Effect of Different Foliar Particle Films (Kaolin and Zeolitite) on Chemical and Sensory Properties of Olive Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Treatments
2.2. Olive and Oil Analyses
2.3. Statistical Analysis
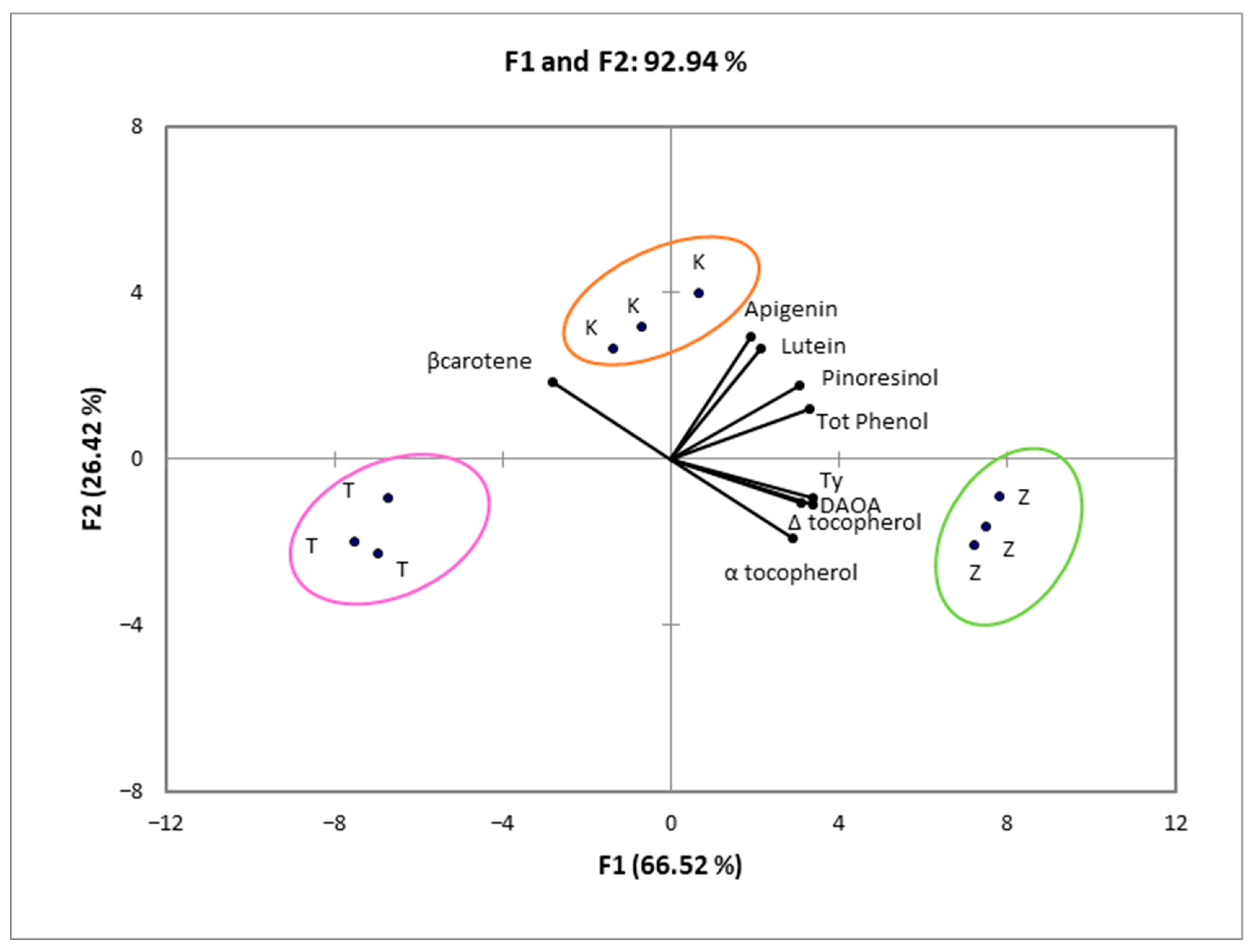
3. Results and Discussion
3.1. Chemical Properties of the Oils
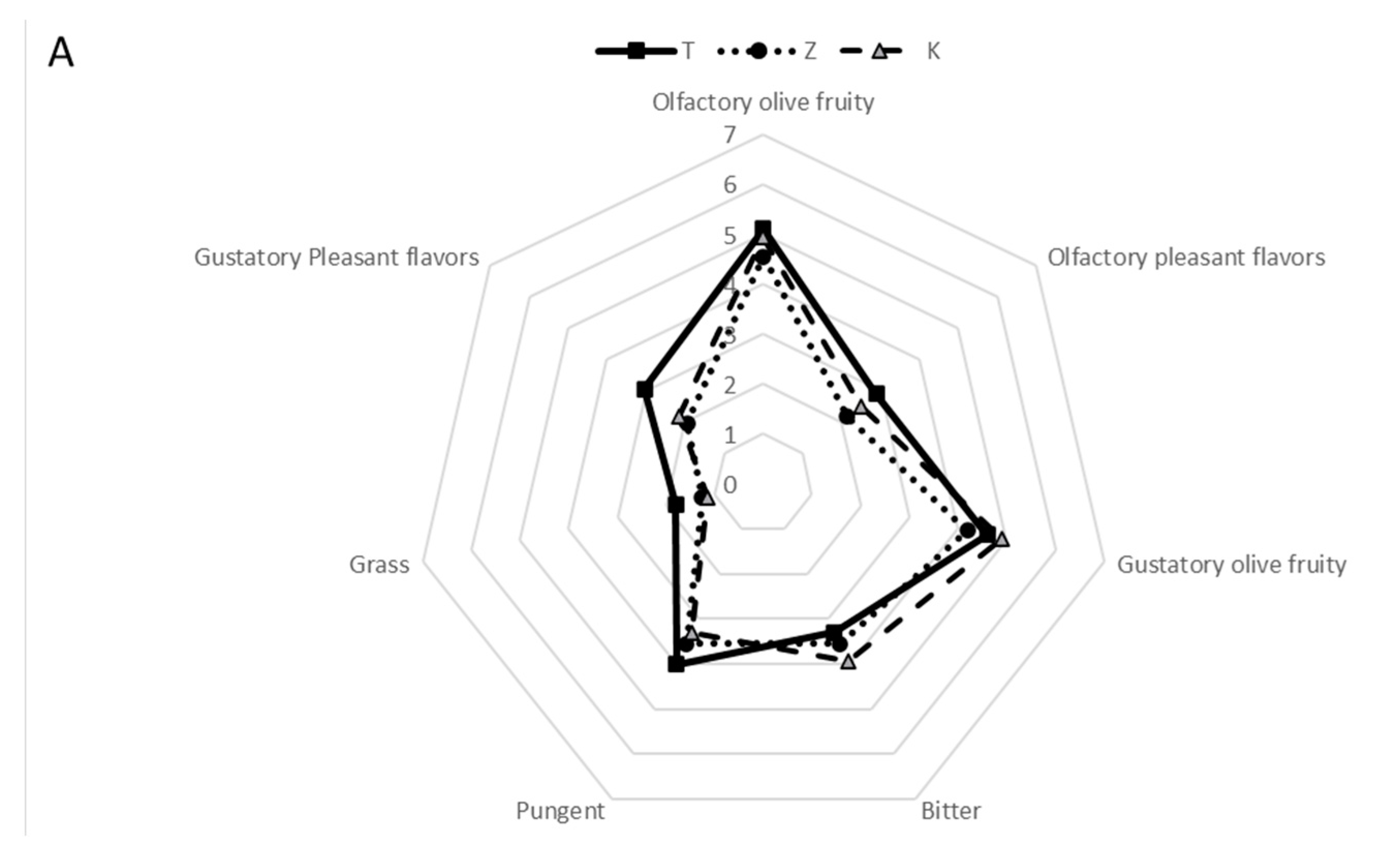
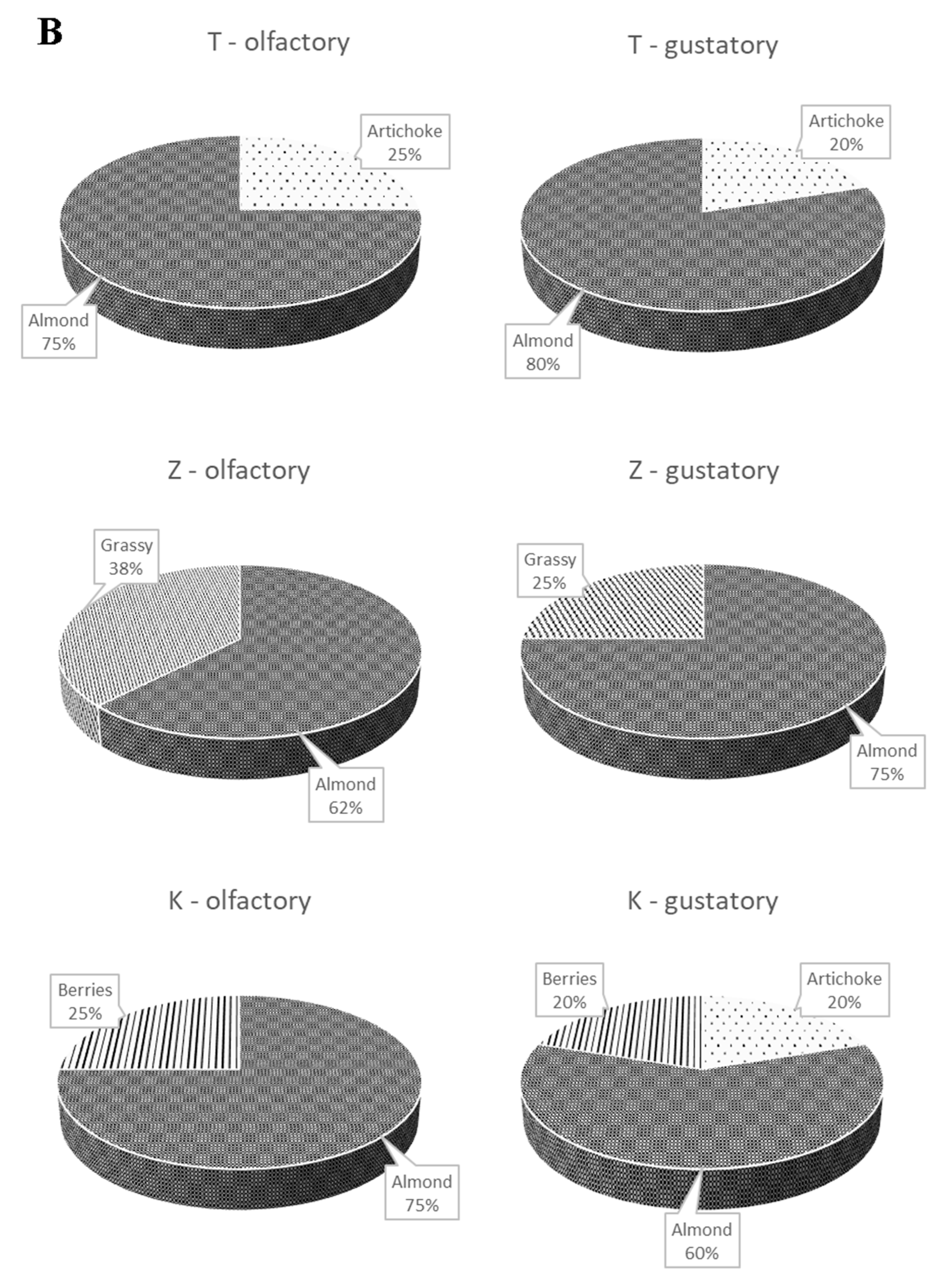
3.2. Sensory Profiles of the Oils
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khaleghi, E.; Arzani, K.; Moallemi, N.; Barzegar, M. The efficacy of kaolin particle film on oil quality indices of olive trees (Olea europaea L.) cv Zard gown under warm and semi-arid region. Food Chem. 2015, 166, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dombrowsky, T. The origins of kaolinite—Implication for utilization. In Science of Whitewares, 2nd ed.; Carty, W.M., Sinton, C.W., Eds.; The American Ceramics Society: Westerville, OH, USA, 2006; pp. 3–12. ISBN 157498067X. [Google Scholar]
- Brindley, G.W.; Robinson, K. Structure of kaolinite. Nature 1945, 156, 661–662. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Z.; Lei, X.; Yan, C. Defects in structure as the sources of the surface charges of kaolinite. Appl. Clay Sci. 2016, 124, 127–136. [Google Scholar] [CrossRef]
- Kahr, G.; Madsen, F.T. Determination of the cation exchange capacity and the surface area of bentonite, illite and kaolinite by methylene blue adsorption. Appl. Clay Sci. 1995, 9, 327–336. [Google Scholar] [CrossRef]
- Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Using zeolitic adsorbents to cleanup special wastewater streams: A review. Micropor. Mesopor. Mat. 2015, 214, 224–241. [Google Scholar] [CrossRef]
- Galli, E.; Passaglia, E. Natural zeolites in environmental engineering. In Zeolites in Chemical Engineering; Holzapfel, H., Ed.; Verlag Processeng Engineering GmbH: Vienna, Austria, 2011; pp. 392–416. ISBN 3902655089. [Google Scholar]
- Gualtieri, A.F.; Passaglia, E. Rietveld structure refinement of NH4-exchanged natural chabazite. Eur. J. Mineral 2006, 18, 351–359. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. In Proceedings of the National Academy of Sciences, Arnold and Mabel Beckman Center, Irvine, CA, USA, 8–9 November 1998; Volume 96, pp. 3463–3470. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, E.C.; Salvi, L.S.; Paoli, F.P.; Fucile, M.F.; Masciandro, G.M.; Manzi, D.M.; Masini, C.M.M.; Matii, G.B.M. Effects of natural clinoptilonite on physiology, water stress, sugar, and anthocyanin content Sanforte (Vitis vinifera) young vineyard. J. Agric. Sci. 2021, 159, 488–499. [Google Scholar] [CrossRef]
- Tekaya, M.; Mechri, B.; Cheheb, H.; Attia, F.; Chraief, I.; Ayachi, M.; Boujneh, D.; Hammami, M. Changes in the profiles of mineral elements, phenols, tocopherols and soluble carbohydrates of olive fruit following foliar nutrient fertilization. LWT-Food Sci. Technol. 2014, 59, 1047–1053. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, Y.; Dutta, P.K. Controlled release of paraquat from surface-modified zeolite Y. Micropourus Mesopourus Mater. 2006, 88, 312–318. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Di Giuseppe, D.; Faccini, B.; Coltorti, M. Mitigation of sodium-risk in a sandy agricultural soil by the use of natural zeolites. Environ. Monit. Assess. 2018, 190, 1–12. [Google Scholar] [CrossRef]
- Colombani, N.; Di Giuseppe, D.; Faccini, B.; Ferretti, G.; Mastrocicco, M.; Coltorti, M. Estimated water savings in an agricultural field amended with natural zeolites. Environ. Process. 2016, 3, 617–628. [Google Scholar] [CrossRef]
- Faccini, B.; Di Giuseppe, D.; Ferretti, G.; Coltorti, M.; Colombani, N.; Mastrocicco, M. Natural and NH4+-enriched zeolitite amendment effects on nitrate leaching from a reclaimed agricultural soil (Ferrara Province, Italy). Nutr. Cycl. Agroecosystems 2018, 110, 327–341. [Google Scholar] [CrossRef]
- Ferretti, G.; Di Giuseppe, D.; Natali, C.; Faccini, B.; Bianchini, G.; Coltorti, M. C-N elemental and isotopic investigation in agricultural soils: Insights on the effects of zeolitite amendments. Geochem 2017, 77, 45–52. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Tzanakakis, V.; Giannakoula, A.; Psoma, P. Inorganic and organic amendements affect soil fertility, nutrition, phosystem II, and fruit weight and may enhance the sustainability of Solanum lycopersicon L. (cv Mountain Fresh) crop. Sustainability 2020, 12, 9028. [Google Scholar] [CrossRef]
- Suwardi, D.F.; Pratiwi, D.F.; Suryaningtyas, D.T. Increasing oil palm (Elaeis guineensis Jacq.) production by the application of humic substances and zeolite as carrier. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 1st International Conference on Sustainable Plantation, Bogor, Indonesia, 20–22 August 2019; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 418, p. 012046. [Google Scholar] [CrossRef]
- Hazrati, S.; Khurizadeh, S.; Sadeghi, A.R. Application of zeolite improves water and nitrogen use efficiency while increasing essential oil yield and quality of Salvia officinalis under water-deficit stress. Saudi J. Biol. Sci. 2021, 29, 1707–1716. [Google Scholar] [CrossRef]
- Rotondi, A.; Morrone, L.; Facini, O.; Faccini, B.; Ferretti, G.; Coltorti, M. Distinct Particle Films Impacts on Olive Leaf Optical Properties and Plant Physiology. Foods 2021, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.T.; Silva, E.; Goncalves, A.; Matos, C.; Rodrigues, M.A.; Moutinho-Pereira, J.; Barros, A.; Correia, C. Kaolin and salicylic acid foliar application modulate yield, quality and phytochemical composition of olive pulp and oil from rainfed trees. Sci. Hortic. 2018, 237, 176–183. [Google Scholar] [CrossRef]
- Tworkoski, T.J.; Glenn, D.M.; Puterka, G. Response of bean to application of hydrophobic mineral particles. Can. J. Plant Sci. 2002, 82, 217–219. [Google Scholar] [CrossRef] [Green Version]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef]
- Valentini, G.; Pastore, C.; Allegro, G.; Muzzi, E.; Seghetti, L.; Filippetti, I. Application of kaolin and Italian natural Chabasite-rich zeolitite to mitigate the effect of global warming in Vitis Vinifera L. cv Sangiovese. Agronomy 2021, 11, 1035. [Google Scholar] [CrossRef]
- Schupp, J.R.; Fallahi, E.; Chun, I. Effect of Particle Film on Fruit Sunburn, Maturity and Quality of `Fuji’ and `Honeycrisp’ Apples. HortTechnology 2002, 12, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Calzarano, F.; Valentini, G.; Arfelli, G.; Seghetti, L.; Manetta, A.C.; Metruccio, E.G.; Di Marco, S. Activity of Italian natural chabazite-rich zeolitites against grey mould, sour rot and grapevine moth, and effect on grape and wine composition. Phytopathol. Mediterr. 2019, 58, 307–321. [Google Scholar]
- Glenn, D.M.; Erez, A.; Puterka, G.J.; Gundrum, P. Particle film affect carbon assimilation and yield in “Empire” apple. J. Am. Soc. Hortic. Sci. 2003, 128, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Zaghloul, A.E.; Ennab, H.A.; El-Shemy, M.E. Influence of kaolin sprays on fruit quality and storability of Balady mandarin. Alex Sci. Exch. J. 2017, 38, 661–670. [Google Scholar] [CrossRef]
- Kahan, B.A.; Damicone, J.P. Kaolin particle film product applications before begins may not improve marketable yields of fresh tomatoes. HorTechnology 2008, 18, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Faghih, S.; Zamani, Z.; Fathi, R.; Omidi, M. Influence of kaolin application on most important fruit and leaf characteristics of two apple cultivars under sustained deficit irrigation. Biol. Res. 2021, 54, 1–15. [Google Scholar] [CrossRef]
- Putri, W.J.; Warsiki, E.; Syamsu, K.; Iskandar, A. Application nanozeolite-molibdate for avocado ripeness indicator. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 6th International Conference on Sustainable Agriculture, Manila, The Philippines, 18–21 October 2018; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 347, p. 012063. [Google Scholar]
- Widayanti, S.M.; Hoerudin, S.M.; Andes, I. Characteristics and postharvest life of snake fruit (Salacca edulis Reinw) during storage as influenced by application of activated nanostructured natural zeolites. In IOP Conference Series: Earth and Environmental Science, Proceedings of the Reframing Food Sovereignty After COVID-19, Semarang, Indonesia, 20 October 2020; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 803, p. 012029. [Google Scholar] [CrossRef]
- Saour, G.; Makee, H. Effects of kaolin particle film on olive fruit yield, oil content and quality. Adv. Hortic. Sci. 2003, 17, 204–206. [Google Scholar]
- Perri, E.; Iannotta, N.; Mazzalupo, I.; Russo, A.; Caravita, M.A.; Pellegrino, M.; Parise, A.; Tucci, P. Kaolin protects olive fruits from Bactrocera oleae (Gmelin) infestations unafeccting olive oil quality. IOBC/WPRS Bull. 2006, 30, 153. [Google Scholar]
- Abdel Ghani, N.A.; Galai, M.A.; El-Sayed, M.E.; Samia, M.; Marsafawy, E.; Omran, M.A. Effect of spraying kaolin and calcium carbonate on the productivity of “Aggezi” and “Picual” olive cvs. Int. J. Plant Prod. 2013, 4, 1035–1050. [Google Scholar] [CrossRef]
- Uceda, M.; Hermoso, M.; Aguilera, M.P. La calidad del aceite de oliva. In El Cultivo del Olivo, 2nd ed.; Barranco, D., Fernandez-Escobar, R., Rallo, L., Eds.; Junta de Andalucıa Ediciones Mundi-Prensa: Madrid, Spain, 1998; pp. 547–572. ISBN 84-7114-707-6. [Google Scholar]
- Rotondi, A.; Morrone, L.; Bertazza, G.; Neri, L. Effect of Duration of Olive Storage on Chemical and Sensory Quality of Extra Virgin Olive Oils. Foods 2021, 10, 2296. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Di Virgilio, F.; Belloni, P.; Lercker, G.; Toschi, T.G. In-process monitoring in industrial olive mill by means of FT-NIR. Eur. J. Lipid. Sci. Technol. 2007, 109, 498–504. [Google Scholar] [CrossRef]
- EEC. Commision Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 1991, L248, 1. [Google Scholar]
- Pirisi, F.M.; Cabras, P.; Falqui Cao, C.; Migliorini, M.; Mugelli, M. Phenolic compounds in virgin olive oil. 2. Reappraisal of the extraction, HPLC separation, and quantification procedures. J. Agric. Food Chem. 2000, 48, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Cerretani, L.; Bendini, A.; Biguzzi, B.; Lercker, G.; Gallina Toschi, T. Evaluation of the oxidative stability of extra-virgin olive oils-obtained by different technological plants-with respect to some qualitative parameters. Ind. Aliment. 2003, 42, 706–711. [Google Scholar]
- Morrone, L.; Pupillo, S.; Neri, L.; Bertazza, G.; Magli, M.; Rotondi, A. Influence of olive ripening degree and crusher typology on chemical and sensory characteristics of Correggiolo virgin olive oil. J. Sci. Food Agric. 2017, 97, 1443–1450. [Google Scholar] [CrossRef]
- ISO 4120; 2004 Sensory Analysis—Methodology—Triangle Test. ISO: Geneva, Switzerland, 2004; Volume 2004.
- Glenn, M.D.; Puterka, G.J.; Drake, S.R.; Unruh, T.R.; Knight, A.L.; Baherle, P.; Prado, E.; Baugher, T.A. Particle film application inflences apple leaf physiology, fruit yield, and fruit quality. J. Am. Soc. Hortic. Sci. 2001, 126, 175–181. [Google Scholar] [CrossRef]
- Makus, D.J. Preliminary Observations on Particle Film and Mycorrhizae Use in Tomato Production. HortScience 2000, 35, 555C–555b. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, H.; Di, Y.; Li, Q.; Shao, D.; Shi, J.; Huang, Q. Antitumor activity of Pinoresinol in vitro: Inducing apoptosis and inhibiting HepG2 invasion. J. Funct. Foods 2018, 45, 206–214. [Google Scholar] [CrossRef]
- Ianni, F.; Volpi, C.; Moretti, S.; Blasi, F.; Mondanelli, G.; Varfaj, I.; Galarini, R.; Sardella, R.; Di Renzo, G.C.; Cossignani, L. In-depth characterization of phenolic profiling of Moraiolo extra-virgin olive oil extract and initial investigation of the inhibitory effect on Indoleamine-2, 3-Dioxygenase (IDO1) enzyme. J. Pharm. Biomed. Anal. 2022, 213, 114688. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Stavrianakou, S.; Karabourniotis, G. Trichome layers versus dehaired lamina of Olea europaea leaves: Differences in flavonoid distribution, UV-absorbing capacity, and wax yield. Environ. Exp. Bot. 2006, 55, 294–304. [Google Scholar] [CrossRef]
- Conde, A.; Pimentel, D.; Neves, A.; Dinis, L.T.; Bernardo, S.; Correia, C.M.; Moutinho-Pereira, J. Kaolin foliar application has a stimulatory effect on phenylpropanoid and flavonoid pathways in grape berries. Front. Plant Sci. 2016, 7, 1150. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Correia, C.; Moutinho-Pereira, J.; Oliveira, H.; Freitas, H.; Silva, A.M.; Santos, C. UV-B radiation modulates physiology and lipophilic metabolite profile in Olea europaea. J. Plant Physiol. 2018, 222, 39–50. [Google Scholar] [CrossRef] [PubMed]

| 18-Sep. | 02-Oct. | 24-Oct. | |
|---|---|---|---|
| T | 21.89 ± 1.6 ab | 29.69 ± 0.4 a | 33.01 ± 0.8 b |
| Z | 28.05 ± 1.4 a | 30.1 ± 0.8 a | 40.97 ± 1.2 a |
| K | 21.13 ± 3.3 b | 26.97 ± 2.4 a | 24.68 ± 0.7 c |
| p value | 0.034 | 0.076 | <0.0001 |
| Acidity | Peroxid Number | K232 | K270 | Total Phenol | |
|---|---|---|---|---|---|
| T | 0.31 ± 0.03 | 10.43 ± 0.31 | 1.93 ± 0.05 | 0.17 ± 0.02 | 239.46 ± 8.48 b |
| Z | 0.37 ± 0.03 | 10.75 ± 0.39 | 1.95 ± 0.01 | 0.16 ± 0.01 | 311.61 ± 6.08 a |
| K | 0.30 ± 0.04 | 11.05 ± 0.55 | 1.95 ± 0.04 | 0.15 ± 0.01 | 294.54 ± 7.82 a |
| p-value | 0.051 | 0.290 | 0.747 | 0.305 | <0.0001 |
| Palmitic Acid | Palmitoleic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | Linolenic Acid | |
|---|---|---|---|---|---|---|
| T | 14.68 ± 0.13 | 1.41 ± 0.04 | 2.09 ± 0.06 | 71.35 ± 0.05 | 9.12 ± 0.06 c | 0.70 ± 0.04 a |
| Z | 14.45 ± 0.16 | 1.32 ± 0.04 | 1.93 ± 0.01 | 71.73 ± 0.20 | 9.46 ± 0.02 b | 0.59 ± 0.01 b |
| K | 15.32 ± 1.46 | 1.32 ± 0.26 | 2.10 ± 0.10 | 69.60 ± 1.58 | 10.45 ± 0.18 a | 0.65 ± 0.01 ab |
| p-value | 0.532 | 0.677 | 0.085 | 0.104 | 0.0003 | 0.023 |
| OhTy | Ty | Vanillic Acid | DAOA | Pinoresinol | Luteolin | Luteolin Aglycone | Apigenin | |
|---|---|---|---|---|---|---|---|---|
| T | 2.01 ± 0.26 | 4.15 ± 0.39 b | 1.28 ± 0.07 a | 117.18 ± 10.07 b | 15.77 ± 1.03 b | 3.29 ± 0.18 | 1.61 ± 0.06 | 0.28 ± 0.04 b |
| Z | 2.24 ± 0.25 | 7.18 ± 0.38 b | 1.16 ± 0.03 ab | 297.09 ± 18.5 a | 28.43 ± 1.67 a | 5.55 ± 0.49 | 1.63 ± 0.06 | 0.43 ± 0.06 ab |
| K | 2.04 ± 0.25 | 4.65 ± 0.23 b | 0.36 ± 0.63 b | 142.63 ± 19.21 b | 27.47 ± 3.43 a | 6.27 ± 2.15 | 1.58 ± 0.03 | 0.52 ± 0.07 a |
| p-value | 0.514 | <0.0001 | 0.042 | <0.0001 | 0.001 | 0.066 | 0.483 | 0.007 |
| Δ Tocopherol | β+γ-Tocopherol | α-Tocopherol | Lutein | β Carotene | |
|---|---|---|---|---|---|
| T | 1.72 ± 0.13 b | 2.4 ± 0.24 | 146.96 ± 3.34 b | 1.79 ± 0.01 c | 1.36 ± 0.03 a |
| Z | 2.41 ± 0.25 a | 2.79 ± 0.18 | 170.56 ± 4.52 a | 2.43 ± 0.08 b | 1.19 ± 0.03 b |
| K | 1.89 ± 0.08 b | 2.37 ± 0.07 | 145.27 ± 5.15 b | 2.77 ± 0.06 a | 1.39 ± 0.05 a |
| p-value | 0.006 | 0.051 | 0.001 | <0.0001 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotondi, A.; Bertazza, G.; Faccini, B.; Ferretti, G.; Morrone, L. Effect of Different Foliar Particle Films (Kaolin and Zeolitite) on Chemical and Sensory Properties of Olive Oil. Agronomy 2022, 12, 3088. https://doi.org/10.3390/agronomy12123088
Rotondi A, Bertazza G, Faccini B, Ferretti G, Morrone L. Effect of Different Foliar Particle Films (Kaolin and Zeolitite) on Chemical and Sensory Properties of Olive Oil. Agronomy. 2022; 12(12):3088. https://doi.org/10.3390/agronomy12123088
Chicago/Turabian StyleRotondi, Annalisa, Gianpaolo Bertazza, Barbara Faccini, Giacomo Ferretti, and Lucia Morrone. 2022. "Effect of Different Foliar Particle Films (Kaolin and Zeolitite) on Chemical and Sensory Properties of Olive Oil" Agronomy 12, no. 12: 3088. https://doi.org/10.3390/agronomy12123088
APA StyleRotondi, A., Bertazza, G., Faccini, B., Ferretti, G., & Morrone, L. (2022). Effect of Different Foliar Particle Films (Kaolin and Zeolitite) on Chemical and Sensory Properties of Olive Oil. Agronomy, 12(12), 3088. https://doi.org/10.3390/agronomy12123088






