Physiological Response of Pea (Pisum sativum L.) Plants to Foliar Application of Biostimulants
Abstract
:1. Introduction
2. Materials and Methods
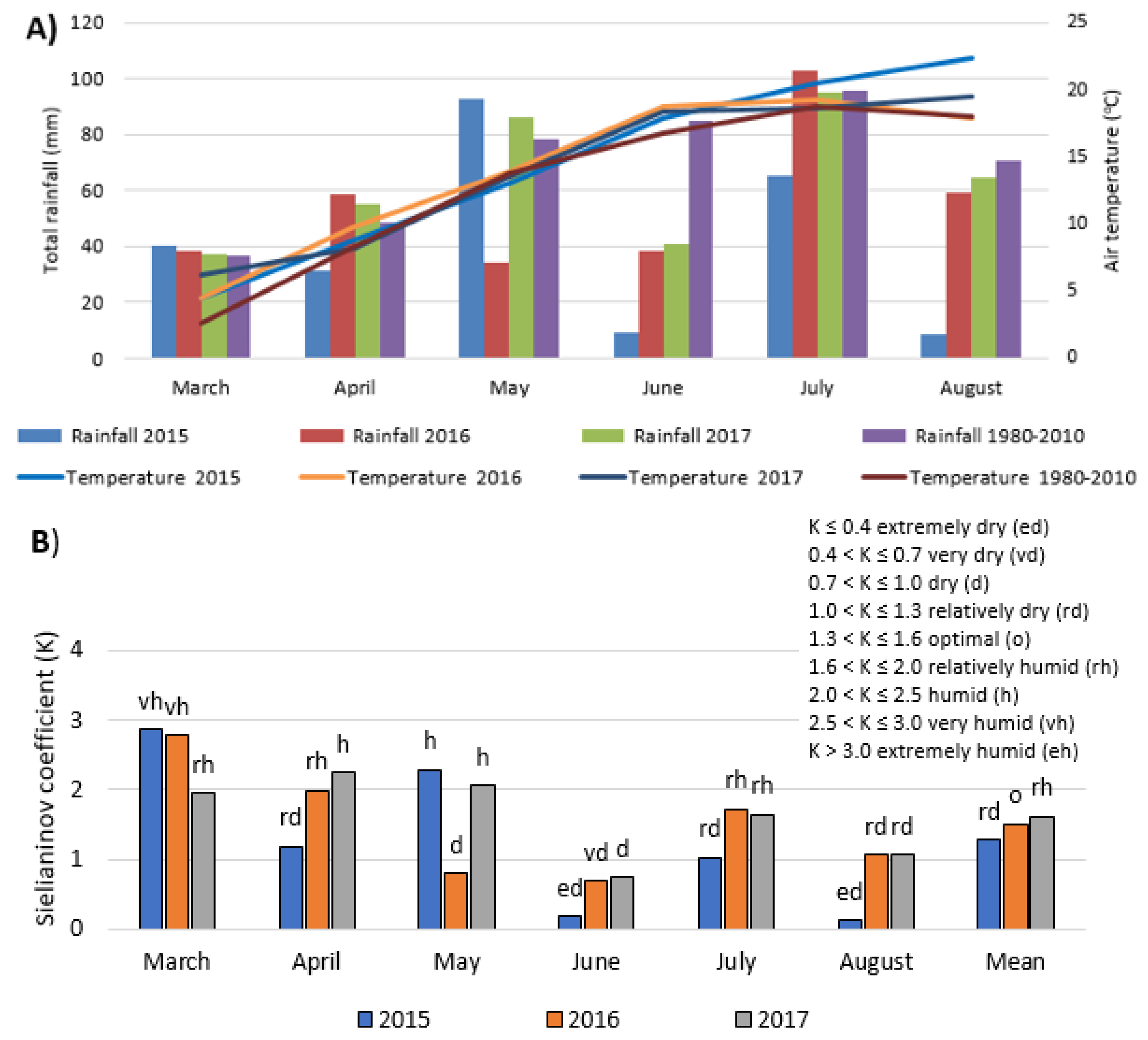
2.1. Experimental Site
2.2. Experimental Design and Crop Management
2.3. Physiological Measurements
- -
- relative chlorophyll (Chl) content (CCI unit)—on 20 randomly selected bracts in each plot, using a Chlorophyll Content Meter CCM-200plus (Opti-Sciences, Hudson, NH, USA),
- -
- chlorophyll (Chl) fluorescence (Fv/Fm—maximum efficiency of PSII, Fv/F0-the maximum quantum yield of primary photochemistry, PI—performance index) on 8 randomly selected pea bracts on each plot, on the adaxial bract lamina after 30 min of adaptation to darkness [24], using a continuous excitation Handy Pocket PEA fluorimeter equipped with leaf-clips (Pocket PEA, Hansatech Instruments, King’s Lynn, Norfolk, UK); a PEA plus (1.10) program was then used to export Chl fluorescence signals,
- -
- gas exchange (Pn—intensity of photosynthesis net, E—transpiration rate, gs—stomatal conductance, Ci—intracellular CO2 concentration), on 4 randomly selected plants in each plot, using the Portable Photosynthesis Measurement System LCpro-SD (ADC BioScientific Ltd., Hoddesdon, UK). In the determination process, the light intensity was 1500 mol m−2 s−1. The LCpro-SD plant leaf photosynthesis chamber has a flow rate accuracy of ±2% of its range.
2.4. Statistical Analysis
3. Results
3.1. Relative Chlorophyll (Chl) Content
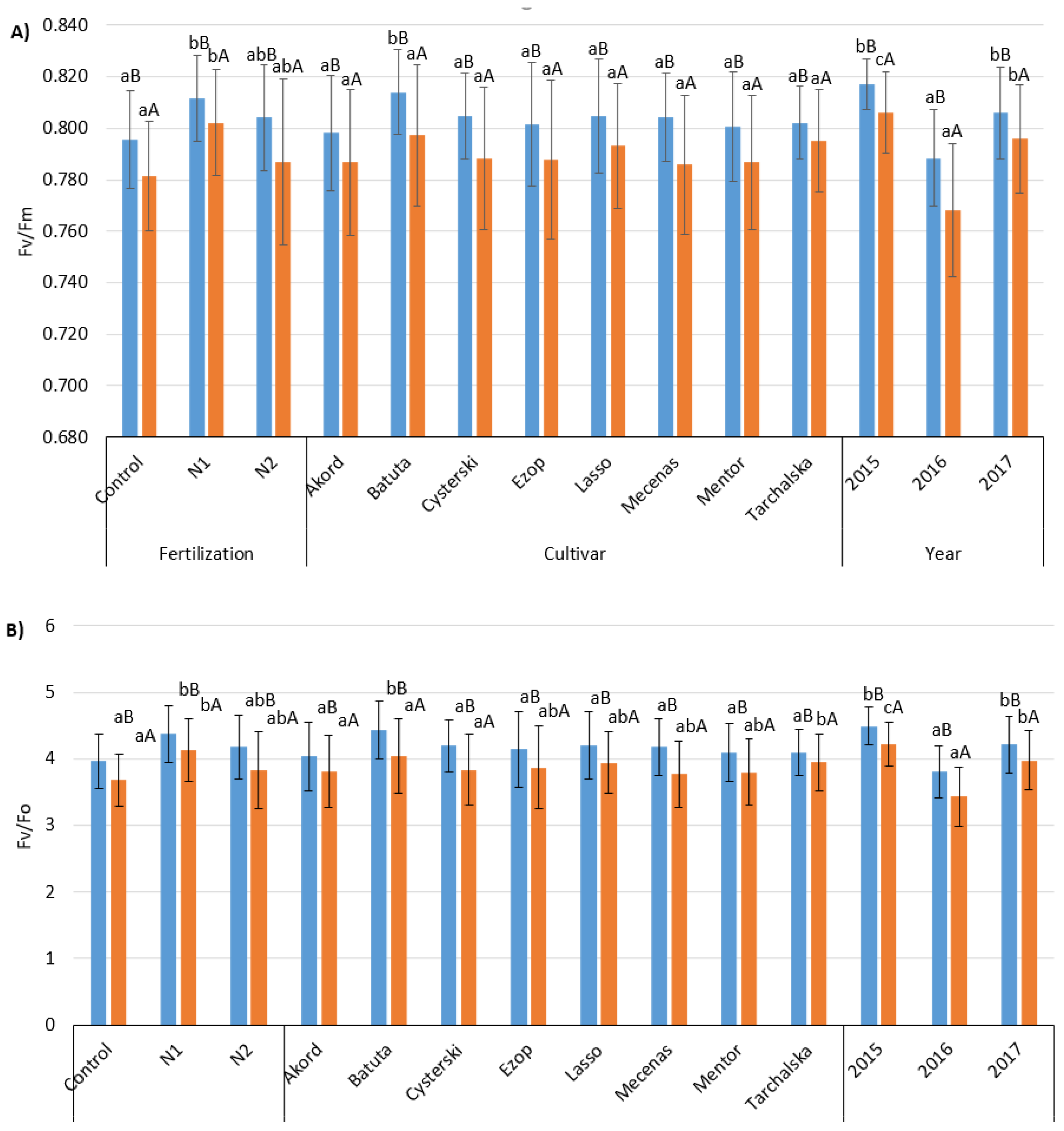
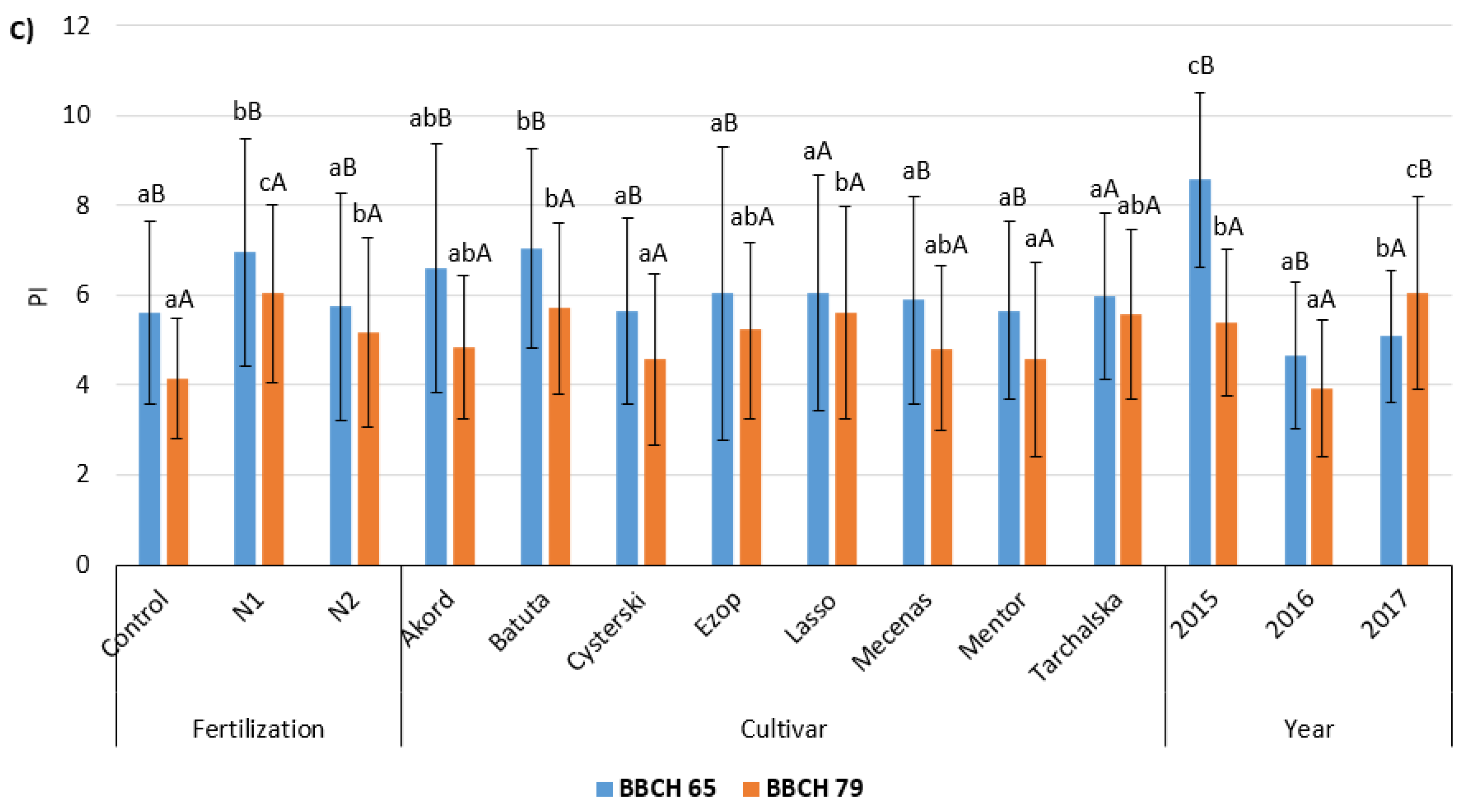
3.2. Chlorophyll (Chl) Fluorescence
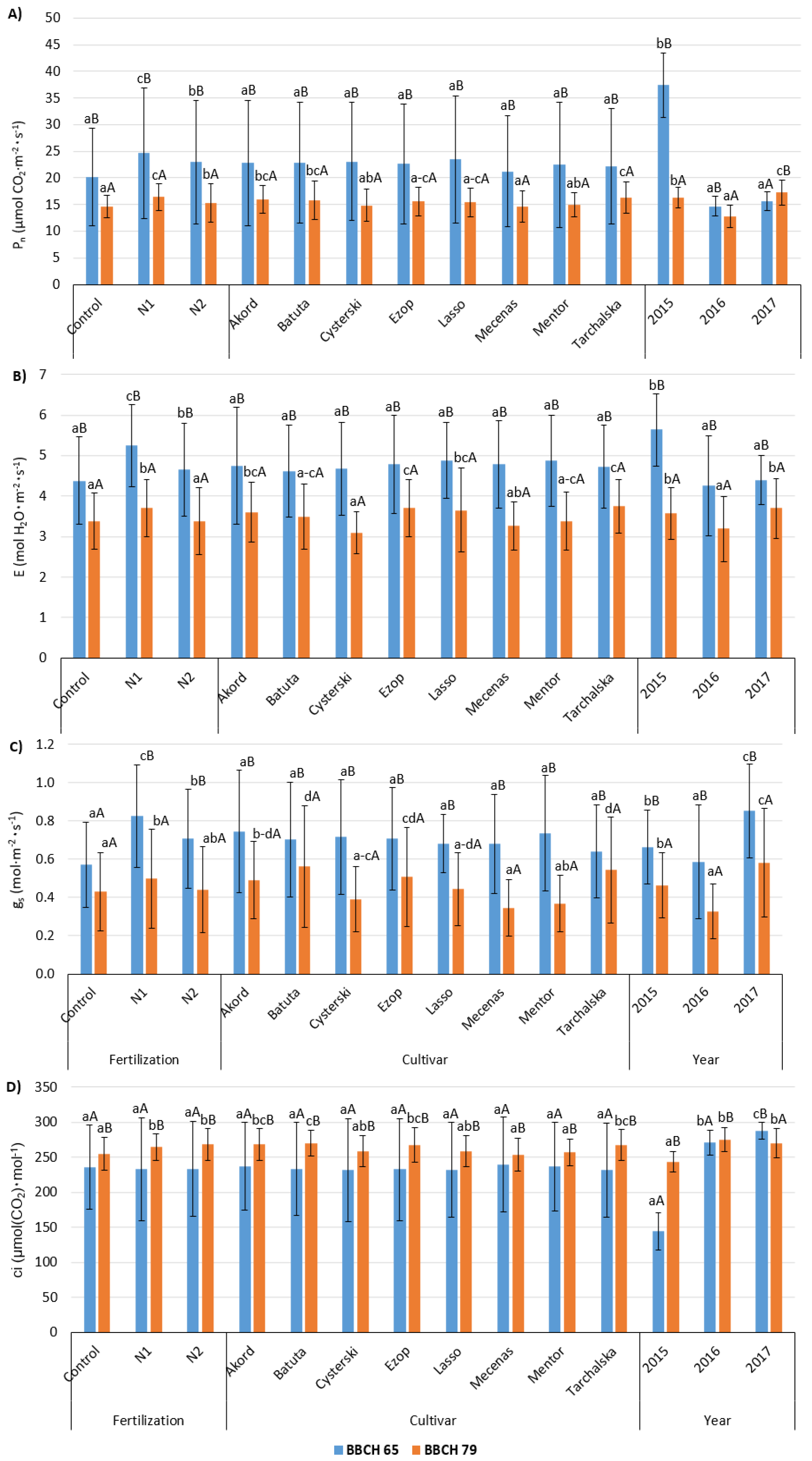
3.3. Gas Exchange
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 28 October 2022).
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and nutritional quality of Vesuvian Piennolo tomato PDO as affected by farming system and biostimulant application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein hydrolysate or plant extract-based biostimulants enhanced yield and quality performances of greenhouse perennial wall rocket grown in different seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Elżbieta Wszelaczyńska, E.; Szczepanek, M.; Pobereżny, J.; Kazula, M.J. Effect of biostimulant application and long-term storage on the nutritional value of carrot. Hortic. Bras. 2019, 37, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Pobereżny, J.; Szczepanek, M.; Wszelaczyńska, E.; Prus, P. The quality of carrot after field biostimulant application and after storage. Sustainability 2020, 12, 1386. [Google Scholar] [CrossRef] [Green Version]
- Osman, M.S.; Badawy, A.A.; Osman, A.I.; Abdel Latef, A.A.H. Ameliorative impact of an extract of the halophyte Arthrocnemum macrostachyum on growth and biochemical parameters of soybean under salinity stress. J. Plant Growth Regul. 2021, 40, 1245–1256. [Google Scholar] [CrossRef]
- Badawy, A.A.; Alotaibi, M.O.; Abdelaziz, A.M.; Osman, M.S.; Khalil, A.M.A.; Saleh, A.M.; Mohammed, A.E.; Hashem, A.H. Enhancement of seawater stress tolerance in barley by the endophytic fungus Aspergillus ochraceus. Metabolites 2021, 11, 428. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants application: A low input cropping management tool for sustainable farming of vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Skrobacz, K.; Bobrecka-Jamro, D.; Balawejder, M. Response of potato (Solanum tuberosum L.) plants to spraying by hydrogen peroxide. Sustainability 2020, 12, 2469. [Google Scholar] [CrossRef] [Green Version]
- Cerdán, M.; Sánchez-Sánchez, A.; Jordá, J.D.; Juárez, M.; Sánchez-Andreu, J. Effect of commercial amino acids on iron nutrition of tomato plants grown under lime-induced iron deficiency. J. Plant. Nutr. Soil Sci. 2013, 176, 859–866. [Google Scholar] [CrossRef]
- Karapouloutidou, S.; Gasparatos, D. Effects of biostimulant and organic amendment on soil properties and nutrient status of Lactuca sativa in a calcareous saline-sodic soil. Agriculture 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Szparaga, A.; Kocira, S.; Kocira, A.; Czerwińska, E.; Świeca, M.; Lorencowicz, E.; Kornas, R.; Koszel, M.; Oniszczuk, T. Modification of growth, yield, and the nutraceutical and antioxidative potential of soybean through the use of synthetic biostimulants. Front. Plant Sci. 2018, 9, 1401. [Google Scholar] [CrossRef]
- Kocira, A.; Lamorska, J.; Kornas, R.; Nowosad, N.; Tomaszewska, M.; Leszczyńska, D.; Kozłowicz, K.; Tabor, S. Changes in biochemistry and yield in response to biostimulants applied in bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Sulewska, H.; Niewiadomska, A.; Ratajczak, K.; Budka, A.; Panasiewicz, K.; Faligowska, A.; Wolna-Maruwka, A.; Dryjański, L. Changes in Pisum sativum L. Plants and in Soil as a Result of Application of Selected Foliar Fertilizers and Biostimulators. Agronomy 2020, 10, 1558. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Pereira, C.; Dias, M.I.; Petropoulos, S.A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Ferreira, I.C.F.R. The effects of biostimulants, biofertilizers and water-stress on nutritional value and chemical composition of two spinach genotypes (Spinacia oleracea L.). Molecules 2019, 24, 4494. [Google Scholar] [CrossRef] [Green Version]
- Schnitkey, G.; Paulson, N.; Swanson, K.; Colussi, J.; Jim, B. Nitrogen Fertiliser Prices and Supply in Light of the Ukraine-Russia 709 Conflict. Department of Agricultural and Consumer Economics, University of Illinois at Urbana-Champaign, farmdoc Dly, 5 April 2022. Available online: https://farmdocdaily.illinois.edu/2022/04/nitrogen-fertilizer-prices-and-supply-in-light-of-the-ukraine-russia-conflict.html (accessed on 28 October 2022).
- Bertrand, C.; Gonzalez-Coloma, A.; Prigent-Combaret, C. Chapter Four—Plant metabolomics to the benefit of crop protection and growth stimulation. Adv. Bot. Res. 2021, 98, 107–132. [Google Scholar] [CrossRef]
- Kumar, H.D.; Aloke, P. Role of Biostimulant Formulations in Crop Production: An Overview. Int. J. Appl. Res. Vet. M. 2020, 8, 38–46. Available online: https://www.researchgate.net/profile/Aloke-Purkait/publication/342095340_Role_of_biostimulant_formulations_in_crop_production_An_overview/links/5ee1c473458515814a5458a5/Role-of-biostimulant-formulations-in-crop-production-An-overview.pdf (accessed on 28 October 2022).
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Physiological effects of liquid applications of a seaweed extract and a humic acid on creeping bentgrass. J. Am. Soc. Hortic. Sci. 2003, 128, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Mauromicale, G.; Ierna, A.; Marchese, M. Chlorophyll fluorescence and chlorophyll content in field-grown potato as affected by nitrogen supply, genotype, and plant age. Photosynthetica 2006, 44, 76–82. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Bojarszczuk, J. The influence of soil tillage system on changes in gas exchange parameters of Pisum sativum L. Agronomy 2021, 11, 1000. [Google Scholar] [CrossRef]
- Lawlor, D. Photosynthesis, productivity and environment. J. Exp. Bot. 1995, 46, 1449–1461. Available online: https://www.jstor.org/stable/23694991 (accessed on 10 November 2022). [CrossRef]
- Kalaji, M.H.; Łoboda, T. Fluorescencja Chlorofilu w Badaniach Stanu Fizjologicznego Roślin/Chlorophyll Fluorescence in the Study of the Physiological State of Plants, 2nd ed.; SGGW: Warsaw, Poland, 2010; p. 116. (In Polish) [Google Scholar]
- Ghaley, B.B.; Hauggaard-Nielsen, H.; Høgh-Jensen, H.; Jensen, E.S. Intercropping of wheat and pea as influenced by nitrogen fertilization. Nutr. Cycl. Agroecosys 2005, 73, 201–212. [Google Scholar] [CrossRef]
- Van Krimpen, M.M.; Leenstra, F.; Maurer, V.; Bestman, M. How to fulfill EU requirements to feed organic laying hens 100% organic ingredients. J. Appl. Poult. Res. 2016, 25, 129–138. [Google Scholar] [CrossRef]
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Review: Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12 (Suppl. 2), 295–309. [Google Scholar] [CrossRef] [Green Version]
- Witten, S.; Grashorn, M.; Aulrich, K. Precaecal digestibility of crude protein and amino acids of a field bean (Vicia faba L.) and a field pea (Pisum sativum L.) variety for broilers. Anim. Feed Sci. Technol. 2018, 243, 35–40. [Google Scholar] [CrossRef]
- Faligowska, A.; Kalembasa, S.; Kalembasa, D.; Panasiewicz, K.; Szymańska, G.; Ratajczak, K.; Skrzypczak, G. The Nitrogen Fixation and Yielding of Pea in Different Soil Tillage Systems. Agronomy 2022, 12, 352. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources 2014. Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Available online: http://www.fao.org/fileadmin/templates/nr/images/resources/pdf_documents/wrb2007_red.pdf (accessed on 29 December 2021).
- Skowera, B.; Puła, J. Extreme pluviothermal conditions in the spring period in Poland in 1971–2000. Acta Agrophys. 2004, 3, 171–177. (In Polish) [Google Scholar]
- Szpunar-Krok, E.; Kuźniar, P.; Pawlak, R.; Migut, D. The effect of foliar fertilization on the resistance of pea (Pisum sativum L.) seeds to mechanical damage. Agronomy 2021, 11, 189. [Google Scholar] [CrossRef]
- Hussein, H.A.A.; Alshammari, S.O.; Kenawy, S.K.M.; Elkady, F.M.; Badawy, A.A. Grain-priming with L-arginine improves the growth performance of wheat (Triticum aestivum L.) plants under drought stress. Plants 2022, 11, 1219. [Google Scholar] [CrossRef]
- Badawy, A.A.; Abdelfattah, N.A.H.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy assessment of biosynthesized copper oxide nanoparticles (CuO-NPs) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.). Plant. Biol. 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Ismail, M.A.; Badawy, A.A.; Awad, M.A.; Hamza, M.F.; Awad, M.F.; Fouda, A. The potency of fungal-fabricated selenium nanoparticles to improve the growth performance of Helianthus annuus L. and control of cutworm Agrotis ipsilon. Catalysts 2021, 11, 1551. [Google Scholar] [CrossRef]
- Omer, A.M.; Osman, M.S.; Badawy, A.A. Inoculation with Azospirillum brasilense and/or Pseudomonas geniculata reinforces flax (Linum usitatissimum) growth by improving physiological activities under saline soil conditions. Bot. Stud. 2022, 63, 15. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artyszak, A. Effect of silicon fertilization on crop yield quantity and quality—A literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of foliar fertilization: A review of current status and future perspectives. J. Soil Sci. Plant. Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Castilla, N.; Romero, L. Nitrogen metabolism in pepper plants applied with different bioregulators. J. Agric. Food Chem. 2000, 48, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Lisiecka, J.; Knaflewski, M.; Spizewski, T.; Fraszczak, B.; Kaluzewicz, A.; Krzesinski, W. The effect of animal protein hydrolysate on quantity and quality of strawberry daughter plants cv.‘Elsanta’. Acta Sci. Pol. Hortorum Cultus. 2011, 10, 31–40. Available online: https://czasopisma.up.lublin.pl/index.php/asphc/article/view/3173 (accessed on 10 November 2022).
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [Green Version]
- Sadak, S.H.M.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C. Biostimulant activities of two protein hydrolysates on the growth and nitrogen metabolism in maize seedlings. J. Plant. Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Baffi, C.; Colla, G. A vegetal biopolymer-based biostimulant promoted root growth in melon while triggering brassinosteroids and stress-related compounds. Front. Plant Sci. 2018, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Desoky, E.-S.M.; Elrys, A.S.; Mansour, E.; Eid, R.S.M.; Selem, E.; Rady, M.M.; Ali, E.F.; Mersal, G.A.M.; Semida, W.M. Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci. Hortic. 2021, 288, 110340. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef] [Green Version]
- Fernández, V.; Bahamonde, H.A.; Peguero-Pina, J.J.; Gil-Pelegrín, E.; Sancho-Knapik, D.; Gil, L.; Goldbach, H.E.; Eichert, T. Physico-chemical properties of plant cuticles and their functional and ecological significance. J. Exp. Bot. 2017, 68, 5293–5306. [Google Scholar] [CrossRef]
- Eichert, T.; Fernández, V. Uptake and release of mineral elements by leaves and other aerial plant parts. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Oxford, UK, 2012; pp. 71–84. [Google Scholar]
- Eichert, T.; Burkhardt, J. Quantification of stomatal uptake of ionic solutes using a new model system. J. Exp. Bot. 2001, 52, 771–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhardt, J.; Basi, S.; Pariyar, S.; Hunsche, M. Stomatal penetration by aqueous solutions–an update involving leaf surface particles. New Phytol. 2012, 196, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, P.; Menzies, N.W.; Lombi, E.; Kopittke, P.M. Effects of changes in leaf properties mediated by methyl jasmonate (MeJA) on foliar absorption of Zn, Mn and Fe. Ann. Bot. 2017, 120, 405–415. [Google Scholar] [CrossRef]
- Khayet, M.; Fernández, V. Estimation of the solubility parameters of model plant surfaces and agrochemicals: A valuable tool for understanding plant surface interactions. Theor. Biol. Med. Model. 2012, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Schönher, J. Calcium chloride penetrates plant cuticles via aqueous pores. Planta 2000, 212, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Eichert, T.; Del Río, V.; López-Casado, G.; Heredia-Guerrero, J.A.; Abadía, A.; Heredia, A.; Abadía, J. Leaf structural changes associated with iron deficiency chlorosis in field-grown pear and peach: Physiological implications. Plant Soil. 2008, 311, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
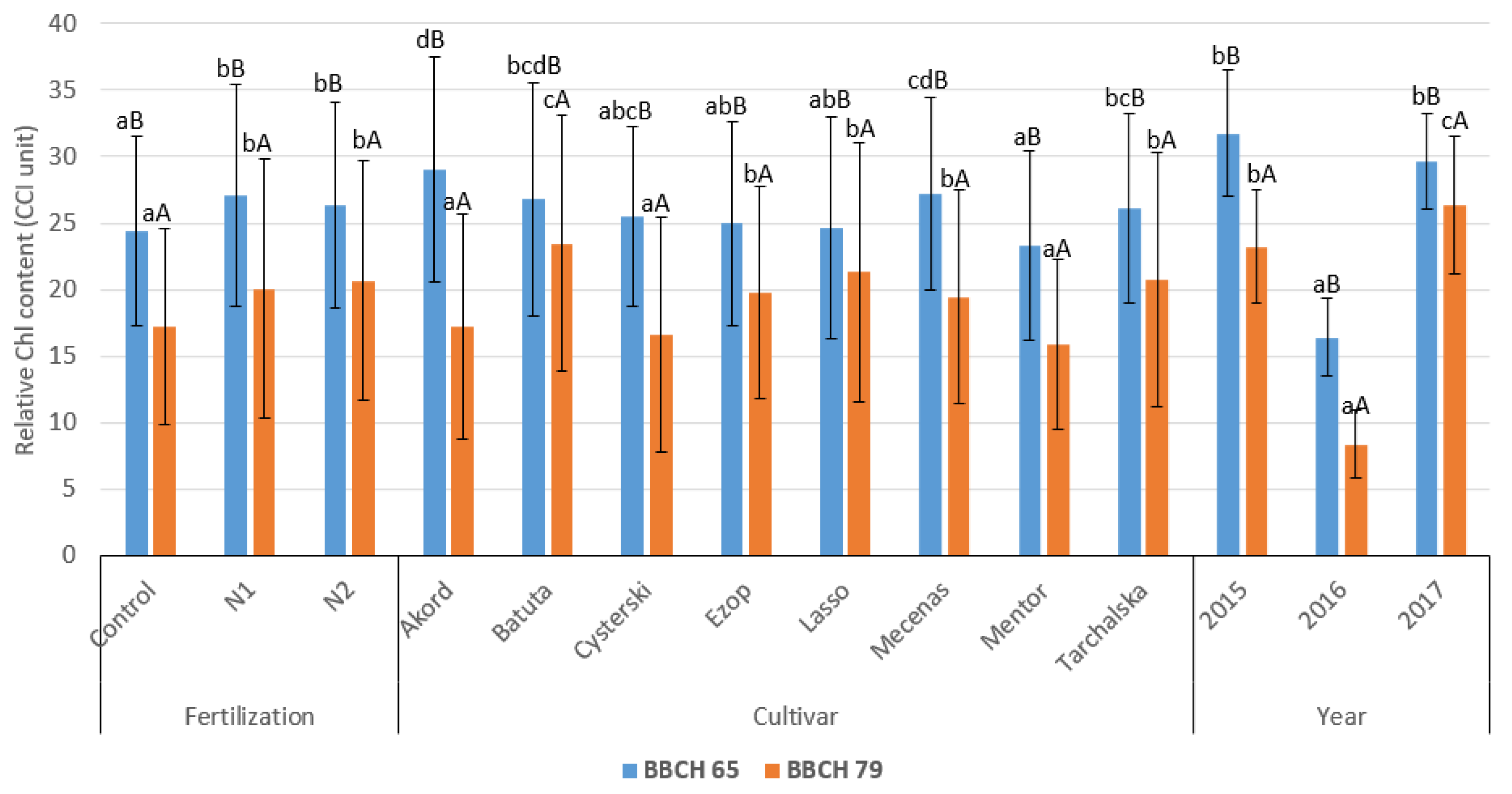
| Source of Variation | Relative Chl Content (CCI Unit) | ||
|---|---|---|---|
| BBCH 65 | BBCH 79 | ||
| Foliar fertilization | Control | 24.4 ± 7.11 aB | 17.3 ± 7.36 aA |
| N1 | 27.0 ± 8.33 bB | 20.1 ± 9.75 bA | |
| N2 | 26.4 ± 7.73 bB | 20.7 ± 9.00 bA | |
| Cultivar | Akord | 29.0 ± 8.47 dB | 17.3 ± 8.47 aA |
| Batuta | 26.8 ± 8.76 b–dB | 23.5 ± 9.62 cA | |
| Cysterski | 25.5 ± 6.76 a–cB | 16.7 ± 8.83 aA | |
| Ezop | 25.0 ± 7.66 a–cB | 19.8 ± 7.95 bA | |
| Lasso | 24.6 ± 8.34 abB | 21.3 ± 9.70 bA | |
| Mecenas | 27.2 ± 7.21 cdB | 19.5 ± 8.03 bA | |
| Mentor | 23.3 ± 7.14 aB | 15.9 ± 6.40 aA | |
| Tarchalska | 26.1 ± 7.10 bcB | 20.8 ± 9.50 bA | |
| Year | 2015 | 31.8 ± 4.71 bB | 23.2 ± 4.23 bA |
| 2016 | 16.4 ± 2.95 aB | 8.4 ± 2.60 aA | |
| 2017 | 29.6 ± 3.62 bB | 26.4 ± 5.14 cA | |
| Mean | 25.9 ± 7.79 B | 19.3 ± 8.86 A | |
| Significance | |||
| Sampling year (Y) | *** | *** | |
| Cultivar (C) | *** | *** | |
| Fertilization (F) | *** | *** | |
| Y × C | NS | *** | |
| Y × F | * | *** | |
| C × F | NS | NS | |
| Y × C × F | NS | NS | |
| Factors | Cultivar | Fv/Fm | Fv/F0 | PI | |||
|---|---|---|---|---|---|---|---|
| BBCH 65 | BBCH 79 | BBCH 65 | BBCH 79 | BBCH 65 | BBCH 79 | ||
| Foliar fertilization | Control | 0.796 ± 0.019 aB | 0.781 ± 0.021 aA | 3.96 ± 0.41 aB | 3.68 ± 0.40 aA | 5.62 ± 2.02 aB | 4.14 ± 1.33 aA |
| N1 | 0.812 ± 0.017 bB | 0.802 ± 0.021 bA | 4.37 ± 0.42 bB | 4.13 ± 0.47 bA | 6.95 ± 2.52 bB | 6.04 ± 1.97 cA | |
| N2 | 0.804 ± 0.021 abB | 0.787 ± 0.032 abA | 4.18 ± 0.48 abB | 3.83 ± 0.58 abA | 5.76 ± 2.53 aB | 5.17 ± 2.11 bA | |
| Cultivar | Akord | 0.798 ± 0.022 aB | 0.787 ± 0.028 A | 4.04 ± 0.52 aB | 3.81 ± 0.55 aA | 6.59 ± 2.76 abB | 4.85 ± 1.60 abA |
| Batuta | 0.814 ± 0.016bB | 0.797 ± 0.028 A | 4.43 ± 0.43 bB | 4.04 ± 0.56 aA | 7.04 ± 2.21 bB | 5.70 ± 1.90 bA | |
| Cysterski | 0.805 ± 0.017 aB | 0.788 ± 0.028 A | 4.19 ± 0.39 aB | 3.83 ± 0.53 aA | 5.64 ± 2.07 aB | 4.58 ± 1.90 aA | |
| Ezop | 0.802 ± 0.024 aB | 0.788 ± 0.031 A | 4.14 ± 0.57 aB | 3.87 ± 0.62 abA | 6.04 ± 3.25 aB | 5.23 ± 1.96 abA | |
| Lasso | 0.805 ± 0.022 aB | 0.793 ± 0.024 A | 4.20 ± 0.51 aB | 3.94 ± 0.46 abA | 6.05 ± 2.62 aA | 5.62 ± 2.36 bA | |
| Mecenas | 0.804 ± 0.017 aB | 0.786 ± 0.027 A | 4.18 ± 0.43 aB | 3.77 ± 0.50 abA | 5.89 ± 2.32 aB | 4.82 ± 1.82 abA | |
| Mentor | 0.801 ± 0.021 aB | 0.787 ± 0.026 A | 4.10 ± 0.44 aB | 3.80 ± 0.50 abA | 5.66 ± 1.97 aB | 4.58 ± 2.17 aA | |
| Tarchalska | 0.802 ± 0.014 aB | 0.795 ± 0.020 A | 4.09 ± 0.34 aB | 3.95 ± 0.43 bA | 5.97 ± 1.86 aA | 5.56 ± 1.89 abA | |
| Year | 2015 | 0.817 ± 0.010 bB | 0.806 ± 0.016 cA | 4.49 ± 0.29 bB | 4.22 ± 0.32 cA | 8.57 ± 1.95 cB | 5.38 ± 1.64 bA |
| 2016 | 0.788 ± 0.019 aB | 0.768 ± 0.026 aA | 3.81 ± 0.39 aB | 3.44 ± 0.44 aA | 4.66 ± 1.64 aB | 3.91 ± 1.52 aA | |
| 2017 | 0.806 ± 0.018 bB | 0.796 ± 0.021 bA | 4.22 ± 0.43 bB | 3.98 ± 0.44 bA | 5.10 ± 1.47 bA | 6.05 ± 2.13 cB | |
| Mean | 0.804 ± 0.020 B | 0.790 ± 0.027 A | 4.17 ± 0.47 B | 3.88 ± 0.52 A | 6.11 ± 2.44 B | 5.12 ± 1.99 A | |
| Significance | |||||||
| Sampling year (Y) | *** | *** | *** | *** | *** | *** | |
| Cultivar (C) | *** | NS | *** | ** | *** | *** | |
| Fertilization (F) | *** | ** | *** | ** | *** | *** | |
| Y × C | *** | NS | *** | ** | *** | NS | |
| Y × F | *** | *** | *** | *** | *** | *** | |
| C × F | NS | NS | NS | NS | NS | NS | |
| Y × C × F | NS | NS | NS | NS | NS | NS | |
| Factor | Cultivar | Pn (µmol CO2·m−2·s−1) | E (mol H2O·m−2·s−1) | gs (mol·m−2·s−1) | Ci (µmol (CO2)·mol−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| BBCH 65 | BBCH 79 | BBCH 65 | BBCH 79 | BBCH 65 | BBCH 79 | BBCH 65 | BBCH 79 | ||
| Foliar fertilization | Control | 20.2 ± 9.2 aB | 14.7 ± 2.09 aA | 4.38 ± 1.08 aB | 3.39 ± 0.70 aA | 0.570 ± 0.222 aA | 0.429 ± 0.205 aA | 236 ± 60.0 A | 255 ± 23.7 aB |
| N1 | 24.6 ± 12.3 cB | 16.4 ± 2.50 cA | 5.25 ± 1.01 cB | 3.71 ± 0.71 bA | 0.826 ± 0.268 cB | 0.497 ± 0.260 bA | 233 ± 73.3 A | 264 ± 18.6 bB | |
| N2 | 23.0 ± 11.5 bB | 15.3 ± 3.59 bA | 4.66 ± 1.15 bB | 3.38 ± 0.83 aA | 0.707 ± 0.260 bB | 0.441 ± 0.225 abA | 234 ± 68.0 A | 269 ± 22.9 bB | |
| Cultivar | Akord | 22.8 ± 11.8 B | 16.0 ± 2.54 bcA | 4.75 ± 1.44 B | 3.60 ± 0.7 bcA | 0.744 ± 0.320 B | 0.490 ± 0.201 b–dA | 237 ± 2.7 A | 268 ± 22.9 bcB |
| Batuta | 22.9 ± 11.4 B | 15.9 ± 3.61 bcA | 4.62 ± 1.14 B | 3.49 ± 0.81 a–cA | 0.703 ± 0.300 B | 0.560 ± 0.318 dA | 234 ± 66.7 A | 270 ± 18.6 cB | |
| Cysterski | 23.1 ± 11.1 B | 14.8 ± 3.02 abA | 4.68 ± 1.15 B | 3.09 ± 0.52 aA | 0.717 ± 0.299 B | 0.391 ± 0.171 a–cA | 232 ± 73.1 A | 259 ± 22.1 abB | |
| Ezop | 22.7 ± 11.2 B | 15.6 ± 2.67 a–cA | 4.78 ± 1.20 B | 3.70 ± 0.70 cA | 0.707 ± 0.268 B | 0.508 ± 0.258 cdA | 233 ± 72.9 A | 267 ± 24.6 bcB | |
| Lasso | 23.5 ± 11.9 B | 15.4 ± 2.75 a–cA | 4.88 ± 0.93 B | 3.65 ± 1.04 bcA | 0.681 ± 0.154 B | 0.443 ± 0.189 a–dA | 232 ± 68.2 A | 259 ± 22.5 abB | |
| Mecenas | 21.2 ± 10.4 B | 14.7 ± 2.95 aA | 4.79 ± 1.08 B | 3.26 ± 0.59 abA | 0.679 ± 0.257 B | 0.345 ± 0.149 aA | 239 ± 67.8 A | 254 ± 23.4 aB | |
| Mentor | 22.5 ± 11.8 B | 15.0 ± 2.27 abA | 4.88 ± 1.12 B | 3.39 ± 0.72 a–cA | 0.735 ± 0.517 B | 0.367 ± 0.146 abA | 236 ± 63.5 A | 257 ± 19.4 aB | |
| Tarchalska | 22.2 ± 10.9 B | 16.3 ± 2.96 cA | 4.73 ± 1.02 B | 3.74 ± 0.67 cA | 0.642 ± 0.242 B | 0.543 ± 0.277 dA | 231 ± 67.4 A | 268 ± 22.0 bcB | |
| Year | 2015 | 37.5 ± 6.1 bB | 16.3 ± 1.94 bA | 5.64 ± 0.89 bB | 3.58 ± 0.64 bA | 0.664 ± 0.193 bB | 0.462 ± 0.170 bA | 144 ± 26.9 aA | 243 ± 14.6 aB |
| 2016 | 14.7 ± 1.9 aB | 12.8 ± 2.10 aA | 4.26 ± 1.24 aB | 3.19 ± 0.81 aA | 0.587 ± 0.507 aB | 0.326 ± 0.144 aA | 271 ± 17.5 bA | 275 ± 17.1 bB | |
| 2017 | 15.6 ± 1.7 aA | 17.3 ± 2.35 cB | 4.39 ± 0.61 aB | 3.70 ± 0.74 bA | 0.852 ± 0.244 cB | 0.580 ± 0.284 cA | 288 ± 11.7 cB | 270 ± 20.9 bA | |
| Mean | 22.6 ± 11.2 B | 15.5 ± 2.89 A | 4.76 ± 1.13 B | 3.49 ± 0.76 A | 0.701 ± 0.271 B | 0.456 ± 0.232 A | 234 ± 7.1 A | 263 ± 22.5 B | |
| Significance | |||||||||
| Sampling year (Y) | *** | *** | *** | *** | *** | *** | *** | *** | |
| Cultivar (C) | NS | *** | NS | *** | NS | *** | NS | *** | |
| Fertilization (F) | *** | *** | *** | *** | *** | ** | NS | *** | |
| Y × C | NS | *** | ** | *** | NS | *** | NS | ** | |
| Y × F | *** | *** | *** | *** | *** | *** | *** | *** | |
| C × F | NS | NS | NS | *** | NS | NS | NS | ** | |
| Y × C × F | NS | NS | ** | ** | ** | NS | NS | *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpunar-Krok, E. Physiological Response of Pea (Pisum sativum L.) Plants to Foliar Application of Biostimulants. Agronomy 2022, 12, 3189. https://doi.org/10.3390/agronomy12123189
Szpunar-Krok E. Physiological Response of Pea (Pisum sativum L.) Plants to Foliar Application of Biostimulants. Agronomy. 2022; 12(12):3189. https://doi.org/10.3390/agronomy12123189
Chicago/Turabian StyleSzpunar-Krok, Ewa. 2022. "Physiological Response of Pea (Pisum sativum L.) Plants to Foliar Application of Biostimulants" Agronomy 12, no. 12: 3189. https://doi.org/10.3390/agronomy12123189
APA StyleSzpunar-Krok, E. (2022). Physiological Response of Pea (Pisum sativum L.) Plants to Foliar Application of Biostimulants. Agronomy, 12(12), 3189. https://doi.org/10.3390/agronomy12123189






