGH3 | +++ | +++ | ++ | ++ | +++ |
| AGH5 | + | + | ++ | + | ++ |
| AGH9 | ++ | ++ | + | + | + |
| Control Plant | AGH3 Treated Plant | |
|---|---|---|
| Shoot Length (cm) | 4.6 ± 1.0 b | 5.2 ± 0.34 a |
| Root Length (cm) | 6.2 ± 0.34 b | 7.68 ± 1.0 a |
| Fresh Weight (g) | 0.21 ± 0.25 b | 0.37 ± 0.3 a |
| Plant Height (cm) | SPAD (Chlorophyll Meter) | |||||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | AVG | ||
| Control (Normal) | 43 | 46 | 49 | 51 | 47.25 | 44.1 |
| Control (Drought) | 25 | 25 | 33 | 30 | 28.25 | 26 |
| AGH3 | 48 | 51 | 51 | 50 | 50 | 50 |
| AGH5 | 46 | 45 | 47 | 45 | 45.85 | 44 |
| AGH9 | 47 | 45 | 46 | 46 | 46 | 45 |
| SL(cm) | RL(cm) | SFW(g) | RFW(g) | SDW | RDW | SPAD | |
|---|---|---|---|---|---|---|---|
| Control | 30 ± 2.5 a | 14.5 ± 2 b | 31.15 ± 1 b | 5.25 ± 0.4 b | 2.74 ± 0.1 b | 0.49 ± 0.01 b | 51.2 ± 2 b |
| AGH3 | 38 ± 3 b | 17 ± 2 a | 37.67 ± 1 a | 8.35 ± 0.5 a | 4.54 ± 0.3 a | 0.58 ± 0.01 a | 55 ± 2 a |
| PEG Stress | |||||||
| 5% | 23 ± 3 cd | 12.1 ± 0.5 bc | 21.61 ± 1 d | 4.7 ± 0.1 c | 2.4 ± 0.1 c | 0.36 ± 0.01 d | 37 ± 1 d |
| 10% | 20 ± 1.5 ed | 10 ± 0.6 de | 14.47 ± 0.5 f | 2.07 ± 0.1 f | 1.8 ± 0.1 d | 0.27 ± 0.01 f | 34 ± 1 ef |
| 15% | 17 ± 0.6 e | 8.2 ± 0.9 e | 14.26 ± 0.6 f | 2.29 ± 0.3 ef | 1.5 ± 0.1 e | 0.25 ± 0.01 g | 31 ± 1 g |
| PEG + AGH3 | |||||||
| AGH3 + 5% | 25 ± 0.9 c | 13.4 ± 1.4 bc | 23.03 ± 0.5 c | 5.8 ± 0.3 b | 2.9 ± 0.1 b | 0.39 ± 0.005 c | 42 ± 1 c |
| AGH3 + 10% | 23 ± 0.6 cd | 11.6 ± 0.4 cd | 18 ± 0.5 e | 3.58 ± 0.3 d | 2.2 ± 0.05 c | 0.32 ± 0.005 e | 36 ± 1.5 de |
| AGH3 + 15% | 20 ± 0.9 ed | 9.7 ± 0.9 de | 15.6 ± 0.4 f | 2.84 ± 0.1 e | 1.9 ± 0.1 d | 0.27 ± 0.01 f | 33 ± 2 fg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siraj, S.; Khan, M.A.; Hamayun, M.; Ali, S.; Khan, S.A.; Hussain, A.; Iqbal, A.; Khan, H.; Kang, S.-M.; Lee, I.-J. Microbacterium oxydans Regulates Physio-Hormonal and Molecular Attributes of Solanum lycopersicum under Drought Stress. Agronomy 2022, 12, 3224. https://doi.org/10.3390/agronomy12123224
Siraj S, Khan MA, Hamayun M, Ali S, Khan SA, Hussain A, Iqbal A, Khan H, Kang S-M, Lee I-J. Microbacterium oxydans Regulates Physio-Hormonal and Molecular Attributes of Solanum lycopersicum under Drought Stress. Agronomy. 2022; 12(12):3224. https://doi.org/10.3390/agronomy12123224
Chicago/Turabian StyleSiraj, Shumaila, Muhammad Aaqil Khan, Muhammad Hamayun, Sajid Ali, Sumera Afzal Khan, Anwar Hussain, Amjad Iqbal, Hamayoon Khan, Sang-Mo Kang, and In-Jung Lee. 2022. "Microbacterium oxydans Regulates Physio-Hormonal and Molecular Attributes of Solanum lycopersicum under Drought Stress" Agronomy 12, no. 12: 3224. https://doi.org/10.3390/agronomy12123224
APA StyleSiraj, S., Khan, M. A., Hamayun, M., Ali, S., Khan, S. A., Hussain, A., Iqbal, A., Khan, H., Kang, S.-M., & Lee, I.-J. (2022). Microbacterium oxydans Regulates Physio-Hormonal and Molecular Attributes of Solanum lycopersicum under Drought Stress. Agronomy, 12(12), 3224. https://doi.org/10.3390/agronomy12123224








