Evaluation of Wood Vinegar as an Herbicide for Weed Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wood Vinegar
2.2. Impact of Wood Vinegar Concentrations on Seed Germination
2.3. Impact of Wood Vinegar Concentrations on Newly Germinated Seedlings
2.4. Impact of Wood Vinegar Application Rate on Motherwort, Spanish Needles, and Tall Fescue
2.5. Impact of Light and Wood Vinegar Concentrations on Common Purslane Seed Germination
2.6. Impact of Light Condition and Wood Vinegar Application Rate on Common Purslane
3. Results
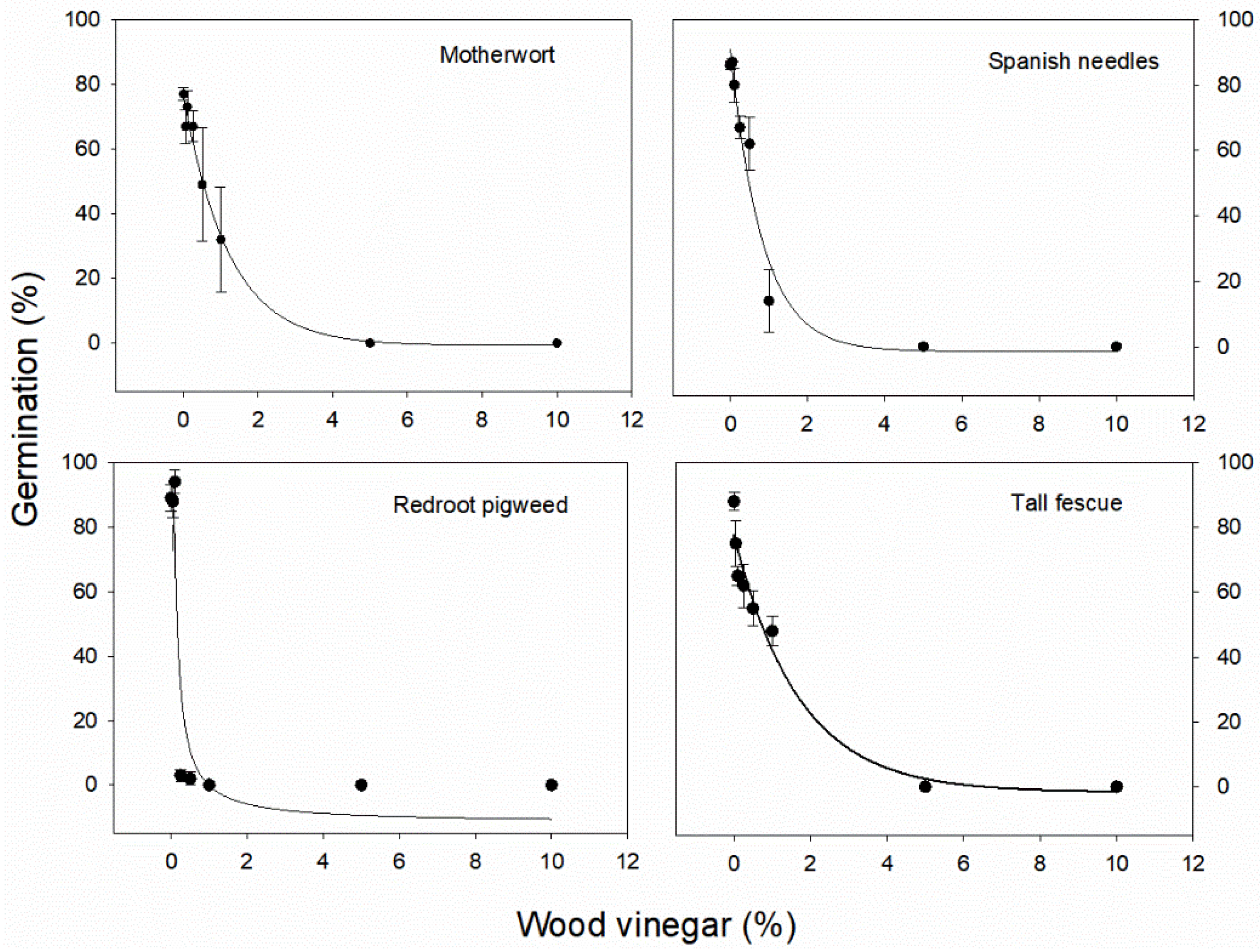
3.1. Impact of Wood Vinegar Concentrations on Weed Seed Germination
3.2. Impact of Wood Vinegar Concentrations on Newly Emerged Seedlings

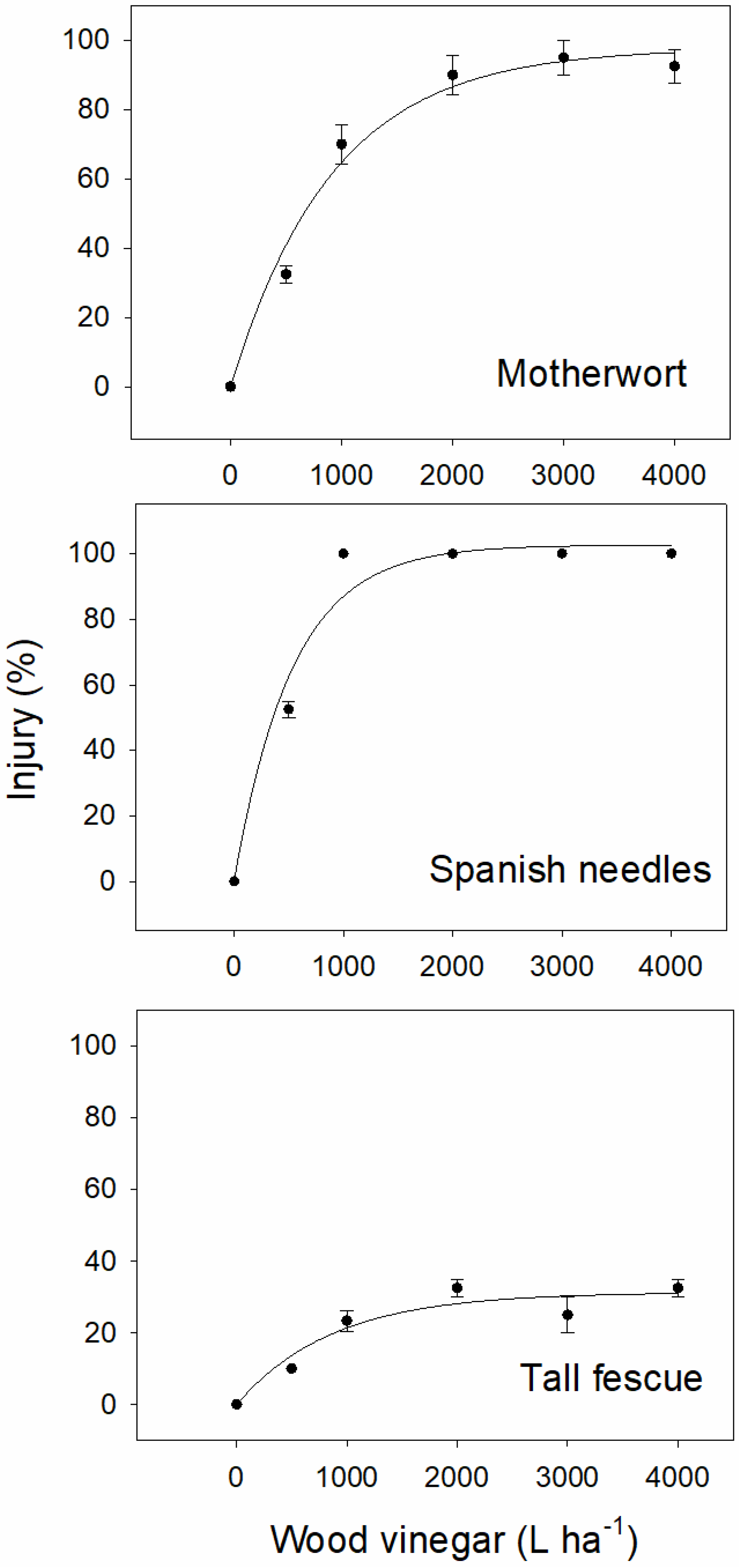
3.3. Impact of Wood Vinegar Application Rate on Motherwort, Spanish Needles, and Tall Fescue
3.4. Impact of Light and Wood Vinegar Concentrations on Common Purslane Seed Germination
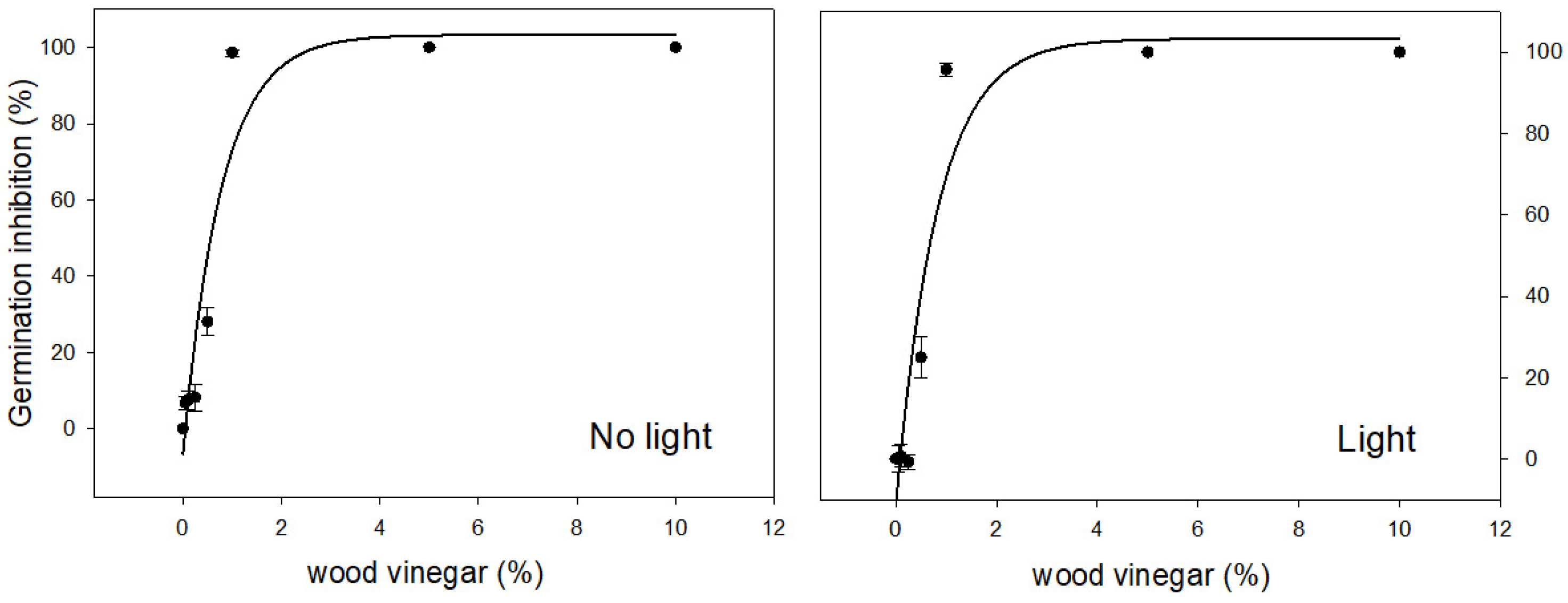
3.5. Impact of Light Condition and Wood Vinegar Application Rate for Control of Common Purslane
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Dar, M.A.; Kaushik, G.; Villareal Chiu, J.F. Pollution status and biodegradation of organophosphate pesticides in the environment. In Abatement of Environmental Pollutants; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Oxford, UK, 2019; pp. 25–66. [Google Scholar]
- Qi, Y.; Donahoe, R.J. The environmental fate of arsenic in surface soil contaminated by historical herbicide application. Sci. Total Environ. 2008, 405, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. Arsenic in Groundwater and the Environment. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2013; pp. 279–310. [Google Scholar]
- USDA-ARS. Guidance Materials for Organic Crop Production. Available online: https://www.ams.usda.gov/sites/default/files/media/NOP-5034-1. (accessed on 2 November 2022).
- Wang, L.; Gao, S.; Yin, X.; Yu, X.; Luan, L. Arsenic accumulation, distribution and source analysis of rice in a typical growing area in north China. Ecotoxicol. Environ. Saf. 2019, 167, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wei, C.; Yang, L.J.M.J. Occurrence of arsenic in two large shallow freshwater lakes in China and a comparison to other lakes around the world. Microchem. J. 2013, 110, 169–177. [Google Scholar] [CrossRef]
- Yu, J.; McCullough, P.E. Efficacy and fate of atrazine and simazine in doveweed (Murdannia nudiflora). Weed Sci. 2016, 64, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Gammon, D.W.; Aldous, C.N.; Carr, W.C., Jr.; Sanborn, J.R.; Pfeifer, K.F. A risk assessment of atrazine use in California: Human health and ecological aspects. Pest Manag. Sci. 2005, 61, 331–355. [Google Scholar] [CrossRef]
- Solomon, K.R.; Baker, D.B.; Richards, R.P.; Dixon, K.R.; Klaine, S.J.; La Point, T.W.; Kendall, R.J.; Weisskopf, C.P.; Giddings, J.M.; Giesy, J.P. Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem. 1996, 15, 31–76. [Google Scholar] [CrossRef]
- Wang, A.; Hu, X.; Wan, Y.; Mahai, G.; Jiang, Y.; Huo, W.; Zhao, X.; Liang, G.; He, Z.; Xia, W.; et al. A nationwide study of the occurrence and distribution of atrazine and its degradates in tap water and groundwater in China: Assessment of human exposure potential. Chemosphere 2020, 252, 126533. [Google Scholar] [CrossRef]
- Sass, J.B.; Colangelo, A. European Union bans atrazine, while the United States negotiates continued use. Int. J. Occup. Med. Environ. Health 2006, 12, 260–267. [Google Scholar] [CrossRef]
- USEPA. Atrazine. Available online: https://www.epa.gov/ingredients-used-pesticide-products/atrazine (accessed on 30 June 2022).
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Vencill, W.K.; Nichols, R.L.; Webster, T.M.; Soteres, J.K.; Mallory-Smith, C.; Burgos, N.R.; Johnson, W.G.; McClelland, M.R. Herbicide resistance: Toward an understanding of resistance development and the impact of herbicide-resistant crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; McCullough, P.E.; Czarnota, M.A. Physiological basis for tall fescue (Festuca arundinacea) tolerance to florasulam. Weed Technol. 2018, 32, 353–359. [Google Scholar] [CrossRef]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef]
- Beckie, H.J.; Ashworth, M.B.; Flower, K.C. Herbicide resistance management: Recent developments and trends. Plants 2019, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Schmölzer, K.; Schachtschabel, D.; Speitling, M.; Nidetzky, B. Selective β-mono-glycosylation of a C15-hydroxylated metabolite of the agricultural herbicide cinmethylin using Leloir glycosyltransferases. J. Agric. Food Chem. 2021, 69, 5491–5499. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef] [Green Version]
- Abouziena, H.F.; Omar, A.A.; Sharma, S.D.; Singh, M. Efficacy comparison of some new natural-product herbicides for weed control at two growth stages. Weed Technol. 2009, 23, 431–437. [Google Scholar] [CrossRef]
- Webber, C.L., III; White, P.M., Jr.; Shrefler, J.W.; Spaunhorst, D.J. Impact of acetic acid concentration, application volume, and adjuvants on weed control efficacy. J. Agric. Sci. 2018, 10, 8. [Google Scholar] [CrossRef]
- Domenghini, J.C. Comparison of acetic acid to glyphosate for weed suppression in the garden. HortTechnology 2020, 30, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Ivany, J.A. Acetic acid for weed control in potato (Solanum tuberosum L.). Can. J. Plant Sci. 2010, 90, 537–542. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Deciduous Fruit Production in China. Available online: http://www.fao.org/3/ab985e/ab985e06.htm (accessed on 10 November 2022).
- Yang, X.; Kang, K.; Qiu, L.; Zhao, L.; Sun, R. Effects of carbonization conditions on the yield and fixed carbon content of biochar from pruned apple tree branches. Renew. Energ. 2020, 146, 1691–1699. [Google Scholar] [CrossRef]
- Duarte, d.; Silva, M.J.; Bezerra, B.S.; Gomes Battistelle, R.A.; De Domenico Valarelli, I. Prospects for the use of municipal tree pruning wastes in particleboard production. Waste Manag. Res. 2013, 31, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, Y.; Wang, Q.; Zhu, A.; Liu, Z.; Wang, Y.; Allen, D.T.; Li, L. Assessment of the effects of straw burning bans in China: Emissions, air quality, and health impacts. Sci. Total Environ. 2021, 789, 147935. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Liu, Y.; Zhang, L.; Hao, L.; Gao, Z. Burning in agricultural landscapes: An emerging natural and human issue in China. Landsc. Ecol. 2014, 29, 1785–1798. [Google Scholar] [CrossRef]
- Teutscherova, N.; Vazquez, E.; Santana, D.; Navas, M.; Masaguer, A.; Benito, M. Influence of pruning waste compost maturity and biochar on carbon dynamics in acid soil: Incubation study. Eur. J. Soil Biol. 2017, 78, 66–74. [Google Scholar] [CrossRef]
- Sahin, H.T.; Arslan, M.B. Properties of orchard pruning and suitability for composite production. Sci. Eng. Compos. Mater. 2013, 20, 337–342. [Google Scholar] [CrossRef]
- Sagani, A.; Hagidimitriou, M.; Dedoussis, V. Perennial tree pruning biomass waste exploitation for electricity generation: The perspective of Greece. Sustain. Energy Technol. Assess. 2019, 31, 77–85. [Google Scholar] [CrossRef]
- Aguirre, J.L.; Baena, J.; Martín, M.; González, S.; Manjón, J.; Peinado, M. Herbicidal effects of wood vinegar on nitrophilous plant communities. Food Energy Secur. 2020, 9, e253. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Feng, X.; Yu, J. Wood vinegar resulting from the pyrolysis of apple tree branches for annual bluegrass control. Ind. Crops Prod. 2021, 174, 114193. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Y.; Li, X.; Li, Y.; Feng, X.; Bagavathiannan, M.; Zhang, C.; Qu, M.; Yu, J. The use of wood vinegar as a non-synthetic herbicide for control of broadleaf weeds. Ind. Crops Prod. 2021, 173, 114105. [Google Scholar] [CrossRef]
- Benzon, H.R.L.; Lee, S.C. Potential of wood vinegar in enhancing fruit yield and antioxidant capacity in tomato. Korean J. Food Preserv. 2016, 29, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Ahn, B.J.; Cho, S.T. Antimicrobial activities of wood vinegar and application as natural fungicides and food preservatives. J. Korean Wood Sci. Technol. 2010, 38, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Fedeli, R.; Vannini, A.; Celletti, S.; Maresca, V.; Munzi, S.; Cruz, C.; Alexandrov, D.; Guarnieri, M.; Loppi, S. Foliar application of wood distillate boosts plant yield and nutritional parameters of chickpea. Ann. Appl. Biol. 2022, in press. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-Based Solutions for Agriculture: Foliar application of wood distillate alone and in combination with other plant-derived corroborants results in different effects on lettuce. Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Hao, Z.; Bagavathiannan, M.; Li, Y.; Qu, M.; Wang, Z.; Yu, J. Wood vinegar for control of broadleaf weeds in dormant turfgrass. Weed Technol. 2021, 35, 901–907. [Google Scholar] [CrossRef]
- Anonymous. Minstry of Agriculture and Rural Affairs of the People’s Republic of China. 2020. Available online: http://www.moa.gov.cn/govpublic/ZZYGLS/202002/t20200212_6336983.htm (accessed on 2 December 2022).
- Tiilikkala, K.; Fagernäs, L.; Tiilikkala, J. History and use of wood pyrolysis liquids as biocide and plant protection product. Open Agric. J. 2010, 4, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Varanasi, A.; Prasad, P.V.; Jugulam, M. Impact of climate change factors on weeds and herbicide efficacy. Adv. Agron. 2016, 135, 107–146. [Google Scholar]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. Reactive oxygen species trigger the fast action of glufosinate. Planta 2019, 249, 1837–1849. [Google Scholar] [CrossRef]
- Petersen, J.; Hurle, K. Influence of climatic conditions and plant physiology on glufosinate-ammonium efficacy. Weed Res. 2008, 41, 31–39. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Fedeli, R.; Fiaschi, T.; de Simone, L.; Vannini, A.; Angiolini, C.; Loppi, S.; Maccherini, S. Effects of wood distillate on seedling emergence and first-stage growth in five threatened arable plants. Diversity 2022, 14, 669. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Zhang, C.; Huang, H.; Wei, S.; Chen, J.; Wang, X. Molecular basis of resistance to imazethapyr in redroot pigweed (Amaranthus retroflexus L.) populations from China. Pestic. Biochem. Physiol. 2015, 124, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.; Letarte, J.; Grainger, C.M.; Rajcan, I.; Tardif, F.J. Widespread herbicide resistance in pigweed species in Ontario carrot production is due to multiple photosystem II mutations. Can. J. Plant Sci. 2019, 100, 56–67. [Google Scholar] [CrossRef]
- Ferguson, G.M.; Hamill, A.S.; Tardif, F.J. ALS inhibitor resistance in populations of Powell amaranth and redroot pigweed. Weed Sci. 2001, 49, 448–453. [Google Scholar] [CrossRef]
- McNaughton, K.E.; Letarte, J.; Lee, E.A.; Tardif, F.J. Mutations in ALS confer herbicide resistance in redroot pigweed (Amaranthus retroflexus) and Powell amaranth (Amaranthus powellii). Weed Sci. 2005, 53, 17–22. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Zhang, L.; Zhao, K.; Ge, L.a.; Lv, X.-C.; Liu, W.; Wang, J. Multiple resistance to thifensulfuron-methyl and fomesafen in redroot pigweed ( Amaranthus retroflexus L.) from China. J. Agric. Res. 2017, 77, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wei, S.; Huang, H.; Li, W.; Zhang, C.; Huang, Z. Target-site mutation and enhanced metabolism confer resistance to thifensulfuron-methyl in a multiple-resistant redroot pigweed (Amaranthus retroflexus) population. Weed Sci. 2021, 69, 161–166. [Google Scholar] [CrossRef]
- Liu, X.; Xiang, S.; Zong, T.; Ma, G.; Wu, L.; Liu, K.; Zhou, X.; Bai, L. Herbicide resistance in China: A quantitative review. Weed Sci. 2019, 67, 605–612. [Google Scholar] [CrossRef]


| Figure | Plant Species | Measurement | Equation b | β0 | β1 | β2 | R2 | SME c | p-Value | C50 or I50 |
|---|---|---|---|---|---|---|---|---|---|---|
| Figure 2 | Motherwort | Germination inhibition | (1) | 77.02 (±7.75) | 0.822 (±0.25) | −0.72 (±6.31) | 0.77 | 16.75 | <0.0001 | 0.51 |
| Figure 2 | Redroot pigweed | Germination inhibition | (1) | 11.92 (±1.61) | −0.33 (±0.08) | −11.72 (±4.26) | 0.87 | 14.73 | <0.0001 | 0.16 |
| Figure 2 | Spanish needles | Germination inhibition | (1) | 92.19 (±5.24) | 1.20 (±0.18) | −1.38 (±4.03) | 0.91 | 11.33 | <0.0001 | 0.48 |
| Figure 2 | Tall fescue | Germination inhibition | (1) | 81.42 (±8.28) | 0.31 (±0.10) | −8.09 (±8.77) | 0.86 | 11.78 | <0.0001 | 1.1 |
| Figure 3 | Motherwort | Visual injury | (2) | −2.90 (±1.67) | 124.99 (±9.85) | 0.17 (±0.03) | 0.99 | 2.96 | <0.0001 | 3.33 |
| Figure 3 | Redroot pigweed | Visual injury | (2) | −15.82 (±10.38) | 114.71 (±11.77) | 1.89 (±0.50) | 0.94 | 10.66 | 0.0014 | 0.47 |
| Figure 3 | Spanish needles | Visual injury | (2) | −19.83 (±8.80) | 119.91 (±9.02) | 3.04 (±0.57) | 0.97 | 7.25 | 0.0003 | 0.42 |
| Figure 3 | Tall fescue | Visual injury | (2) | −1.27 (±5.65) | 111.38 (±12.93) | 0.30 (±0.12) | 0.96 | 9.12 | 0.0009 | 2.00 |
| Figure 4 | Motherwort | Visual injury | (3) | 73.28 (±7.22) | 0.0006 (±0.0001) | - | 0.88 | 9.18 | <0.0001 | 1911 |
| Figure 4 | Spanish needles | Visual injury | (3) | 83.42 (±3.76) | 0.0014 (±0.0002) | - | 0.90 | 10.53 | <0.0001 | 653 |
| Figure 4 | Tall fescue | Visual injury | (3) | 20.51 (±0.47) | 0.0017 (±0.0002) | - | 0.96 | 1.46 | <0.0001 | NA |
| Figure 5 | Common purslane | Germination under dark | (4) | −5.45 (±3.22) | 108.80 (±4.35) | 1.72 (±0.18) | 0.93 | 11.54 | <0.0001 | 0.49 |
| Figure 5 | Common purslane | Germination under light | (4) | −11.28 (±3.63) | 114.83 (±4.94) | 1.65 (±0.19) | 0.92 | 13.11 | <0.0001 | 0.61 |
| Wood Vinegar Rate (L ha−1) | Common Purslane Control | |
|---|---|---|
| 1 DAT | 3 DAT | |
| % | ||
| 500 | 51 c | 68 c |
| 1000 | 70 b | 79 b |
| 2000 | 78 a | 94 a |
| p-value | <0.0001 | <0.0001 |
| Weed Control | Aboveground Biomass | ||
|---|---|---|---|
| % | % of nontreated control | ||
| Light condition | Dark | 95 a | 57 a |
| Light | 87 b | 79 b | |
| Wood vinegar | 500 L ha−1 | 83 a | 62 |
| 1000 L ha−1 | 91 b | 69 | |
| 2000 L ha−1 | 99 c | 75 | |
| Light condition | <0.0001 | <0.0001 | |
| Wood vinegar | <0.0001 | 0.9382 | |
| Light condition × wood vinegar | 0.0612 | 0.4622 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, L.; Liu, H.; Zhang, Z.; Zhan, Y.; Wang, K.; Yang, D.; Liu, Z.; Yu, J. Evaluation of Wood Vinegar as an Herbicide for Weed Control. Agronomy 2022, 12, 3120. https://doi.org/10.3390/agronomy12123120
Chu L, Liu H, Zhang Z, Zhan Y, Wang K, Yang D, Liu Z, Yu J. Evaluation of Wood Vinegar as an Herbicide for Weed Control. Agronomy. 2022; 12(12):3120. https://doi.org/10.3390/agronomy12123120
Chicago/Turabian StyleChu, Lei, Haifeng Liu, Zhenyu Zhang, Yue Zhan, Kang Wang, Deyu Yang, Ziqiang Liu, and Jialin Yu. 2022. "Evaluation of Wood Vinegar as an Herbicide for Weed Control" Agronomy 12, no. 12: 3120. https://doi.org/10.3390/agronomy12123120
APA StyleChu, L., Liu, H., Zhang, Z., Zhan, Y., Wang, K., Yang, D., Liu, Z., & Yu, J. (2022). Evaluation of Wood Vinegar as an Herbicide for Weed Control. Agronomy, 12(12), 3120. https://doi.org/10.3390/agronomy12123120





