Characterizing the Mechanism of Serotonin Alleviates Rice Resistance to Brown Planthopper Nilaparvata lugens (Homoptera: Delphacidae) Nymphs
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Transcriptome Analysis
2.3. Quantification of Serotonin in Rice after BPH Nymph Infestation
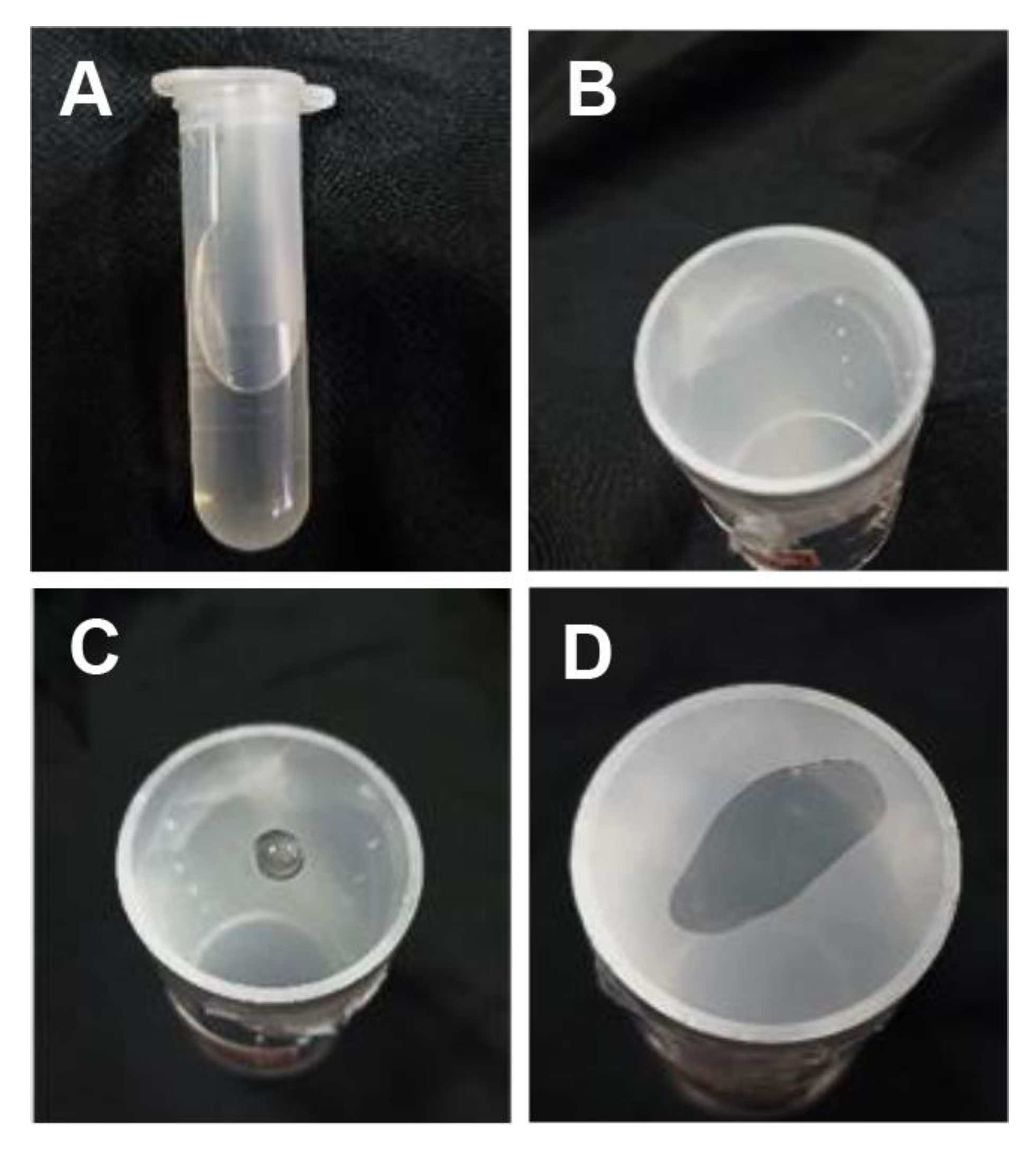
2.4. Measurement of BPH Feeding Preference and Honeydew Production
2.5. Direct Effects of Serotonin on BPH Nymphs
2.6. Determination of Rice Average Injury Scales and Functional Plant Loss Indices
2.7. Quantification of Soluble Sugar, Free Amino Acid, and Flavonoid Content
2.8. Measurement of the Activities of Antioxidant Enzymes
2.9. Statistical Analysis
3. Results
3.1. BPH Nymph Infestation Induces the Expression of the Key Genes in Serotonin Biosynthesis
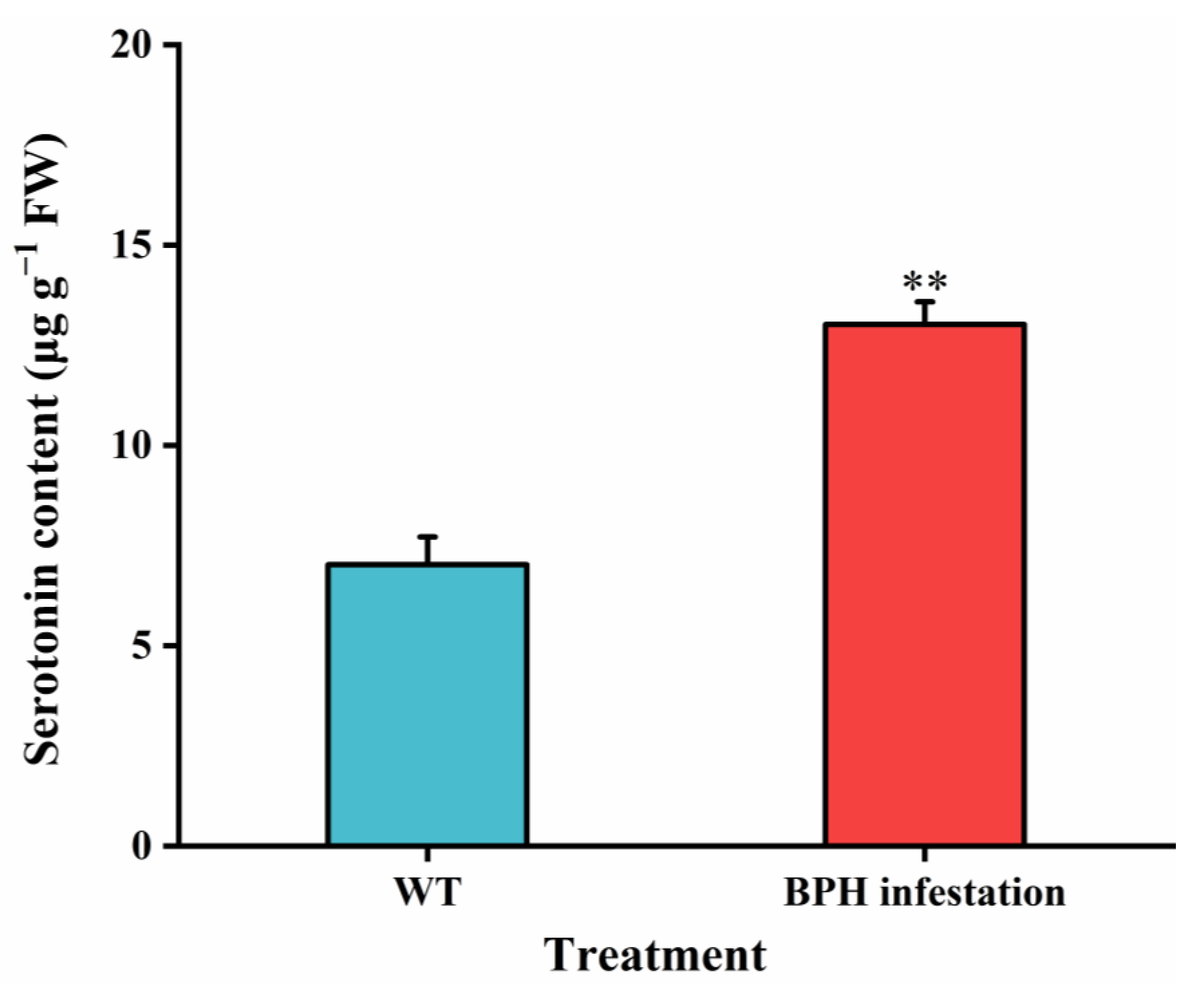
3.2. BPH Nymph Infestation Induces Accumulation of Serotonin in Rice
3.3. Exogenous Serotonin Affects BPH Performance
3.4. Exogenous Serotonin Alleviates Rice Tolerance to BPH Nymphs
3.5. Exogenous Serotonin Affects Levels of Rice Soluble Sugars, Free Amino Acids and Flavonoids
3.6. Exogenous Serotonin Affects the Activities of Antioxidant Enzymes in Rice
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furstenberg-Hagg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hedo, M.; Alonso-Valiente, M.; Vacas, S.; Gallego, C.; Rambla Nebot, J.L.; Navarro-Llopis, V.; Granell Richart, A.; Urbaneja García, A. Eliciting tomato plant defenses by exposure to herbivore induced plant volatiles. Entomol. Gen. 2021, 41, 209–218. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Li, Y.Y.; Dang, P.Q.; Zhao, S.J.; Lai, D.W.; Zhou, L.G. Rice secondary metabolites: Structures, roles, biosynthesis, and metabolic regulation. Molecules 2018, 23, 3098. [Google Scholar] [CrossRef]
- Widemann, E.; Bruinsma, K.; Walshe-Roussel, B.; Rioja, C.; Arbona, V.; Saha, R.K.; Letwin, D.; Zhurov, V.; Gómez-Cadenas, A.; Bernards, M.A. Multiple indole glucosinolates and myrosinases defend Arabidopsis against Tetranychus urticae herbivory. Plant Physiol. 2021, 187, 116–132. [Google Scholar] [CrossRef]
- Zeng, T.; Su, H.A.; Liu, Y.L.; Li, J.F.; Jiang, D.X.; Lu, Y.Y.; Qi, Y.X. Serotonin modulates insect gut bacterial community homeostasis. BMC Biol. 2022, 20, 105. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Turi, C.E.; Saxena, P.K. Serotonin: An ancient molecule and an important regulator of plant processes. Biotechnol. Adv. 2016, 34, 1347–1361. [Google Scholar] [CrossRef]
- Bhowal, B.; Bhattacharjee, A.; Goswami, K.; Sanan-Mishra, N.; Singla-Pareek, S.L.; Kaur, C.; Sopory, S. Serotonin and melatonin biosynthesis in plants: Genome-wide identification of the genes and their expression reveal a conserved role in stress and development. Int. J. Mol. Sci. 2021, 22, 11034. [Google Scholar] [CrossRef]
- Kang, S.; Kang, K.; Lee, K.; Back, K. Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 2007, 227, 263–272. [Google Scholar] [CrossRef]
- Kanjanaphachoat, P.; Wei, B.Y.; Lo, S.F.; Wang, I.W.; Wang, C.S.; Yu, S.M.; Yen, M.L.; Chiu, S.H.; Lai, C.C.; Chen, L.J. Serotonin accumulation in transgenic rice by over-expressing tryptophan decarboxylase results in a dark brown phenotype and stunted growth. Plant Mol. Biol. 2012, 78, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Negri, S.; Commisso, M.; Avesani, L.; Guzzo, F. The case of tryptamine and serotonin in plants: A mysterious precursor for an illustrious metabolite. J. Exp. Bot. 2021, 72, 5336–5355. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Wang, B.; Ren, J.X.; Zhou, Y.T.; Han, Y.; Niu, S.Y.; Zhang, Y.Y.; Shi, Y.H.; Zhou, J.J.; Yang, C.K.; et al. Osbzip18, a positive regulator of serotonin biosynthesis, negatively controls the UV-B tolerance in rice. Int. J. Mol. Sci. 2022, 23, 3215. [Google Scholar] [CrossRef] [PubMed]
- Erland, L.A.E.; Yasunaga, A.; Li, I.T.S.; Murch, S.J.; Saxena, P.K. Direct visualization of location and uptake of applied melatonin and serotonin in living tissues and their redistribution in plants in response to thermal stress. J. Pineal Res. 2019, 66, e12527. [Google Scholar] [CrossRef]
- Lu, H.P.; Gao, Q.; Han, J.P.; Guo, X.H.; Wang, Q.; Altosaar, I.; Barberon, M.; Liu, J.X.; Gatehouse, A.M.R.; Shu, Q.Y. An ABA-serotonin module regulates root suberization and salinity tolerance. New Phytol. 2022, 236, 958–973. [Google Scholar] [CrossRef]
- He, H.; Lei, Y.; Yi, Z.; Raza, A.; Zeng, L.; Yan, L.; Ding, X.Y.; Yong, C.; Zou, X.L. Study on the mechanism of exogenous serotonin improving cold tolerance of rapeseed (Brassica napus L.) seedlings. Plant Growth Regul. 2021, 94, 161–170. [Google Scholar] [CrossRef]
- Erland, L.A.; Turi, C.E.; Saxena, P.K. Serotonin in plants: Origin, functions, and implications. Serotonin 2019, 2019, 23–46. [Google Scholar]
- Abbasi, B.H.; Younas, M.; Anjum, S.; Ahmad, N.; Ali, M.; Fazal, H.; Hano, C. Serotonin in plant signalling and communication. In Neurotransmitters in Plant Signaling and Communication; Springer: Cham, Switzerland, 2020; pp. 75–92. [Google Scholar]
- Ishihara, A.; Kumeda, R.; Hayashi, N.; Yagi, Y.; Sakaguchi, N.; Kokubo, Y.; Ube, N.; Tebayashi, S.; Ueno, K. Induced accumulation of tyramine, serotonin, and related amines in response to Bipolaris sorokiniana infection in barley. Biosci. Biotechnol. Biochem. 2017, 81, 1090–1098. [Google Scholar] [CrossRef]
- Ishihara, A.; Hashimoto, Y.; Tanaka, C.; Dubouzet, J.G.; Nakao, T.; Matsuda, F.; Nishioka, T.; Miyagawa, H.; Wakasa, K. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef]
- Hayashi, K.; Fujita, Y.; Ashizawa, T.; Suzuki, F.; Nagamura, Y.; Hayano-Saito, Y. Serotonin attenuates biotic stress and leads to lesion browning caused by a hypersensitive response to Magnaporthe oryzae penetration in rice. Plant J. 2016, 85, 46–56. [Google Scholar] [CrossRef]
- Dangol, A.; Shavit, R.; Yaakov, B.; Strickler, S.R.; Jander, G.; Tzin, V. Characterizing serotonin biosynthesis in Setaria viridis leaves and its effect on aphids. Plant Mol. Biol. 2022, 109, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Hashimoto, Y.; Miyagawa, H.; Wakasa, K. Induction of serotonin accumulation by feeding of rice striped stem borer in rice leaves. Plant Signal. Behav. 2008, 3, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.P.; Luo, T.; Fu, H.W.; Wang, L.; Tan, Y.Y.; Huang, J.Z.; Wang, Q.; Ye, G.Y.; Gatehouse, A.M.R.; Lou, Y.G.; et al. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat. Plants 2018, 4, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Yu, Z.X.; Meng, J.P.; Zhou, P.Y.; Luo, T.; Zhang, J.; Wu, J.; Lou, Y.G. Rice phenolamindes reduce the survival of female adults of the white-backed planthopper Sogatella furcifera. Sci. Rep. 2020, 10, 5778. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhu, L.; He, G.C. Towards understanding of molecular interactions between rice and the brown planthopper. Mol. Plant 2013, 6, 621–634. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, F.M.; Wang, M.Q. Transgenic expression of Bt in rice does not affect feeding behavior and population density of the brown planthopper, Nilaparvata lugens Stål (Hemiptera: Delphacidae). Entomol. Gen. 2018, 37, 35–45. [Google Scholar] [CrossRef]
- Li, Y.; Gao, H.; Zhang, Y.W.; Lin, X.D. Role of the transcription factor taiman in moulting and ovarian development of Nilaparvata lugens. Entomol. Gen. 2021, 41, 169–177. [Google Scholar] [CrossRef]
- Hu, D.B.; Luo, B.Q.; Li, J.; Han, Y.; Jiang, T.R.; Liu, J.; Wu, G.; Hua, H.X.; Xiong, Y.F.; Li, J.S. Genome-wide analysis of Nilaparvata lugens nymphal responses to high-density and low-quality rice hosts. Insect Sci. 2013, 20, 703–716. [Google Scholar] [CrossRef]
- Sōgawa, K. The rice brown planthopper: Feeding physiology and host plant interactions. Annu. Rev. Entomol. 1982, 27, 49–73. [Google Scholar] [CrossRef]
- Shah, A.Z.; Ma, C.; Zhang, Y.Y.; Zhang, Q.X.; Xu, G.; Yang, G.Q. Decoyinine induced resistance in rice against small brown planthopper Laodelphax striatellus. Insects 2022, 13, 104. [Google Scholar] [CrossRef]
- Hao, Z.N.; Wang, L.P.; He, Y.P.; Liang, J.G.; Tao, R.X. Expression of defense genes and activities of antioxidant enzymes in rice resistance to rice stripe virus and small brown planthopper. Plant Physiol. Biochem. 2011, 49, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.Y.; Tan, J.; Zhou, C.; Yang, X.F.; Yang, F.; Zhang, S.J.; Sun, S.C.; Miao, X.X.; Shi, Z.Y. The OsmiR396–OsGRF8–OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa). Plant Biotechnol. J. 2019, 17, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, J.; Sun, J.R.; Shi, W.P.; Harwood, J.D.; Monticelli, L.S.; Tan, X.L.; Chen, J.L. Effects of field simulated warming on feeding behavior of Sitobion avenae (Fabricius) and host defense systems. Entomol. Gen. 2021, 41, 567–578. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H. Laboratory Manual for Physiological Studies of Rice, 3rd ed.; International Rice Research Institute: Los Baños, Philippines, 1976. [Google Scholar]
- Li, J.T.; Chen, L.; Ding, X.; Fan, W.Y.; Liu, J.L. Transcriptome analysis reveals crosstalk between the abscisic acid and jasmonic acid signaling pathways in rice-mediated defense against Nilaparvata lugens. Int. J. Mol. Sci. 2022, 23, 6319. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. Tbtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Ji, R.; Ye, W.F.; Chen, H.D.; Zeng, J.M.; Li, H.; Yu, H.X.; Li, J.C.; Lou, Y.G. A salivary endo-β-1,4-glucanase acts as an effector that enables the brown planthopper to feed on rice. Plant Physiol. 2017, 173, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Chen, X.; Zhang, H.M.; Yang, X.; Wong, A. Effects of exogenous plant growth regulator abscisic acid-induced resistance in rice on the expression of vitellogenin mRNA in Nilaparvata lugens (Hemiptera: Delphacidae) adult females. J. Insect Sci. 2014, 14, 213. [Google Scholar] [CrossRef]
- Sun, L.T.; Li, J.T.; Liu, Y.Y.; Noman, A.; Chen, L.; Liu, J.L. Transcriptome profiling in rice reveals a positive role for OsNCED3 in defense against the brown planthopper, Nilaparvata lugens. BMC Genom. 2022, 23, 634. [Google Scholar] [CrossRef]
- Huang, Q.L.; Li, L.; Zheng, M.H.; Chen, F.; Long, H.; Deng, G.B.; Pan, Z.F.; Liang, J.J.; Li, Q.; Yu, M.Q.; et al. The tryptophan decarboxylase 1 gene from Aegilops variabilis No. 1 regulate the resistance against cereal cyst nematode by altering the downstream secondary metabolite contents rather than auxin synthesis. Front. Plant Sci. 2019, 9, 1297. [Google Scholar] [CrossRef]
- Pasquet, J.C.; Chaouch, S.; Macadre, C.; Balzergue, S.; Huguet, S.; Martin-Magniette, M.L.; Bellvert, F.; Deguercy, X.; Thareau, V.; Heintz, D.; et al. Differential gene expression and metabolomic analyses of Brachypodium distachyon infected by deoxynivalenol producing and non-producing strains of Fusarium graminearum. BMC Genom. 2014, 15, 629. [Google Scholar] [CrossRef]
- Sun, P.Y.; Teng, D.; Lu, B.B.; Zhang, H.; Zhang, Y.J. Temporal and spatial dynamics of 5-hydroxytryptamine content in cotton and its effects on the growth and development of cotton bollworm Helicoverpa armigera. J. Plant Prot. 2021, 48, 1075–1080. (In Chinese) [Google Scholar]
- Chen, L.; Cao, T.T.; Zhang, J.; Lou, Y.G. Overexpression of OsGID1 enhances the resistance of rice to the brown planthopper Nilaparvata lugens. Int. J. Mol. Sci. 2018, 19, 2744. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C. Comparison of Defense Responses in Rice Induced by Feeding and Oviposition of the Brown Planthopper Nilaparvata lugens and Their Underlying Mechanisms. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2015. [Google Scholar]
- Jayasimha, G.; Nalini, R.; Chinniah, C.; Muthamilan, M.; Mini, M. Evaluation of biochemical constituents in healthy and brown planthopper, Nilaparvata lugens Stål (Hemiptera: Delphacidae) damaged rice plants. Curr. Biot. 2015, 9, 129–136. [Google Scholar]
- Liu, C.X.; Du, B.; Hao, F.H.; Lei, H.H.; Wan, Q.F.; He, G.C.; Wang, Y.L.; Tang, H.R. Dynamic metabolic responses of brown planthoppers towards susceptible and resistant rice plants. Plant Biotechnol. J. 2017, 15, 1346–1357. [Google Scholar] [CrossRef] [PubMed]
- Jannoey, P.; Channei, D.; Kotcharerk, J.; Pongprasert, W.; Nomura, M. Expression analysis of genes related to rice resistance against brown planthopper, Nilaparvata lugens. Rice Sci. 2017, 24, 163–172. [Google Scholar] [CrossRef]
- Duan, C.X.; Yu, J.J.; Bai, J.Y.; Zhu, Z.D.; Wang, X.M. Induced defense responses in rice plants against small brown planthopper infestation. Crop J. 2014, 2, 55–62. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Feng, L.; Liang, X.; Li, J.; Liao, G.; Zhu, L.; Fu, K.; Fan, W.; Wang, S.; Liu, J. Characterizing the Mechanism of Serotonin Alleviates Rice Resistance to Brown Planthopper Nilaparvata lugens (Homoptera: Delphacidae) Nymphs. Agronomy 2022, 12, 3191. https://doi.org/10.3390/agronomy12123191
Chen L, Feng L, Liang X, Li J, Liao G, Zhu L, Fu K, Fan W, Wang S, Liu J. Characterizing the Mechanism of Serotonin Alleviates Rice Resistance to Brown Planthopper Nilaparvata lugens (Homoptera: Delphacidae) Nymphs. Agronomy. 2022; 12(12):3191. https://doi.org/10.3390/agronomy12123191
Chicago/Turabian StyleChen, Lin, Ling Feng, Xinyan Liang, Jitong Li, Guangrong Liao, Lei Zhu, Kang Fu, Wenyan Fan, Shuang Wang, and Jinglan Liu. 2022. "Characterizing the Mechanism of Serotonin Alleviates Rice Resistance to Brown Planthopper Nilaparvata lugens (Homoptera: Delphacidae) Nymphs" Agronomy 12, no. 12: 3191. https://doi.org/10.3390/agronomy12123191
APA StyleChen, L., Feng, L., Liang, X., Li, J., Liao, G., Zhu, L., Fu, K., Fan, W., Wang, S., & Liu, J. (2022). Characterizing the Mechanism of Serotonin Alleviates Rice Resistance to Brown Planthopper Nilaparvata lugens (Homoptera: Delphacidae) Nymphs. Agronomy, 12(12), 3191. https://doi.org/10.3390/agronomy12123191





