Influence of Reduced Tillage, Fertilizer Placement, and Soil Afforestation on CO2 Emission from Arable Sandy Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of the Experiments and CO2 Mitigation Practices
2.1.1. Tillage Systems
2.1.2. Fertilizer Placement
2.1.3. Afforestation of the Infertile Soils
2.2. Climatic Conditions
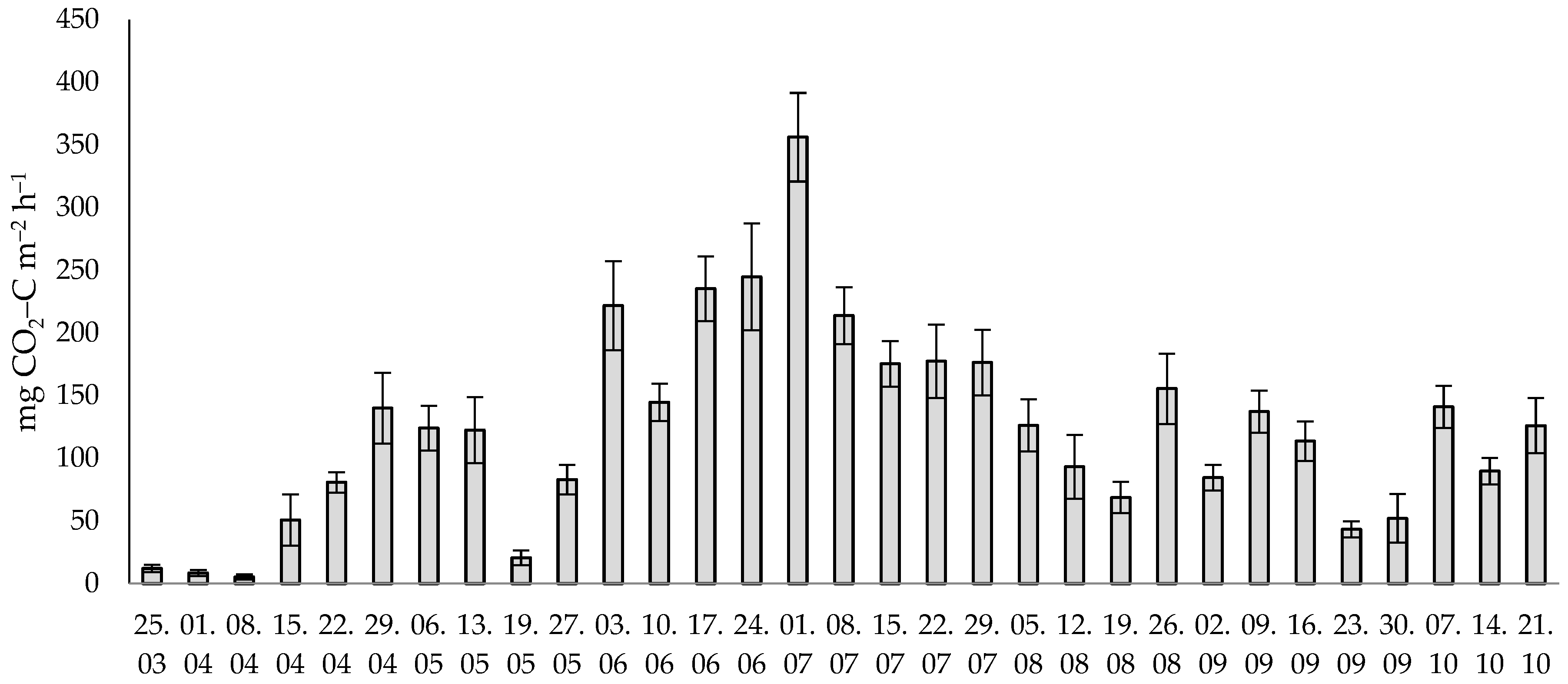
2.3. CO2 Soil Emissions Measurements
2.4. Statistical Analysis
3. Results and Discussion
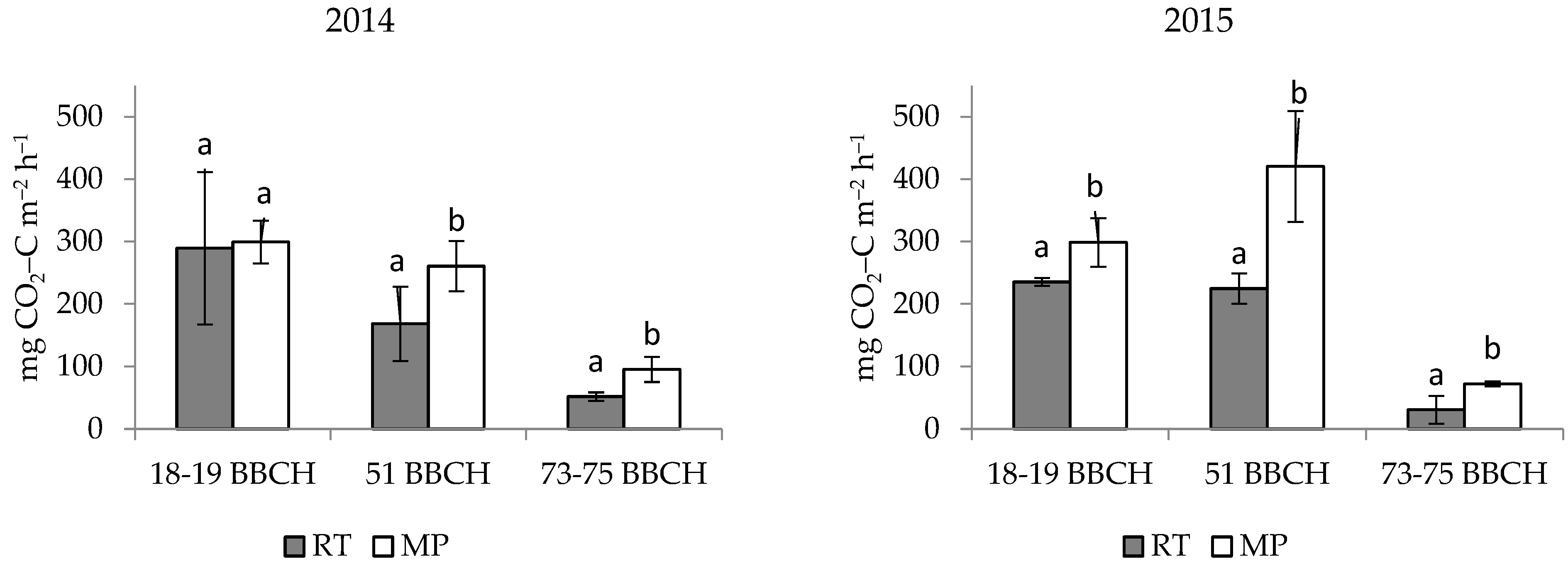
3.1. Effect of the Tillage Systems
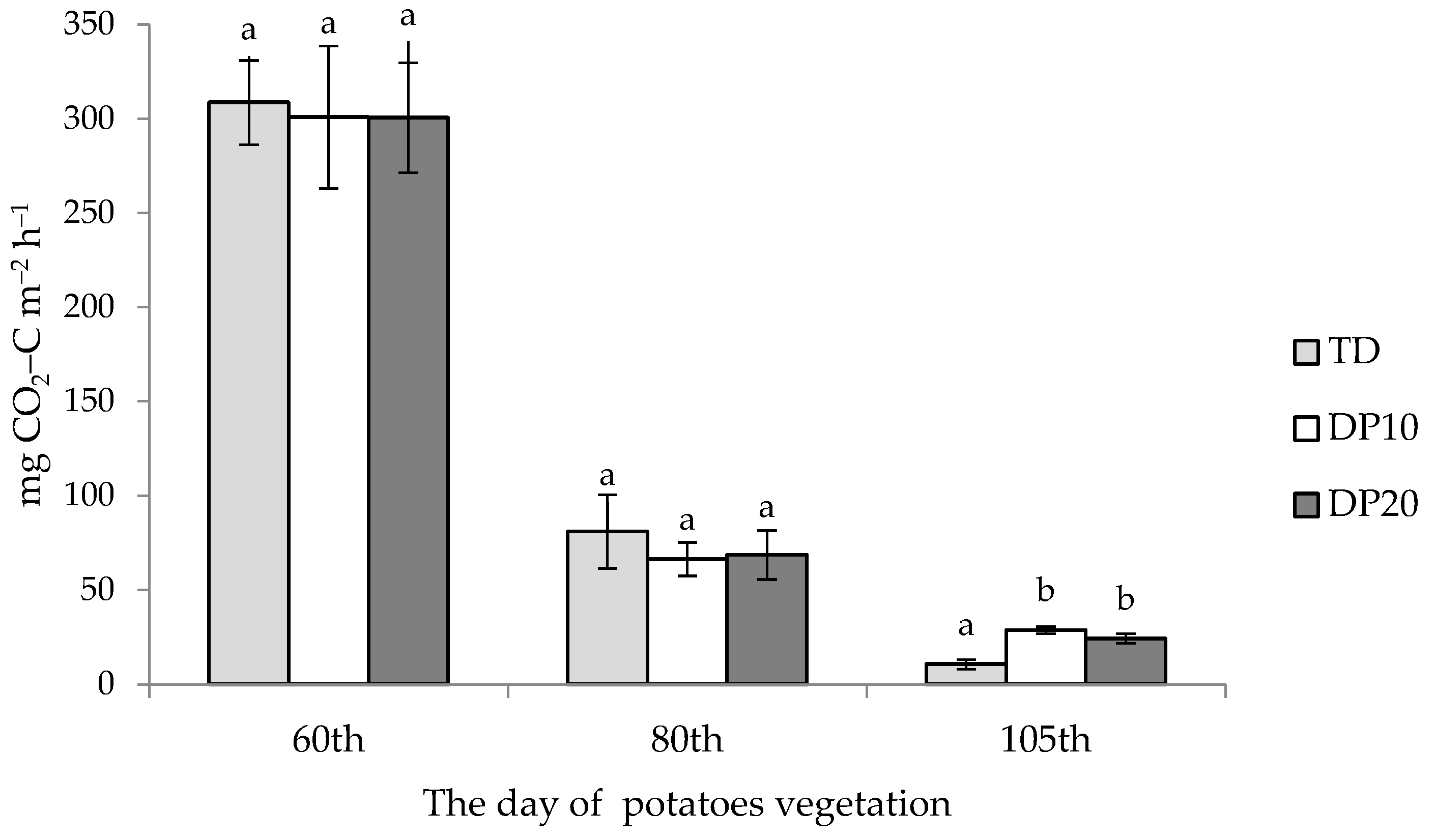
3.2. Effect of Fertilizer Placement
3.3. Prediction of Soil Afforestation Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Communication from The Commission to The European Parliament, The European Council, The Council, The European Economic And Social Committee, And The Committee of The Regions. The European Green Deal. Brussels, 11.12.2019 COM(2019) 640 final. p. 24. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0002.02/DOC_1&format=PDF (accessed on 31 July 2022).
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; The Working Group I Contribution to the Sixth Assessment Report Addresses the Most up-to-Date Physical Understanding of the Climate System and Climate Change, Bringing Together the Latest Advances in Climate Science. Available online: https://www.ipcc.ch/report/sixth-assessment-report-working-group-i (accessed on 31 July 2022).
- FAO. FAOSTAT Analytical Brief 18. Emissions due to Agriculture. Global, Regional, and Country Trends 2000–2018. 2021. Available online: https://www.fao.org/3/cb3808en/cb3808en.pdf (accessed on 31 July 2022).
- Sanz-Cobena, A.; Lassaletta, L.; Aguilera, E.; del Prado, A.; Garnier, J.; Billen, G.; Iglesias, A.; Sánchez, B.; Guardia, G.; Abalos, D.; et al. Strategies for greenhouse gas emissions mitigation in Mediterranean agriculture: A review. Agric. Ecosyst. Environ. 2017, 238, 5–24. [Google Scholar] [CrossRef] [Green Version]
- Panchasara, H.; Samrat, N.; Islam, N. Greenhouse Gas Emissions Trends and Mitigation Measures in Australian Agriculture Sector—A Review. Agriculture 2021, 11, 85. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, X.; Liu, J.; Sun, N.; Wu, L.; Li, Z.; Xu, M. A synthetic analysis of greenhouse gas emissions from manure amended agricultural soils in China. Sci. Rep. 2017, 7, 8123. [Google Scholar] [CrossRef]
- Mohammed, S.; Mirzaei, M.; Pappné Törő, Á.; Anari, M.G.; Moghiseh, E.; Asadi, H.; Szabó, S.; Kakuszi-Széles, A.; Harsányi, E. Soil carbon dioxide emissions from maize (Zea mays L.) fields as influenced by tillage management and climate. Irrig. Drain. 2021, 71, 228–240. [Google Scholar] [CrossRef]
- Reicosky, D. Tillage-induced CO2 emission from soil. Nutr. Cycl. Agroecosystems 1997, 49, 273–285. [Google Scholar] [CrossRef]
- Krasowicz, S.; Oleszek, W.; Horabik, J.; Dębicki, R.; Jankowiak, J.; Stuczyński, T.; Jadczyszyn, J. Rational management of the soil environment in Poland. Pol. J. Agron. 2011, 7, 43–58. [Google Scholar]
- Król, A.; Żyłkwski, T.; Kozyra, J.; Książak, J. Soil moisture under no-tillage and tillage systems in maize long-term experiment. Pol. J. Soil Sci. 2018, 51, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Sosulski, T.; Korc, M. Effects of different mineral and organic fertilization on the content of nitrogen and carbon in soil organic matter fractions. Ecol. Chem. Eng. 2011, 18, 601–609. [Google Scholar]
- Ochal, P.; Jadczyszyn, T.; Jurga, B.; Kopiński, J.; Matyka, M.; Madej, A.; Rutkowska, A.; Smreczak, B.; Łysiak, M. Środowiskowe Aspekty Zakwaszenia Gleb w Polsce; Institute of Soil Science and Plant Cultivation State Research Institute: Puławy, Poland, 2017; p. 43. (In Polish) [Google Scholar]
- Körschens, M. The importance of long-term field experiments for soil science and environmental research—A review. Plant. Soil Environ. 2006, 52, 1–8. [Google Scholar]
- Ballabio, C.; Panagos, P.; Monatanarella, L. Mapping topsoil physical properties at European scale using the LUCAS database. Geoderma 2016, 261, 110–123. [Google Scholar] [CrossRef]
- Lasy w Polsce. Centrum Informacyjne Lasów Państwowych. Dyrekcja Generalna Lasów Państwowych. 2012; p. 55. Available online: https://www.lasy.gov.pl/pl/informacje/publikacje/do-poczytania/lasy-w-polsce-1/lasy-w-polsce-2012/ (accessed on 31 July 2022). (In Polish)
- de Castro, P.; Miglietta, P.P.; Vecchio, Y. The Common Agricultural Policy 2021–2027: A new history for European agriculture. Ital. Rev. Agric. Econ. 2020, 75, 5–12. [Google Scholar]
- Doroszewski, A.; Jadczyszyn, J.; Kozyra, J.; Pudełko, R.; Stuczyński, T.; Mizak, K.; Łopatka, A.; Koza, P.; Górski, T.; Wróblewska, E. Fundamentals of the agricultural drought monitoring system. Water Environ. Rural. Areas. 2012, 12, 77–91. (In Polish) [Google Scholar]
- SMSR IUNG PIB. 2012. Available online: https://susza.iung.pulawy.pl/kbw (accessed on 31 July 2022).
- Adamiak, M. Informacja o Wynikach Kontroli. Pomoc Rolnikom Poszkodowanym w Wyniku Suszy. NIK. KRR.430.008,2021. Nr ewid.163/2021/P/21/048/KRR. 2022; p. 85. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj5q7a7v-b7AhU2gVYBHdcqBNwQFnoECA4QAQ&url=https%3A%2F%2Fwww.nik.gov.pl%2Fkontrole%2Fwyniki-kontroli-nik%2Fpobierz%2Ckrr~p_21_048_202111180815061637219706~01%2Ctyp%2Ckk.pdf&usg=AOvVaw3PJ2hQnC3BRPajetQRgtxL (accessed on 31 July 2022). (In Polish)
- Abęcki, L. Foreseen climate changes and irrigation development in Poland. Infrastruct. Ecol. Rural. Areas. 2009, 3, 7–18. [Google Scholar]
- Wirtschafts Woche. Dürrehilfe: Knapp 292 Millionen Euro an Bauern Ausgezahlt. 2020. Available online: https://www.wiwo.de/unternehmen/handel/landwirtschaft-duerrehilfe-knapp-292-millionen-euro-an-bauern-ausgezahlt/25549774.html (accessed on 31 July 2022). (In German).
- Sosulski, T.; Szymańska, M.; Szara, E. Assessment of various practices of the mitigation of N2O emissions from the arable soils of Poland. Soil Sci. Annu. 2017, 68, 55–64. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, X.; Yang, W.; Ding, S.; Ren, G.; Li, M.; Zhu, A. Soil respiration and net carbon flux response to long-term reduced/no-tillage with and without residues in a wheat-maize cropping system. Soil Tillage Res. 2021, 214, 105182. [Google Scholar] [CrossRef]
- Oorts, K.; Merckx, R.; Gréhan, E.; Labreuche, J.; Nicolardot, B. Determinants of annual fluxes of CO2 and N2O in long-term no-tillage and conventional tillage systems in northern France. Soil Tillage Res. 2007, 95, 133–148. [Google Scholar] [CrossRef]
- Jia, S.; Liang, A.; Zhang, S.; Chen, X.; McLaughlin, N.B.; Sun, B.; Zhang, X.; Wu, D. Effect of tillage system on soil CO2 flux, soil microbial community and maize (Zea mays L.) yield. Geoderma 2021, 384, 114813. [Google Scholar] [CrossRef]
- Du, K.; Li, F.; Leng, P.; Li, Z.; Tian, C.; Qiao, Y.; Li, Z. Differential Influence of no-tillage and precipitation pulses on soil het-erotrophic and autotrophic respiration of summer maize in the North China Plain. Agronomy 2020, 10, 2004. [Google Scholar] [CrossRef]
- Flessa, H.; Ludwig, B.; Heil, B.; Merbach, W. The Origin of Soil Organic C, Dissolved Organic C and Respiration in a Long-Term Maize Experiment in Halle, Germany, Determined by13C Natural Abundance. J. Plant. Nutr. Soil Sci. 2000, 163, 157–163. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Qiao, Y.; Du, K.; Li, Z.; Tian, C.; Zhu, N.; Leng, P.; Yue, Z.; Cheng, H.; et al. Evaluation of no-tillage impacts on soil respiration by 13C-isotopic signature in North China Plain. Sci. Total Environ. 2022, 824, 153852. [Google Scholar] [CrossRef]
- Shakoor, A.; Shahbaz, M.; Farooq, T.H.; Sahar, N.E.; Shahzad, S.M.; Altaf, M.M.; Ashraf, M. A global meta-analysis of greenhouse gases emission and crop yield under no-tillage as compared to conventional tillage. Sci. Total. Environ. 2020, 750, 142299. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Yu, Q.; Zhang, Y.; Wang, R.; Li, J.; Wang, X. No tillage increases soil organic carbon storage and decreases carbon dioxide emission in the crop residue-returned farming system. J. Environ. Manag. 2020, 261, 110261. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yin, W.; Chai, Q.; Fan, Z.; Hu, F.; Fan, H.; Zhao, C.; Yu, A.; Coulter, J.A. No tillage with previous plastic covering increases water harvesting and decreases soil CO2 emissions of wheat in dry regions. Soil Tillage Res. 2020, 208, 104883. [Google Scholar] [CrossRef]
- Abdalla, K.; Chivenge, P.; Ciais, P.; Chaplot, V. No-tillage lessens soil CO2 emissions the most under arid and sandy soil con-ditions: Result from a meta-analysis. Biogeosci. Discuss. 2015, 12, 15495–15535. [Google Scholar]
- Gaihre, Y.K.; Singh, U.; Huda, A.; Islam, S.M.M.; Islam, M.R.; Biswas, J.C.; DeWald, J. Nitrogen use efficiency, crop productivity and environmental impacts of urea deep placement in lowland rice fields. In Proceedings of the 2016 International Nitrogen Initiative Conference, “Solution to Improve Nitrogen use Efficiency for the World”, Melbourne, VI, Australia, 4–8 December 2016; Volume 1, pp. 1–4. [Google Scholar]
- Sosulski, T.; Stępień, W.; Wąs, A.; Szymańska, M. N2O and CO2 Emissions from Bare Soil: Effect of Fertilizer Manage-ment. Agriculture 2020, 10, 602. [Google Scholar] [CrossRef]
- Ťupek, B.; Lehtonen, A.; Mäkipää, R.; Peltonen-Sainio, P.; Huuskonen, S.; Palosuo, T.; Heikkinen, J.; Regina, K. Extensification and afforestation of cultivated mineral soil for climate change mitigation in Finland. For. Ecol. Manag. 2021, 501, 119672. [Google Scholar] [CrossRef]
- Luyssaert, S.; Ciais, P.; Piao, S.L.; Schulze, E.-D.; Jung, M.; Zaehle, S.; Schelhaas, M.J.; Reichstein, M.; Churkina, G.; Papale, D.; et al. The European carbon balance. Part 3: Forests. Glob. Chang. Biol. 2010, 16, 1429–1450. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L. How strongly can forest management influence soil C sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Eurostat Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Archive:Forestry_and_climate_change (accessed on 20 October 2022).
- Talarczyk, W.; Szulc, T.; Szczepaniak, J.; Łowński, Ł. Functional verification of unit for strip tillage, fertilization and corn sowing. J. Res. Appl. Agric. Eng. 2016, 61, 110–113. [Google Scholar]
- Milewski, W. Lasy w Polsce. Lasy Państwowe. Centrum Informacyjne Lasów Państwowych. Warszawa. 2012; p. 58. Available online: https://www.lasy.gov.pl/pl/informacje/publikacje/do-poczytania/lasy-w-polsce-1/lasy-w-polsce-2012/ (accessed on 31 July 2022). (In Polish)
- Sosulski, T.; Szara, E.; Szymańska, M.; Stępień, W.; Rutkowska, B.; Szulc, W. Soil N2O emissions under conventional tillage conditions and from forest soil. Soil Tillage Res. 2019, 190, 86–91. [Google Scholar] [CrossRef]
- Bickel, K.; Köhl, M.; IPCC. Default Climate and Soil Classifications. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories.Ch. 3. Consistent Representation of Lands. Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_03_Ch3_Representation.pdf (accessed on 30 July 2022).
- Institute of Meteorology and Water Management IMGW-PIB. Available online: https://www.imgw.pl. (accessed on 31 July 2022).
- Hutchinson, G.L.; Livingston, G.P. Use of Chamber Systems to Measure Trace Gas; Fluxes, L.A., Mosier, A.R., Duxbury, J.M., Rolston, D.E., Eds.; Agricultural Ecosystem Effects on Trace Gases and Global Climate Change; Special Publication: Madison, WI, USA, 1993; pp. 63–78. [Google Scholar]
- Frouz, J.; Bujalský, L. Flow of CO2 from soil may not correspond with CO2 concentration in soil. Sci. Rep. 2018, 8, 10099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosulski, T.; Szymańska, M.; Szara, E. CO2 Emissions from Soil Under Fodder Maize Cultivation. Agronomy 2020, 10, 1087. [Google Scholar] [CrossRef]
- Buragiené, S.; Šaruskis, E.; Romaneckas, K.; Adamavičiené, A.; Kriaučiüniené, Z.; Zvižienyté, D.; Mazoras, V.; Naujokiené, V. Relationship between CO2 emissions and soil properties of differently tilled soils. Sci. Total Environ. 2019, 662, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Sainju, U.; Jabro, J.D.; William, B.S. Soil carbon dioxide emissions and carbon content as affected by irrigation, tillage, cropping system and nitrogen fertilization. J. Environ. Qual. 2008, 37, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q. Characteristics of soil profile CO2 concentrations in karst areas and their significance for global carbon cycles and climate change. Earth Syst. Dyn. 2019, 10, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Bogužas, V.; Sinkevičiene, A.; Romaneckas, K.; Steponavičiené, V.; Skinuliené, L.; Butkevičiené, L.M. The impact of tillage intensity and meteorological conditions on soil temperature, moisture content and CO2 efflux in maize and spring barley cul-tivation. Zemdirbyste 2018, 105, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Adviento-Borbe, M.A.A.; Haddix, M.L.; Binder, D.L.; Walters, D.T.; Dobermann, A. Soil greenhouse gas fluxes and global warming potential in four high-yielding maize systems. Glob. Chang. Biol. 2007, 13, 1972–1988. [Google Scholar] [CrossRef]
- Amos, B.; Arkebauer, T.J.; Doran, J.W. Soil Surface Fluxes of Greenhouse Gases in an Irrigated Maize-Based Agroecosystem. Soil Sci. Soc. Am. J. 2005, 69, 387–395. [Google Scholar] [CrossRef]
- Fang, H.J.; Yu, G.R.; Cheng, S.L.; Zhu, T.H.; Wang, Y.S.; Yan, J.H.; Wang, M.; Cao, M.; Zhou, M. Effects of multiple environmental factors on CO2 emission and CH4 uptake from old-growth forest soils. Biogeosciences 2010, 7, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Pumpanen, J.; Ilvesniemi, H.; Perämäki, M.; Hari, P. Seasonal patterns of soil CO2 efflux and soil air CO2 concentration in a Scots pine forest: Comparison of two chamber techniques. Glob. Chang. Biol. 2003, 9, 371–382. [Google Scholar] [CrossRef]
- Grüning, M.M.; Germeshausen, F.; Thies, C.; Arnold, A.I.M. Increased forest soil CO2 and N2O emissions during insect infes-tation. Forests 2018, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zang, S.; Ma, D.; Ren, J.; Chen, Q.; Dong, X. Emissions of CO2, CH4, and N2O Fluxes from Forest Soil in Permafrost Region of Daxing’an Mountains, Northeast China. Int. J. Environ. Res. Public Health 2019, 16, 2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, E.A.; Savage, K.E.; Trumbore, S.E.; Borken, W. Vertical partitioning of CO2 production within a temperate forest soil. Glob. Chang. Biol. 2006, 12, 944–956. [Google Scholar] [CrossRef] [Green Version]
- Muhr, J.; Goldberg, S.D.; Borken, W.; Gebauer, G. Repeated drying–rewetting cycles and their effects on the emission of CO2, N2O, NO, and CH4 in a forest soil. J. Plant. Nutr. Soil. Sci. 2008, 171, 719–728. [Google Scholar] [CrossRef]
- Leitner, S.; Sae-Tun, O.; Kranzinger, L.; Zechmeister-Boltenstern, S.; Zimmermann, M. Contribution of litter layer to soil greenhouse gas emissions in a temperate beech forest. Plant. Soil 2016, 403, 455–469. [Google Scholar] [CrossRef] [Green Version]
- Bolinder, M.A.; Janzen, H.H.; Gregorich, E.G.; Angers, D.A.; Vanden Bygaart, A.J. An approach for estimating net primary productivity and annual crops in Canada. Agric. Ecosyst. 2007, 118, 29–42. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, S.; Li, Z.; Zhang, D.; Tang, X.; Zhou, C.; Yan, J.; Mo, J. Old-Growth Forests Can Accumulate Carbon in Soils. Science 2006, 314, 1417. [Google Scholar] [CrossRef] [Green Version]
- Rodtassana, C.; Unawong, W.; Yaemphum, S.; Chanthorn, W.; Chawchai, S.; Nathalang, A.; Brockelman, W.Y.; Tor-Ngern, P. Different responses of soil respiration to environmental factors across forest stages in a Southeast Asian forest. Ecol. Evol. 2021, 11, 15430–15443. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Y.; Xu, Z.; Wu, Q. Soil respiration in a subtropical forest of southwestern China: Components, patterns and controls. PLoS ONE 2018, 13, e0204341. [Google Scholar] [CrossRef]


| Location | Year of Investigation | April | May | Jun | July | August | September | October | Growing Period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mm | °C | mm | °C | mm | °C | mm | °C | mm | °C | mm | °C | mm | °C | mm | °C | |||
| Western Poland | Baborówko | 2014 | 46.8 | 10.4 | 67.9 | 13.4 | 30.0 | 16.1 | 51.6 | 21.5 | 91.6 | 17.5 | 29.5 | 14.9 | 35.3 | 10.5 | 352.7 | 14.9 |
| 2015 | 16.3 | 8.5 | 26.8 | 12.7 | 41.3 | 15.9 | 71.3 | 19.2 | 12.8 | 22.3 | 20.1 | 14.5 | 21.6 | 7.6 | 210.2 | 14.4 | ||
| Central Poland | Kuklówka Zarzeczna | 2015 | 45.8 | 7.5 | 51.2 | 13.0 | 16.1 | 16.3 | 64.0 | 18.8 | 6.4 | 21.1 | 37.2 | 14.1 | 44.5 | 6.4 | 265.2 | 13.7 |
| Mikanów | 2012 | 41.7 | 9.4 | 45,4 | 15.6 | 10.3 | 17.3 | 56.0 | 21.1 | 0.0 | 19.0 | 28.5 | 14.7 | 48.3 | 8.2 | 230.1 | 15.0 | |
| Meteorological Conditions | Location | Treatment | mg CO2-C m−2 h−1 | |
|---|---|---|---|---|
| Mean | Median | |||
| Normal | Baborówko | RT | 169.7 ± 118.7 | 168.4 |
| MP | 218.4 ± 108.4 | 260.6 | ||
| Extremely dry | RT | 163.6 ± 115.2 | 224.9 | |
| MP | 263.7 ± 176.6 | 298.6 | ||
| Extremaly dry | Kuklówka Zarzeczna | TD | 133.7 ± 155.8 | 81.0 |
| DP10 | 132.0 ± 147.5 | 66.0 | ||
| DP20 | 131.0 ± 148.1 | 69.0 | ||
| Dry | Mikanów | Forest | 123.3 ± 79.0 | 79.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosulski, T.; Niedziński, T.; Jadczyszyn, T.; Szymańska, M. Influence of Reduced Tillage, Fertilizer Placement, and Soil Afforestation on CO2 Emission from Arable Sandy Soils. Agronomy 2022, 12, 3102. https://doi.org/10.3390/agronomy12123102
Sosulski T, Niedziński T, Jadczyszyn T, Szymańska M. Influence of Reduced Tillage, Fertilizer Placement, and Soil Afforestation on CO2 Emission from Arable Sandy Soils. Agronomy. 2022; 12(12):3102. https://doi.org/10.3390/agronomy12123102
Chicago/Turabian StyleSosulski, Tomasz, Tomasz Niedziński, Tamara Jadczyszyn, and Magdalena Szymańska. 2022. "Influence of Reduced Tillage, Fertilizer Placement, and Soil Afforestation on CO2 Emission from Arable Sandy Soils" Agronomy 12, no. 12: 3102. https://doi.org/10.3390/agronomy12123102
APA StyleSosulski, T., Niedziński, T., Jadczyszyn, T., & Szymańska, M. (2022). Influence of Reduced Tillage, Fertilizer Placement, and Soil Afforestation on CO2 Emission from Arable Sandy Soils. Agronomy, 12(12), 3102. https://doi.org/10.3390/agronomy12123102





