ACT-Toxin, the Key Effector for the Virulence of Alternaria alternata Tangerine Pathotype to Specific Citrus Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Plants
2.2. Medium or Solution
2.3. Conidiation
2.4. ACTT5 Knockout
2.5. Determination of Pathogenicity
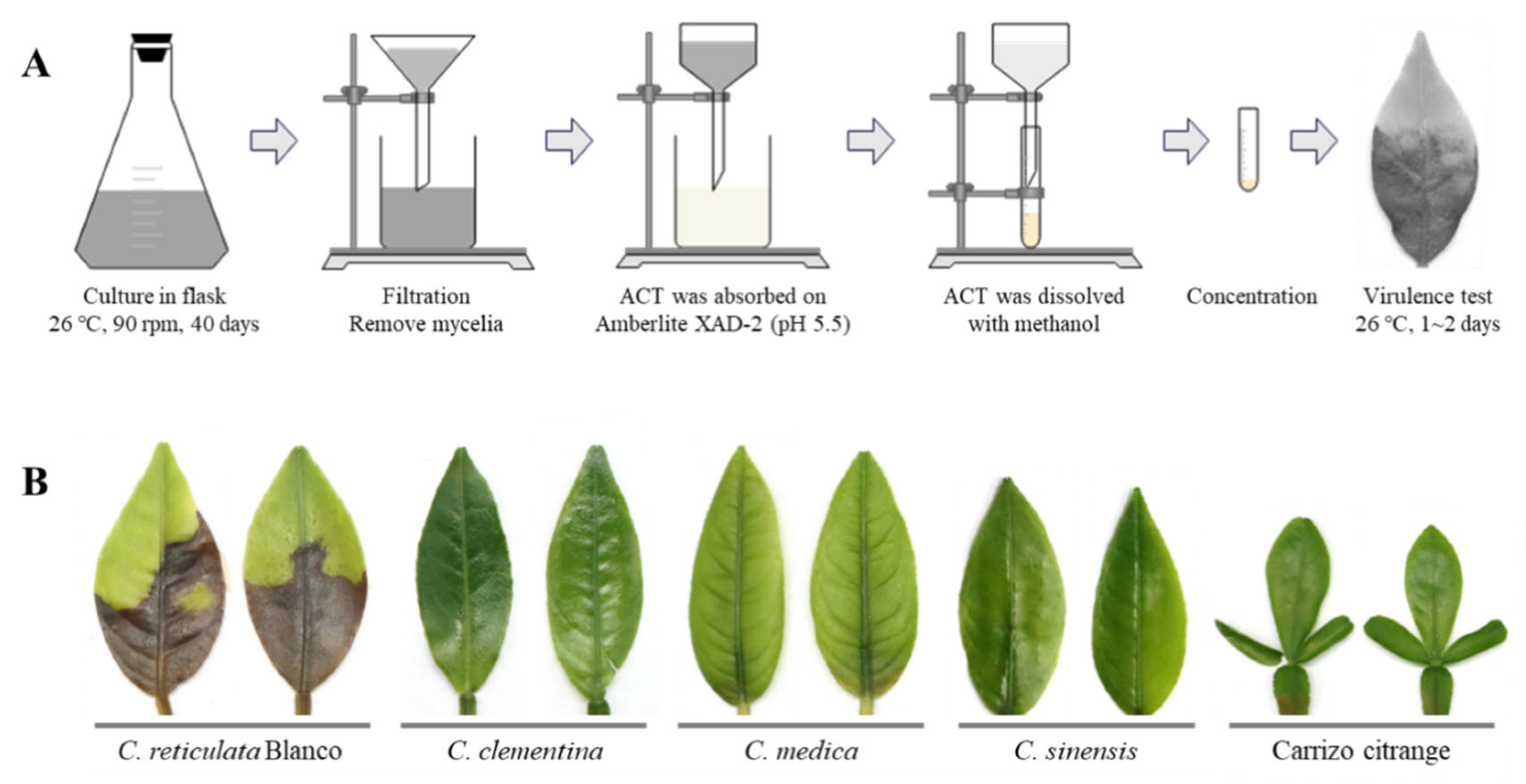
2.6. ACT-Toxin Extraction and Virulence Assay
2.7. Phenotypic Analysis
2.8. Statistical Analysis
3. Results
3.1. ACT-Toxin Biosynthesis and ROS Detoxification-Related Genes Are Involved in the Pathogenicity of A. alternata
3.2. Different Citrus Species Display Distinct Sensitivity to ACT-Toxin
3.3. ACTT5 Is Required for the Pathogenicity of A. alternata
3.4. ACTT5 Is Not Involved in Conidiation, Vegetative Growth, and Multi-Stress Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meena, M.; Gupta, S.K.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Alternaria toxins: Potential virulence factors and genes related to pathogenesis. J. Front. Microbiol. 2017, 8, 1451. [Google Scholar] [CrossRef] [PubMed]
- Kohmoto, K.; Itoh, Y.; Shimomura, N.; Shimomura, Y.; Kondoh, Y.; Otani, H.; Kodama, M.; Nishimura, S.; Nakatsuka, S. Isolation and biological activities of two host-bpecific toxins from the tangerine pathotype of Alternaria alternata. Phytopathology 1993, 83, 495–502. [Google Scholar] [CrossRef]
- Parada, R.; Sakuno, E.; Mori, N.; Oka, K.; Egusa, M.; Kodama, M.; Otani, H. Alternaria brassicae produces a host-specific protein toxin from germinating spores on host leaves. Phytopathology 2008, 98, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Kono, Y.; Takeuchi, S.; Daly, J.M. Structure of HV-toxin M, a host-specific toxin-related compound produced by Helminthosporium victoriae. Agric. Biol. Chem. 1989, 53, 1283–1290. [Google Scholar] [CrossRef]
- Holden, M.J.; Sze, H. Helminthosporium maydis T toxin increased membrane permeability to Ca2+ in susceptible corn mitochondria. Plant Physiol. 1984, 75, 235–237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishimura, S.; Kohmoto, K. Host-specific toxins and chemical structures from Alternaria species. Annu. Rev. Phytopathol. 1983, 21, 87–116. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, R.P.; Livingston, R.S. Host-selective toxins and their role in plant diseases. Science 1984, 223, 17–21. [Google Scholar] [CrossRef]
- Walton, J.D. Host-selective toxins: Agents of compatibility. Plant Cell 1996, 8, 1723. [Google Scholar]
- Wolpert, T.J.; Dunkle, L.D.; Ciuffetti, L.M. Host-selective toxins and avirulence determinants: What’s in a name? Annu. Rev. Phytopathol. 2002, 40, 251–285. [Google Scholar] [CrossRef]
- Yoder, O. Toxins in pathogenesis. Annu. Rev. Phytopathol. 1980, 18, 103–129. [Google Scholar] [CrossRef]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef]
- Thomma, B.P. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Patriarca, A.; Magan, N. Alternaria in food, Ecophysiology, mycotoxin production and toxicology. Mycobiology 2015, 43, 93–106. [Google Scholar] [CrossRef]
- Tanahashi, M.; Nakano, T.; Akamatsu, H.; Kodama, M.; Otani, H.; Osaki-Oka, K. Alternaria alternata apple pathotype (A. mali) causes black spot of European pear. Eur. J. Plant Pathol. 2016, 145, 787–795. [Google Scholar] [CrossRef]
- Tsuge, T.; Harimoto, Y.; Akimitsu, K.; Ohtani, K.; Kodama, M.; Akagi, Y.; Egusa, M.; Yamamoto, M.; Otani, H. Host-selective toxins produced by the plant pathogenic fungus Alternaria alternata. FEMS Microbiol. Rev. 2013, 37, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Shiotani, H.; Yamamoto, M.; Tsuge, T. Insertional mutagenesis and cloning of the genes required for biosynthesis of the host-specific AK-toxin in the Japanese pear pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 1999, 12, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, S.; Ueda, K.; Goto, T.; Yamamoto, M.; Nishimura, S.; Kohmoto, K. Structure of AF-toxin II, one of the host-specific toxins produced by Alternaria alternata strawberry pathotype. Tetrahedron Lett. 1986, 27, 2753–2756. [Google Scholar] [CrossRef]
- Gilchrist, D.; Grogan, R. Production and nature of a host-specific toxin from Alternaria alternata f. sp. lycopersici. Phytopathology 1976, 66, 165–171. [Google Scholar] [CrossRef]
- Izumi, Y.; Ohtani, K.; Miyamoto, Y.; Masunaka, A.; Fukumoto, T.; Gomi, K.; Tada, Y.; Ichimura, K.; Peever, T.L.; Akimitsue, K. A polyketide synthase gene, ACRTS2, is responsible for biosynthesis of host-selective ACR-toxin in the rough lemon pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 2012, 25, 1419–1429. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Akimitsu, K. Functional analysis of a multicopy host-selective ACT-toxin biosynthesis gene in the tangerine pathotype of Alternaria alternata using RNA silencing. Mol. Plant-Microbe Interact. 2008, 21, 1591–1599. [Google Scholar] [CrossRef]
- Akimitsu, K.; Tsuge, T.; Kodama, M.; Yamamoto, M.; Otani, H. Alternaria host-selective toxins: Determinant factors of plant disease. J. Gen. Plant Pathol. 2014, 80, 109–122. [Google Scholar] [CrossRef]
- Ajiro, N.; Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Izumi, Y.; et al. Role of the host-selective ACT-toxin synthesis gene ACTTS2 encoding an enoyl-reductase in pathogenicity of the tangerine pathotype of Alternaria alternata. Phytopathology 2010, 100, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Kamei, E.; Miyamoto, Y.; Ohtani, K.; Masunaka, A.; Fukumoto, T.; Gomi, K.; Tada, Y.; Ichimura, K.; Peever, T.L.; et al. Role of the pathotype-specific ACRTS1 gene encoding a hydroxylase involved in the biosynthesis of host-selective ACR-toxin in the rough lemon pathotype of Alternaria alternata. Phytopathology 2012, 102, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Ishii, Y.; Honda, A.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; et al. Function of genes encoding acyl-CoA synthetase and enoyl-CoA hydratase for host-selective ACT-toxin biosynthesis in the tangerine pathotype of Alternaria alternata. Phytopathology 2009, 99, 369–377. [Google Scholar] [CrossRef]
- Masunaka, A. Studies on genes for biosynthesis of host-selective ACT-toxin in the tangerine pathotype of Alternaria alternata. J. Gen. Plant Pathol. 2007, 73, 424–425. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Yu, D.; Xu, J.; Chung, K.; Li, H. Genomic and transcriptomic analyses of the tangerine pathotype of Alternaria alternata in response to oxidative stress. Sci. Rep. 2016, 6, 32437. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Tada, Y.; Ichimura, K.; et al. ACTTS3 encoding a polyketide synthase is essential for the biosynthesis of ACT-toxin and pathogenicity in the tangerine pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 2010, 23, 406–414. [Google Scholar] [CrossRef]
- Li, L.; Ma, H.; Zheng, F.; Chen, Y.; Wang, M.; Jiao, C.; Li, H.; Gai, Y. The transcription regulator ACTR controls ACT-toxin biosynthesis and pathogenicity in the tangerine pathotype of Alternaria alternata. Microbiol. Res. 2021, 248, 126747. [Google Scholar] [CrossRef]
- Gai, Y.; Ma, H.; Chen, Y.; Li, L.; Cao, Y.; Wang, M.; Sun, X.; Jiao, C.; Riely, B.K.; Li, H.; et al. Chromosome-scale genome sequence of Alternaria alternata causing Alternaria brown spot of citrus. Mol. Plant-Microbe Interact. 2021, 34, 726–732. [Google Scholar] [CrossRef]
- Akimitsu, K.; Kohmoto, K.; Otani, H.; Nishimura, S. Host-specific effects of toxin from the rough lemon pathotype of Alternaria alternata on mitochondria. Plant Physiol. 1989, 89, 925–931. [Google Scholar] [CrossRef]
- Yamagishi, D.; Akamatsu, H.; Otani, H.; Kodama, M. Pathological evaluation of host-specific AAL-toxins and fumonisin mycotoxins produced by Alternaria and Fusarium species. J. Gen. Plant Pathol. 2006, 72, 323–327. [Google Scholar] [CrossRef]
- Yang, S.L.; Chung, K.R. Similar and distinct roles of NADPH oxidase components in the tangerine pathotype of Alternaria alternata. Mol. Plant Pathol. 2013, 14, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.R. Reactive oxygen species in the citrus fungal pathogen Alternaria alternata: The roles of NADPH-dependent oxidase. Physiol. Mol. Plant Pathol. 2014, 88, 10–17. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.L.; Wang, N.Y.; Chung, K.R. The FUS3 MAPK signaling pathway of the citrus pathogen Alternaria alternata functions independently or cooperatively with the fungal redox-responsive AP1 regulator for diverse developmental, physiological and pathogenic processes. Fungal Genet. Biol. 2010, 47, 381–391. [Google Scholar] [CrossRef]
- Lin, C.H.; Chung, K.R. Specialized and shared functions of the histidine kinase-and HOG1 MAP kinase-mediated signaling pathways in Alternaria alternata, a filamentous fungal pathogen of citrus. Fungal Genet. Biol. 2010, 47, 818–827. [Google Scholar] [CrossRef]
- Chen, L.H.; Lin, C.H.; Chung, K.R. Roles for SKN7 response regulator in stress resistance, conidiation and virulence in the citrus pathogen Alternaria alternata. Fungal Genet. Biol. 2012, 49, 802–813. [Google Scholar] [CrossRef]
- Ma, H.; Wang, M.; Gai, Y.; Fu, H.; Zhang, B.; Ruan, R.; Chung, K.-R.; Li, H. Thioredoxin and glutaredoxin systems required for oxidative stress resistance, fungicide sensitivity, and virulence of Alternaria alternata. Appl. Environ. Microbiol. 2018, 84, e00086-18. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, B.; Gai, Y.; Sun, X.; Chung, K.R.; Li, H. Cell-wall-degrading enzymes required for virulence in the host selective toxin-producing necrotroph Alternaria alternata of Citrus. Front. Microbiol. 2019, 10, 2514. [Google Scholar] [CrossRef]
- Fu, H.; Chung, K.R.; Gai, Y.; Mao, L.; Li, H. The basal transcription factor II H subunit Tfb5 is required for stress response and pathogenicity in the tangerine pathotype of Alternaria alternata. Mol. Plant Pathol. 2020, 21, 1337–1352. [Google Scholar] [CrossRef]
- Huang, F.; Fu, Y.; Nie, D.; Stewart, J.E.; Peever, T.L.; Li, H. Identification of a novel phylogenetic lineage of Alternaria alternata causing citrus brown spot in China. Fungal Biol. 2015, 119, 320–330. [Google Scholar] [CrossRef]
- Chung, K.-R.; Shilts, T.; Li, W.; Timmer, L.W. Engineering a genetic transformation system for Colletotrichum acutatum, the causal fungus of lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol. Lett. 2002, 213, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kohmoto, K.; Scheffer, R.P.; Whiteside, J.O. Host-selective toxins from Alternaria citri. Phytopathology 1979, 69, 667–671. [Google Scholar] [CrossRef]
- Masunaka, A.; Tanaka, A.; Tsuge, T.; Peever, T.L.; Timmer, L.W.; Yamamoto, M.; Yamamoto, H.; Akimitsu, K. Distribution and characterization of AKT homologs in the tangerine pathotype of Alternaria alternata. Phytopathology 2000, 90, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Ueno, T.; Fukami, H.; Taga, T.; Masuda, H.; Osaki, K.; Otani, H.; Kohmoto, K.; Nishimura, S. Isolation and structures of AK-Toxin I and II, host-specific phytotoxic metabolites produced by Alternaria alternata Japanese pear pathotype. Agric. Biol. Chem. 1985, 49, 807–815. [Google Scholar] [CrossRef]
- Kohmoto, K.; Otani, H.; Kodama, M.; Nishimura, S. Host recognition: Can accessibility to fungal invasion be induced by host-specific toxins without necessitating necrotic cell death? Phytotoxins Plant Pathog. 1989, 27, 249–265. [Google Scholar]
- Gardner, J.; Mansour, I.S.; Scheffer, R. Effects of the host-specific toxin of Periconia circinata on some properties of sorghum plasma membranes. Physiol. Plant Pathol. 1972, 2, 197–206. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Zhu, Z.; Wang, X. Expression of Ht2-related genes in response to the HT-Toxin of Exserohilum turcicum in Maize. Ann. Appl. Biol. 2010, 156, 111–120. [Google Scholar] [CrossRef]
- Okuno, T.; Ishita, Y.; Sawai, K.; Matsumoto, T. Characterization of alternariolide, a host-specific toxin produced by Alternaria mali roberts. Chem. Lett. 1974, 3, 635–638. [Google Scholar] [CrossRef]
- Akimitsu, K.; Peever, T.L.; Timmer, L.W. Molecular, ecological and evolutionary approaches to understanding Alternaria diseases of citrus. Mol. Plant Pathol. 2003, 4, 435–446. [Google Scholar] [CrossRef]
- Schröter, H.; Novacky, A.; Macko, V. Effect of Helminthosporium sacchari-toxin on cell membrane potential of susceptible sugarcane. Physiol. Plant Pathol. 1985, 26, 165–174. [Google Scholar] [CrossRef]
- Kohmoto, K.; Akimitsu, K.; Otani, H. Correlation of resistance and susceptibility of citrus to Alternaria alternata with sensitivity to host-specific toxins. Phytopathology 1991, 81, 719–722. [Google Scholar] [CrossRef]
- Maekawa, N.; Yamamoto, M.; Nishimura, S.; Kohmoto, K.; Kuwada, M.; Watanabe, Y. Studies on host-specific AF-toxins produced by Alternaria alternata strawberry pathotype causing Alternaria black spot of strawberry (1) production of host-specific toxins and their biological activities. Jpn. J. Phytopathol. 1984, 50, 600–609. [Google Scholar] [CrossRef]
- Slavov, S.; Mayama, S.; Atanassov, A. Toxin production of Alternaria alternata tobacco pathotype. Biotechnol. Biotechnol. Equip. 2004, 18, 90–95. [Google Scholar] [CrossRef]
- Walton, J.D.; Earle, E.D.; Gibson, B.W. Purification and structure of the host-specific toxin from Helminthosporium carbonum race 1. Biochem. Biophys. Res. Commun. 1982, 107, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Ciuffetti, L.M.; Manning, V.A.; Pandelova, I.; Betts, M.F.; Martinez, J.P. Host-selective toxins, Ptr ToxA and Ptr ToxB, as necrotrophic effectors in the Pyrenophora tritici-repentis–wheat interaction. New Phytol. 2010, 187, 911–919. [Google Scholar] [CrossRef]
- Liakopoulou-Kyriakides, M.; Lagopodi, A.; Thanassoulopoulos, C.; Stavropoulos, G.S.; Magafa, V. Isolation and synthesis of a host-selective toxin produced by Alternaria alternata. Phytochemistry 1997, 45, 37–40. [Google Scholar] [CrossRef]
- Bobylev, M.M.; Bobyleva, L.I.; Strobel, G.A. Synthesis and bioactivity of analogs of maculosin, a host-specific phytotoxin produced by Alternaria alternata on spotted knapweed (Centaurea maculosa). J. Agric. Food Chem. 1996, 44, 3960–3964. [Google Scholar] [CrossRef]
- Bottini, A.T.; Gilchrist, D.G. Phytotoxins. I. A 1-aminodimethylheptadecapentol from Alternaria alternata f. sp. lycopersici. Tetrahedron Lett. 1981, 22, 2719–2722. [Google Scholar] [CrossRef]
- Johnson, R.; Johnson, L.; Itoh, Y.; Kodama, M.; Otani, H.; Kohmoto, K. Cloning and characterization of a cyclic peptide synthetase gene from Alternaria alternata apple pathotype whose product is involved in AM-toxin synthesis and pathogenicity. Mol. Plant-Microbe Interact. 2000, 13, 742–753. [Google Scholar] [CrossRef]
- Yakimova, E.T.; Yordanova, Z.P.; Slavov, S.; Kapchina-Toteva, V.M.; Woltering, E.J. Alternaria alternata AT toxin induces programmed cell death in tobacco. J. Phytopathol. 2009, 157, 592–601. [Google Scholar] [CrossRef]
- Stierle, A.C.; Cardellina, J.H.; Strobel, G.A. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8011. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.L.; Chung, K.R. The YAP1 homolog–mediated oxidative stress tolerance is crucial for pathogenicity of the necrotrophic fungus Alternaria alternata in citrus. Mol. Plant-Microbe Interact. 2009, 22, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Yu, P.L.; Chung, K.R. The glutathione peroxidase-mediated reactive oxygen species resistance, fungicide sensitivity and cell wall construction in the citrus fungal pathogen Alternaria alternata. Environ. Microbiol. 2016, 18, 923–935. [Google Scholar] [CrossRef]
- Gai, Y.; Liu, B.; Ma, H.; Li, L.; Chen, X.; Moenga, S.; Riely, B.; Fayyaz, A.; Wang, M.; Li, H. The methionine biosynthesis regulator AaMetR contributes to oxidative stress tolerance and virulence in Alternaria alternata. Microbiol. Res. 2019, 219, 94–109. [Google Scholar] [CrossRef]
- Yu, P.L.; Chen, L.H.; Chung, K.R. How the pathogenic fungus Alternaria alternata copes with stress via the response regulators SSK1 and SHO1. PLoS ONE 2016, 11, e0149153. [Google Scholar] [CrossRef]
- Wu, P.C.; Choo, C.Y.L.; Lu, H.Y.; Wei, X.Y.; Chen, Y.K.; Yago, J.I.; Chung, K.R. Pexophagy is critical for fungal development, stress response, and virulence in Alternaria alternata. Mol. Plant Pathol. 2022, 23, 1538–1554. [Google Scholar] [CrossRef]
- Chung, K.R.; Wu, P.C.; Chen, Y.K.; Yago, J.I. The siderophore repressor SreA maintains growth, hydrogen peroxide resistance, and cell wall integrity in the phytopathogenic fungus Alternaria alternata. Fungal Genet. Biol. 2020, 139, 103384. [Google Scholar] [CrossRef]
- Chung, K.-R. Stress response and pathogenicity of the necrotrophic fungal pathogen Alternaria alternata. Scientifica 2012, 2012, 635431. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.L.; Chung, K.R. Cellular responses required for oxidative stress tolerance, colonization, and lesion formation by the necrotrophic fungus Alternaria alternata in citrus. Curr. Microbiol. 2011, 62, 807–815. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Luo, Z.; Gao, L.; Li, R.; Zhang, Y.; Kalaji, H.M.; Qiang, S.; Chen, S. Recent advances in Alternaria phytotoxins: A review of their occurrence, structure, bioactivity, and biosynthesis. J. Fungi 2022, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.; Kono, Y.; Chandler, J. Bioassay and host-selectivity of Alternaria citri toxins affecting rough lemon and mandarins. Physiol. Mol. Plant Pathol. 1986, 29, 293–304. [Google Scholar] [CrossRef]
- Solel, Z.; Kimchi, M. Susceptibility and resistance of citrus genotypes to Alternaria alternata pv. citri. J. Phytopathol. 1997, 145, 389–391. [Google Scholar] [CrossRef]
- Peever, T.L.; Olsen, L.; Ibañez, A.; Timmer, L.W. Genetic differentiation and host specificity among populations of Alternaria spp. causing brown spot of grapefruit and tangerine × grapefruit hybrids in florida. Phytopathology 2000, 90, 407–414. [Google Scholar] [CrossRef]
- Vicent, A.; Badal, J.; Asensi, M.J.; Sanz, N.; Armengol, J.; García-Jiménez, J. Laboratory evaluation of citrus cultivars susceptibility and influence of fruit size on fortune mandarin to infection by Alternaria alternata pv. citri. Eur. J. Plant Pathol. 2004, 110, 245–251. [Google Scholar] [CrossRef]


| No | Pathogen | Toxin | Host | Reference |
|---|---|---|---|---|
| 1 | A. alternata Japanese pear pathotype | AK | Pear | [44,45] |
| 2 | Helminthosporium victoriae | HV | Oat | [4] |
| 3 | Periconia circinata | PC | Sorghum | [46] |
| 4 | Helminthosporium turcicum | HT | Maize | [47] |
| 5 | A. alternata apple pathotype | AM | Apple | [48,49] |
| 6 | A. alternata tangerine pathotype | ACT | Citrus | [2,49] |
| 7 | Helminthosporium maydis | HMT | Maize | [5] |
| 8 | Helminthosporium sacchari | HS | Sugarcane | [50] |
| 9 | A. alternata tomato pathotype | AAL | Tomato | [18] |
| 10 | A. alternata rough lemon pathotype | ACR | Rough lemon | [42,51] |
| 11 | A. alternata strawberry pathotype | AF | Strawberry | [52] |
| 12 | A. alternata tobacco pathotype | AT | Tobacco | [53] |
| 13 | Helminthosporium carbonum | HC | Maize | [54] |
| 14 | Pyrenophora tritici-repentis | PTR | Wheat | [55] |
| 15 | A. alternata sunflower pathotype | AS-I | Sunflower | [56] |
| 16 | A. alternata spotted knapweed pathotype | Maculosin | Knapweed | [57] |
| 17 | A. brassicae | ABR | Brassica spp. | [3] |
| No | Pathotype | HSTs | Disease | Chemical Characteristics | Reference |
|---|---|---|---|---|---|
| 1 | Tomato pathotype | AAL | Alternaria stem canker of tomato | Aminopentol esters | [1,18,58] |
| 2 | Tangerine pathotype | ACT | Citrus brown spot | Epoxy-decatrienoic esters | [2] |
| 3 | Rough lemon pathotype | ACR | Leaf spot of rough lemon | Terpenoid | [19] |
| 4 | Strawberry pathotype | AF | Black spot of strawberry | Epoxy-decatrienoic esters | [17] |
| 5 | Japanese pear pathotype | AK | Black spot of Japanese pear | Epoxy-decatrienoic esters | [16] |
| 6 | Apple pathotype | AM | Alternaria blotch of apple | Cyclic peptide | [59] |
| 7 | Sunflower pathotype | AS-I | Leaf spot of sunflower | Tetrapeptide | [56] |
| 8 | Tobacco pathotype | AT | Brown spot of tobacco | --- | [60] |
| 9 | Spotted knapweed pathotype | Maculosin | Black leaf blight of knapweed | Tetrapeptide | [61] |
| No | Gene | Name | Copy Number | Accession Number | Reference |
|---|---|---|---|---|---|
| 1 | ACTT2 | Hydrolase | ≥2 | AALT_g11743 | [20] |
| 2 | ACTTS2 | Enoyl-reductase | ≥2 | AALT_g12031 | [22] |
| 3 | ACTT3 | HMG-CoA hydrolase | ≥2 | AALT_g11755 | [25] |
| 4 | ACTTS3 | Polyketide synthase | ≥3 | AALT_g11750 | [27] |
| 5 | ACTT5 | Acyl-CoA synthetase | ≥3 | AALT_g11751 | [24] |
| 6 | ACTT6 | Enoyl-CoA hydratase | 2 | AALT_g12047 | [24] |
| 7 | ACTTR | Zn(II)2Cys6 transcription factor | ≥2 | AALT_g11754 | [28] |
| No | Gene | Vegetative Growth | Conidiation | Pathogenicity | Accession Number | Reference |
|---|---|---|---|---|---|---|
| 1 | Hog1 | Required | Required | Required | GQ414509 | [35] |
| 2 | Skn7 | Required | Required | Required | JQ716919 | [36] |
| 3 | Ap1 | Required | --- | Required | FJ376607 | [62] |
| 4 | Gpx3 | Required | Required | Required | ACY73852 | [63] |
| 5 | Tsa1 | Not required | Not required | Required | MG593564 | [37] |
| 6 | Trr1 | Required | Required | Required | MG593563 | [37] |
| 7 | Glr1 | Required | Required | Required | MG593559 | [37] |
| 8 | NoxA | Required | Required | Required | JN900389 | [32] |
| 9 | NoxB | Required | Required | Required | JX136700 | [32] |
| 10 | NoxR | Required | Required | Required | JX207117 | [32] |
| 11 | MetR | Required | Required | Required | Aa03030 | [64] |
| 12 | SSK1 | Required | Required | Required | KU170060 | [65] |
| 13 | Tbf1 | Required | Required | Required | MT184174 | [39] |
| 14 | Atg8 | Required | Required | Required | OK617334 | [66] |
| 15 | SreA | Required | Required | Required | OWY49902.1 | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Jia, Z.; Li, H.; Zhang, S.; Shen, J.; Gai, Y.; Jiao, C.; Sun, X.; Duan, S.; Wang, M.; et al. ACT-Toxin, the Key Effector for the Virulence of Alternaria alternata Tangerine Pathotype to Specific Citrus Species. Agronomy 2022, 12, 3181. https://doi.org/10.3390/agronomy12123181
Huang S, Jia Z, Li H, Zhang S, Shen J, Gai Y, Jiao C, Sun X, Duan S, Wang M, et al. ACT-Toxin, the Key Effector for the Virulence of Alternaria alternata Tangerine Pathotype to Specific Citrus Species. Agronomy. 2022; 12(12):3181. https://doi.org/10.3390/agronomy12123181
Chicago/Turabian StyleHuang, Suya, Zhaohui Jia, Hangfei Li, Shuting Zhang, Junying Shen, Yunpeng Gai, Chen Jiao, Xuepeng Sun, Shuo Duan, Min Wang, and et al. 2022. "ACT-Toxin, the Key Effector for the Virulence of Alternaria alternata Tangerine Pathotype to Specific Citrus Species" Agronomy 12, no. 12: 3181. https://doi.org/10.3390/agronomy12123181
APA StyleHuang, S., Jia, Z., Li, H., Zhang, S., Shen, J., Gai, Y., Jiao, C., Sun, X., Duan, S., Wang, M., & Ma, H. (2022). ACT-Toxin, the Key Effector for the Virulence of Alternaria alternata Tangerine Pathotype to Specific Citrus Species. Agronomy, 12(12), 3181. https://doi.org/10.3390/agronomy12123181







