Yam Staking Reduces Soil Loss Due to Crop Harvesting under Agronomic Management System: Environmental Effect of Soil Carbon Loss
Abstract
1. Introduction
2. Materials and Methods
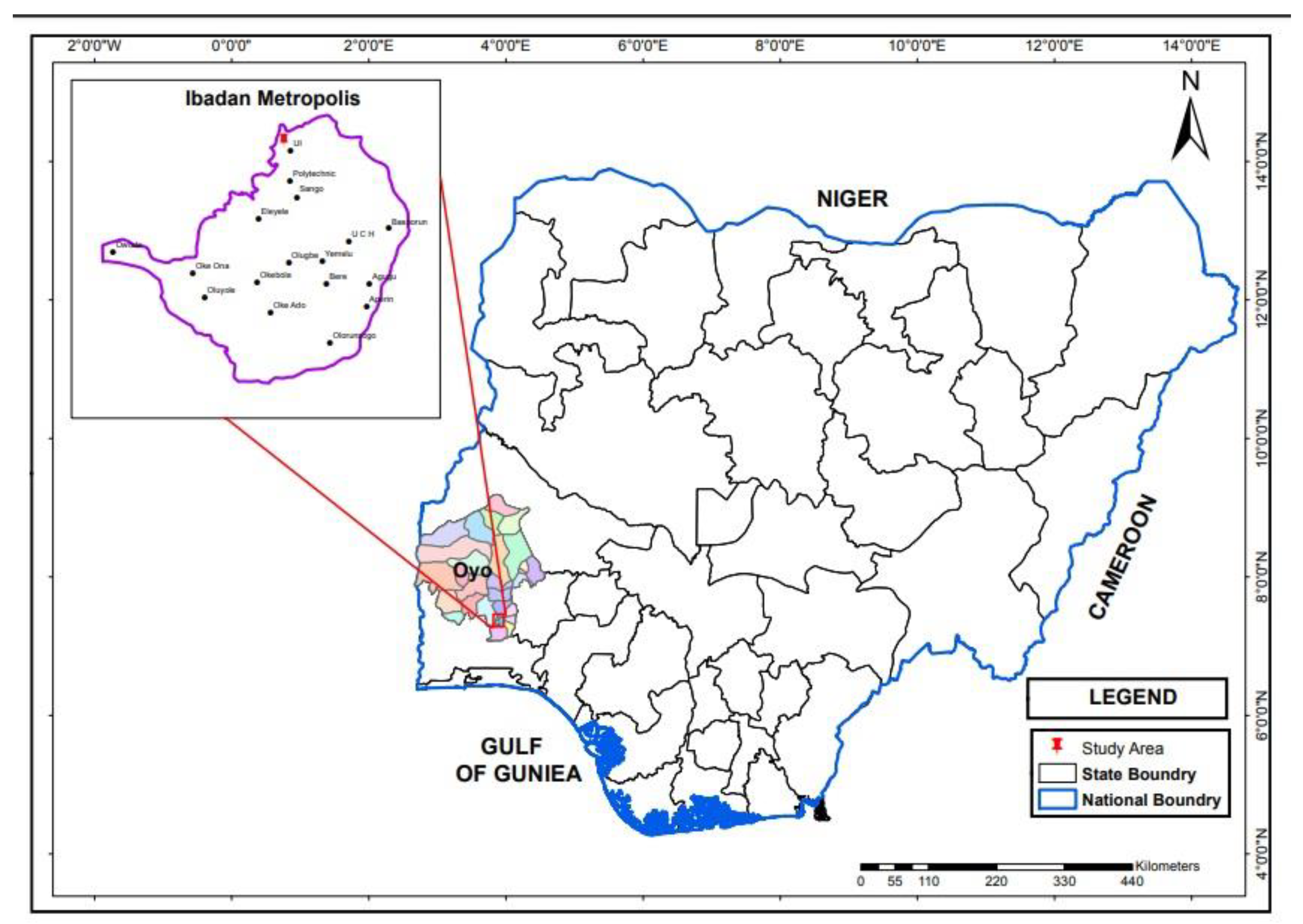
2.1. Site Description
2.2. Soil Sampling and Analysis
2.3. Planting and Staking of Yam Vines
2.4. Estimation of above Ground Total Biomass, Tuber Yield, and Soil and Nutrient Losses
2.5. Data Analysis
3. Results
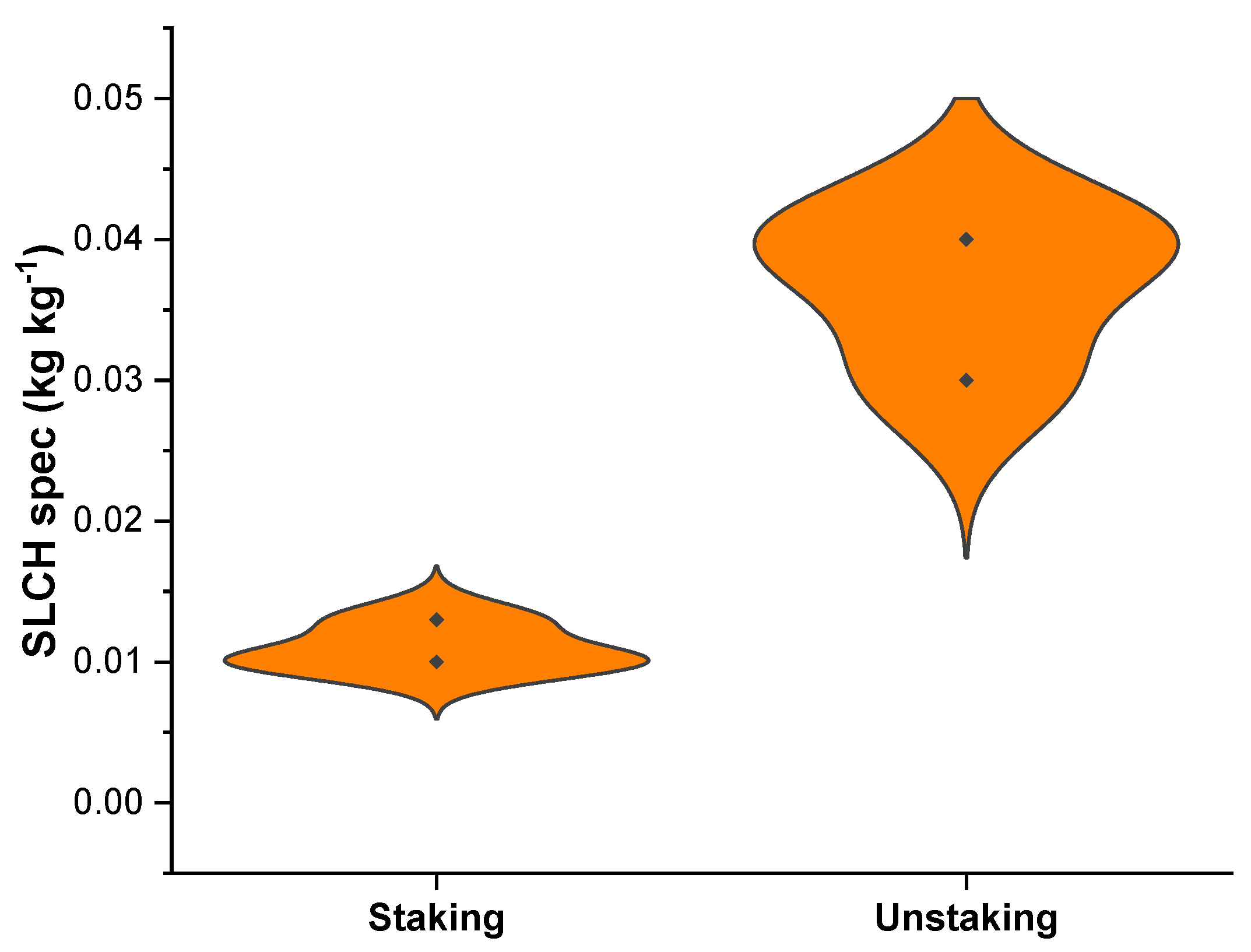
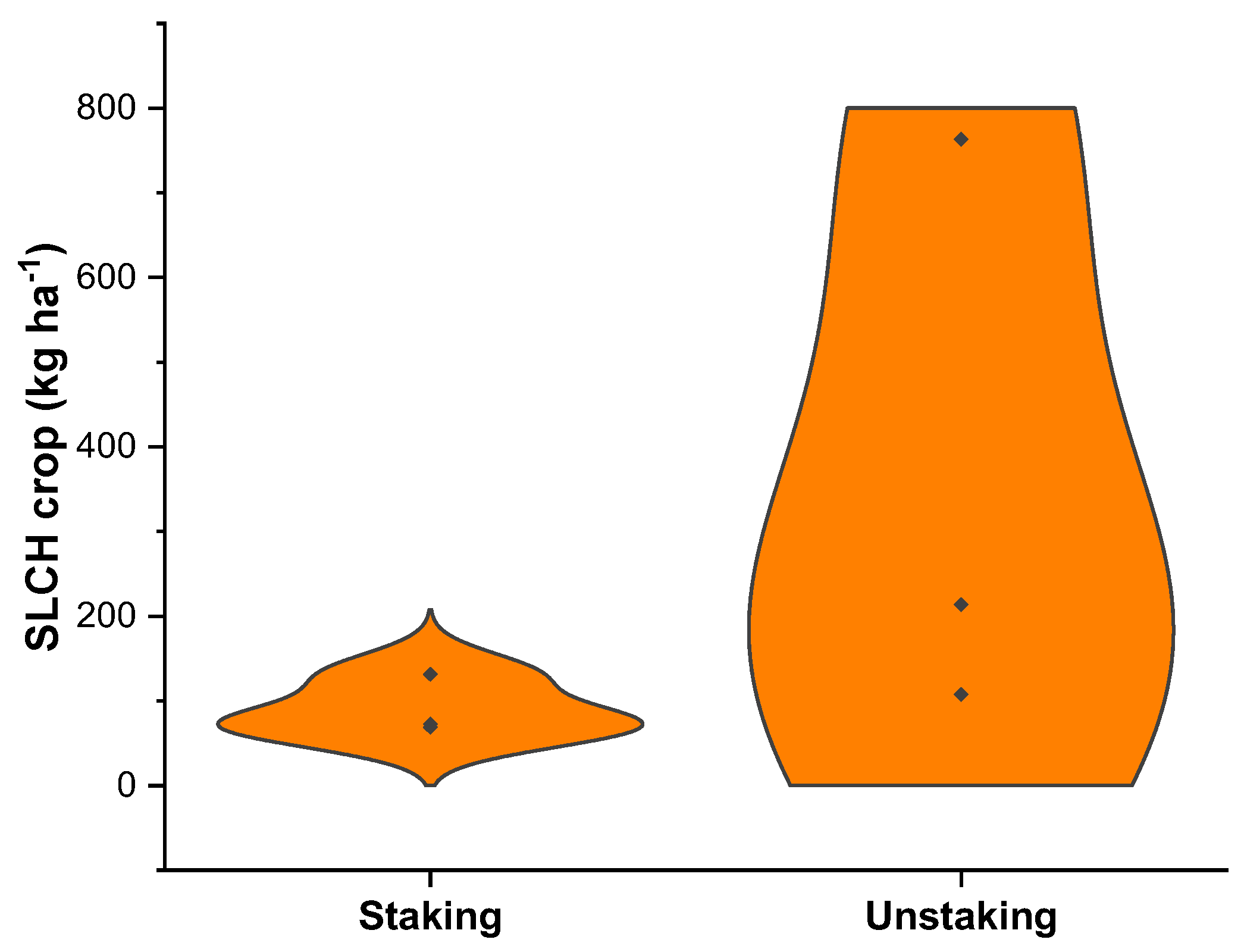
3.1. Soil and Nutrient Losses Due to Crop Harvesting
3.2. Above-Ground Biomass, Root Hair Weight and Tuber Yield
3.3. Relationships of SLCH with Soil Physical Indices and Root Crop Morphology
4. Discussion
4.1. Effects of Mound Moisture and Root Hairs on SLCH
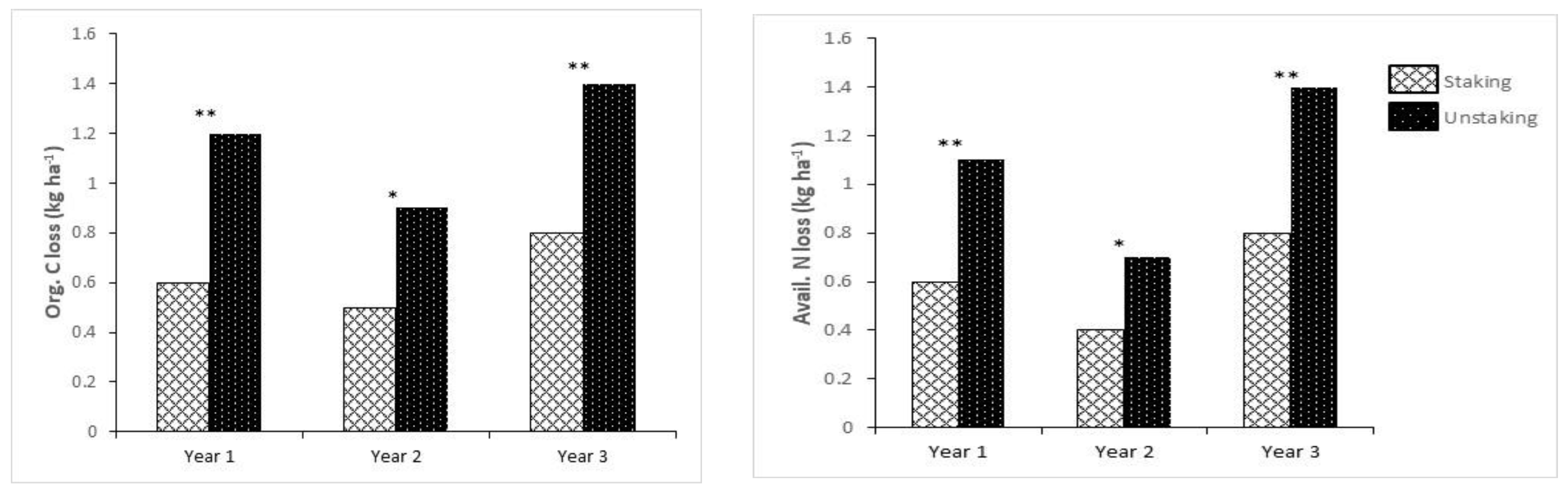
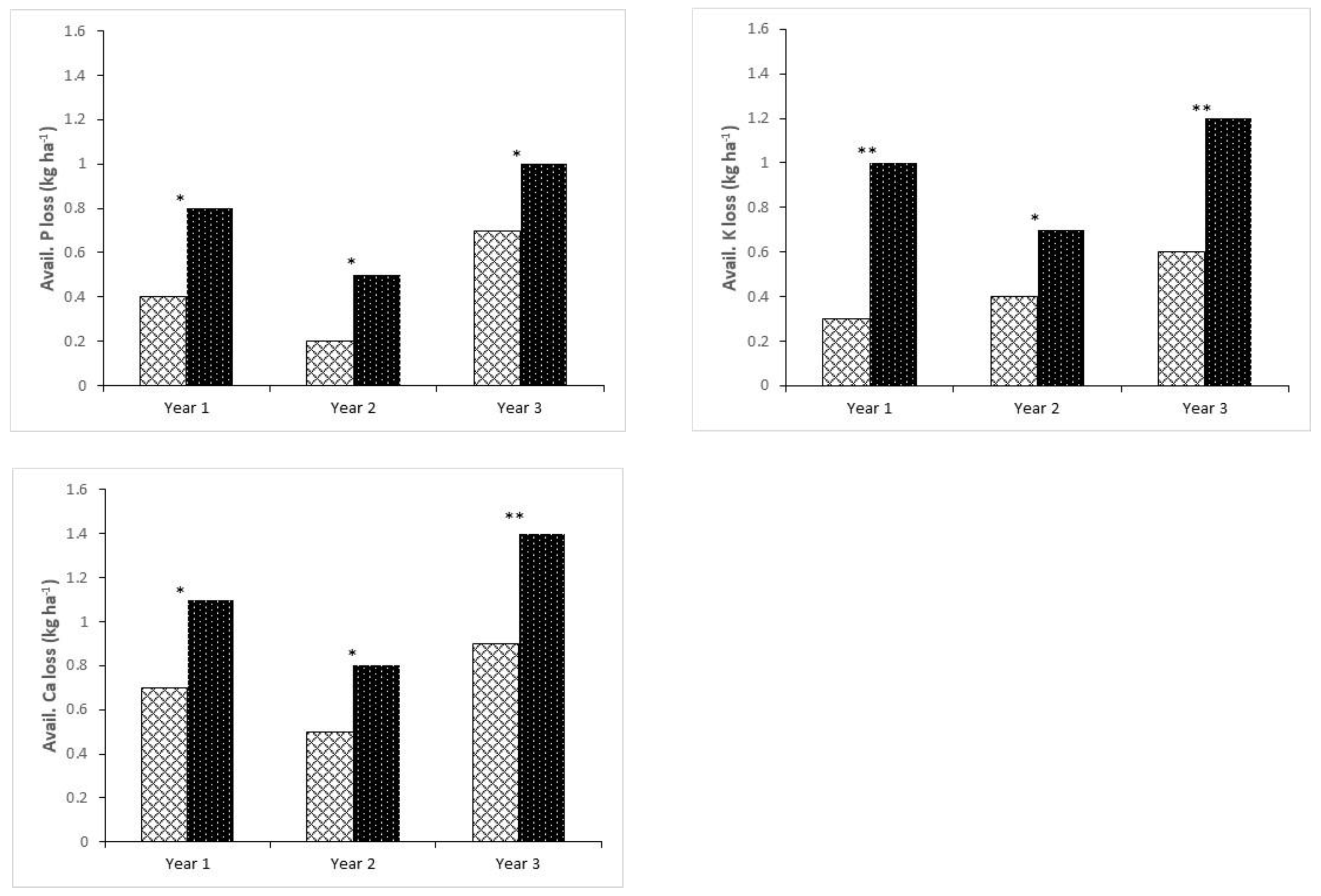
4.2. Influence of Staking on Nutrient Loss
4.3. Environmental Implication of Soil Carbon Loss
4.4. Possible Effect of above-Ground Biomass on Crop Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Degefa, I.; Anabose, B. Traditional agronomic practices of yam (Dioscorea species) in Abaya Woreda, Southern Ethiopia. Int. J. Chem. Nat. Sci. 2017, 5, 499–503. [Google Scholar]
- Cornet, D.; Sierra, J.; Tournewze, R.; Ney, B. Yams (Discorea spp.) plant size hierarchy and yield variability: Emergence time is critical. Eur. J. Agron. 2014, 55, 100–107. [Google Scholar] [CrossRef]
- Mekbib, Y.; Deressa, T. Exploration and collection of root and tuber crops in East Wollega and Ilu Ababora zones: Rescuing declining genetic resources. Indian J. Tradit. Knowl. 2016, 15, 86–92. [Google Scholar]
- Neina, D. Ecological and edaphic drivers of yam production in West Africa. Appl. Environ. Soil Sci. 2021, 21, 13. [Google Scholar] [CrossRef]
- Devi, E.; Jahan, M.N.; Shafiqul, I.M.; Das, M.; Khayer, A.; Sultana, S.E.; Debnath, P.; Zeba, N. Influence of Staking and Non-Staking on Tomatillo (Physalis ixocarpa Brot.) Cultivation in Coastal Areas; Science Academy: Nairobi, Kenya, 2020. [Google Scholar]
- Lamptey, S.; Koomson, E. The role of staking and pruning methods on yield and profitability of tomato (Solanum lycopersicum L.) production in the guinea savanna zone of Ghana. Adv. Agric. 2021, 21, 7. [Google Scholar] [CrossRef]
- Verter, N.; Becvárová, V. The impact of agricultural exports on economic growth in Nigeria. Acta Univ. Agric. et Silvic. Mendel. Brun. 2016, 64, 691–700. [Google Scholar] [CrossRef]
- Norman, P.; Whyte, J.; Samura, A.E. Effect of staking and non-staking systems on disease severity, yield and quality attributes of yams (Discorea alata). J. Agric. Ecol. 2019, 2, 219–229. [Google Scholar]
- Maliki, R.; Toukourou, M.; Sinsin, B.; Vernier, P. Productivity of yam-based system with herbaceous legumes and shot fallows in the Guinea-Sudan transition zone of Benin. Nutr. Cycl. Agroecosystems 2012, 92, 9–19. [Google Scholar] [CrossRef]
- Ennin, S.A.; Issaka, R.N.; Acheampong, P.P.; Numato, M.; Danquah, E.O. Staking options for sustainable yam production in Ghana. Sustain. Agric. Res. 2014, 9, 2222–2230. [Google Scholar]
- Scott, G.J. A review of root, tuber and banana crops in developing countries: Past, present and future. Int. J. Food Sci. Technol. 2020, 56, 1093–1114. [Google Scholar] [CrossRef]
- Ecoma, C.S.; Ecoma, L.E. Edele festival of the itigidi people of cross river state and its impact. Int. J. Asian Soc. Sci. 2013, 3, 1781–1792. [Google Scholar]
- Kuhwald, M.; Busche, F.; Saggau, P.; Duttmann, R. Is soil loss due to crop harvesting the most disregarded soil erosion process? A review of harvest erosion. Soil Tillage Res. 2022, 215, 1–12. [Google Scholar] [CrossRef]
- Poesen, J.; Verstraeten, G.; Soenens, R.; Seynaeve, L. Soil losses due to harvesting of chicory roots and sugar beet: An underrated geomorphic process. Catena 2001, 43, 35–47. [Google Scholar] [CrossRef]
- Biesmans, M. Bodemverlies door het rooien van suikerbieten en aardappelen: Ruimtelijke Variatie op Perceels–en Regionaal niveau. Unplublished M.Sc. Thesis, Department of Geography, K.U. Leuven, Belgium, 2012. [Google Scholar]
- Oztas, T.; Ozbek, A.K.; Turan, M. The cost of soil lost from fields due to removal on harvested sugar beet: A case study in Turkey. Soil Use Manag. 2002, 18, 236–237. [Google Scholar] [CrossRef]
- Ruysschaert, G.; Poesen, J.; Verstraeten, G.; Govers, G. Soil loss due to crop harvesting: Significance and determining factors. Prog. Phys. Geogr. Earth Environ. 2004, 28, 467–501. [Google Scholar] [CrossRef]
- Mwango, S.B.; Msanya, B.M.; Mtakwa, P.W.; Kimaro, D.N.; Deckers, J.; Poesen, J.; Massawe, J.S. Effectiveness of selected soil conservation practices on soil erosion control and crop yields in the Usambara Mountains, Tanzania. J. Agric. Econ. Res. 2015, 2, 129–144. [Google Scholar] [CrossRef]
- Dada, P.O.O.; Adeyanju, O.R.; Adeosun, O.J.; Adewumi, J.K. Effects of soil physical properties on soil loss due to manual yam harvesting under a sandy loam environment. Int. Soil Water Conserv. Res. 2016, 4, 121–125. [Google Scholar] [CrossRef]
- Oshunsanya, S.O. Alternative methods of reducing soil loss due to harvesting of sweet potato: A case study of low input agriculture in Nigeria. Soil Tillage Res. 2016, 158, 49–56. [Google Scholar] [CrossRef]
- Oshunsanya, S.O. Quantification of soil loss due to white cocoyam (Colocasia esculentus) and red cocoyam (Xanthosoma sagittifolium) harvesting in traditional farming practice. Catena 2016, 137, 134–143. [Google Scholar] [CrossRef]
- Parlak, M.; Palta, C.; Yokus, S.; Blanco-Canqui, H.; Carkaci, D.A. Soil losses due to carrot harvesting in south central Turkey. Catena 2016, 140, 24–30. [Google Scholar] [CrossRef]
- Yu, H.Q.; Li, Y.; Zhou, N.; Chappell, A.; Li, X.Y.; Poesen, J. Soil nutrient loss due to tuber crop harvesting and its environment impact in the North China Plain. J. Integr. Agric. 2016, 15, 1612–1624. [Google Scholar] [CrossRef]
- Oshunsanya, S.O.; Yu, H.; Li, Y. Soil loss due to root crop harvesting increases with tillage operations. Soil Tillage Res. 2018, 181, 93–101. [Google Scholar] [CrossRef]
- Oshunsanya, S.O.; Yu, H.Q.; Li, Y.; Saggar, S. Root hairs and cortex contribute to soil loss due to root crop harvesting. Catena 2018, 174, 514–523. [Google Scholar] [CrossRef]
- Thomaz, E.Z.; Bereze, J. Soil loss due to crop harvest in Southern Brazil: Effect of potato morphology. Plant Soil 2021, 468, 67–76. [Google Scholar] [CrossRef]
- Eswaran, H.; Lal, R.; Reich, P.F. Land degradation: An overview. Responses to land degradation. In Proceedings of the Second International Conference on Land Degradation and Desertification, Khon Kaen, Thailand, 12–16 October 2021; Oxford Press: New Delhi, India, 2021; p. 77. [Google Scholar]
- Li, Y.; Ruysschaert, G.; Poesen, J.; Zhang, Q.W.; Bai, L.Y.; Li, L.; Sun, L.F. Soil losses due to potato and sugar beet harvesting in NE China. Earth Surf. Process. Landforms 2006, 31, 1003–1016. [Google Scholar] [CrossRef]
- FAO, FAOSTAT Database. Food and Agriculture Organization, Roma, Italy. Available online: http://faostst.fao.org (accessed on 21 February 2022).
- Pascuzzi, S.; Santoro, F. Analysis of the almond harvesting and hulling mechanization process: A case study. Agriculture 2017, 7, 100. [Google Scholar] [CrossRef]
- Bulgakov, V.; Pascuzzi, S.; Santoro, F.; Anifantis, A.S. Mathematical model of the plane-parallel movement of the self-propelled root-harvesting machine. Sustainability 2018, 10, 3614. [Google Scholar] [CrossRef]
- Parlak, M.; Cicek, G.; Blano-Canqui, H. Celery harvesting causes losses of soil: A case study in Turkey. Soil Tillage Res. 2018, 180, 204–209. [Google Scholar] [CrossRef]
- Mclean, E.O. Soil pH and lime requirements. In Methods of Soil Analysis, Part 2, Chemical and Microbial Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph 9; ASA; SSSA: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Walkley, A.; Black, C.A. An examination of the different method for determining soil organic matter and a proposed modification to the chronic titration method. Soil Sci. 1932, 37, 29–38. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.W. Total carbon, organic carbon, organic matter. In Methods of soil Analysis, Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph 9; Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Miller, P.H., Keeney, D.R., Eds.; Chemical and Microbiological Properties, Agronomy 9/2; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–427. [Google Scholar]
- Rhodes, J.D. Cation exchange capacity. In Methods of Soil Analysis, PART 2, Chemical Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 149–157. [Google Scholar]
- Grossman, R.B.; Reinsch, T.G. Bulk density and linear extensibility: Core method. In Methods of Soil Analysis. Part 4, Physical Methods, SSSA.; Dane, J.H., Topp, G.C., Eds.; Incorporated: Madison, WI, USA, 2002; pp. 208–228. [Google Scholar]
- Asiedu, R.; Sartie, A. Crops that feed the world 1. Yams. Yams for income and food security. Food Secur. 2010, 2, 305–315. [Google Scholar] [CrossRef]
- Faraji, M.; Chakan, A.A.; Jafarizadeh, M.; Behbahani, A.M. Soil and nutrient losses due to root crops harvesting: A case study from Southwestern Iran. Arch. Agron. Soil Sci. 2017, 63, 1523–1534. [Google Scholar] [CrossRef]
- Oshunsanya, S.O.; Samson, V.M.; Yu, H. Soil loss due to harvesting of peanut and cassava under traditional farming system: Cost implications of soil nutrient loss. J. Soil Water Conserv. 2022, 77, 240–248. [Google Scholar] [CrossRef]
- Parlak, M.; Everest, T.; Blanco, H. Soil loss due to sugar beet harvesting in Northwestern Turkey. J. Soil Sci. Plant Nutr. 2021, 21, 2993–3001. [Google Scholar] [CrossRef]
- Isabirye, M.; Ruysschaert, G.; Van linden, L.; Poesen, J.; Magunda, M.K.; Deckers, J. Soil losses due to cassava and sweet potato harvesting: A case study from low input traditional agriculture. Soil Tillage Res. 2007, 92, 96–103. [Google Scholar] [CrossRef]
- Mwango, S.B.; Msanya, B.M.; Mkatwa, P.W.; Kimaro, D.N.; Deckers, D.N.; Poesen, J.; Sanga, E. Soil loss due to crop harvesting in Usambara Mountains Tanzania. The case of carrot, onion and potato. Int. J. Plant Soil Sci. 2015, 4, 18–28. [Google Scholar] [CrossRef]
- Parlak, M.; Everest, T.; Ruis, S.J.; Blanco, H. Impact of urbanization on soil loss: A case study from sod production. Environ. Monit. Assess. 2020, 192, 588. [Google Scholar] [CrossRef]
- Sumithra, R.; Thushyanthy, M.; Srivaratharasan, T. Assessment of soil loss and nutrient depletion due to cassava harvesting: A case study from low input traditional agriculture. Int. Soil Water Conserv. Res. 2013, 1, 72–79. [Google Scholar] [CrossRef]
- Ahmadi, J.; Pour-Aboughadareh, A.; Fabriki-Ourang, S.; Mehrabi, A.; Siddique, K.H.M. Screening wheat germplasm for seedling root architectural traits under contrasting water regimes: Potential sources of variability for drought adaptation. Arch. Agron. Soil Sci. 2018, 64, 1351–1365. [Google Scholar] [CrossRef]
- Cucci, G.; Lacolla, G.; Caranfa, G. Spatial distribution of roots and cracks in soils cultivated with sunflower. Arch. Agron. Soil Sci. 2018, 64, 1. [Google Scholar] [CrossRef]
- Tugrul, K.M.; Icoz, E.; Perendeci, N.A. Determination of soil loss by sugar beet harvesting. Soil Tillage Res. 2012, 123, 71–77. [Google Scholar] [CrossRef]
- Orkwor, G.C.; Asadu, C.L.A. Agronomy. In Food Yams: Advances in Research; Science Academy: Washington, DC, USA, 1998; pp. 42–51. [Google Scholar]
- Kathabwalika, D.M.; Chilembwe, E.H.C.; Mwale, V.M.; Kambewa, D.; Njoloma, J.P. Plant growth and yield stability of orange fleshed sweet potato (Ipomoea batatas) in three agro-ecological zones of Malawi. Int. J. Agric. Sci. 2013, 3, 383–392. [Google Scholar]
- Ahmed, M.; Nigussie-Dechassa, R.; Abebie, B. Effects of planting methods and vine harvesting on shoots and tuberous root yields of sweet potato (Ipomoea batatas Lam.) in the Afar region of Ethiopia. Afr. J. Agric. Res. 2012, 7, 1129–1141. [Google Scholar]
- Kumar, J. Lager yam. In Underutilized and Underexploited Horticultural Crops; Peter, K.V., Ed.; New India Publishing Agency: New Delhi, India, 2007; Volume 1, pp. 37–56. [Google Scholar]
- Vermeulen, G.D.; Koolen, A.J. Soil dynamics of the origination of soil tare during sugar beet lifting. Soil Tillage Res. 2002, 65, 169–184. [Google Scholar] [CrossRef]

| Parameter | Staking | Un-Staking | T-Test (p-Value) | Staking | Un-Staking | T-Test (p-Value) | Staking | Un-Staking | T-Test (p-Value) |
|---|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 2 | |||||||
| AGBDM (kg ha−1) | 1624.61 | 1225.37 | 2.45 (0.005) | 1462.18 | 831.42 | 21.4 (0.001) | 2765.45 | 2067.11 | 19.32 (0.001) |
| RHW (kg ha−1) | 8.82 | 14.10 | 1.37 (0.001) | 6.40 | 9.87 | 1.39 (0.001) | 29.70 | 32.60 | 8.16 (0.001) |
| Tuber yield (t ha−1) | 7.22 | 5.34 | 1.09 (0.001) | 6.86 | 3.58 | 1.05 (0.001) | 13.11 | 9.27 | 1.79 (0.001) |
| RHW/Tuber yield | 1.22 × 10−3 | 2.64 × 10−3 | 0.001 (0.01) | 9.33 × 10−4 | 2.76 × 10−3 | 0.001 (0.01) | 2.27 × 10−3 | 3.52 × 10−3 | 0.001 (0.05) |
| Parameter | Staking | Un-Staking | T-Test (p-Value) | Staking | Un-Staking | T-Test (p-Value) | Staking | Un-Staking | T-Test (p-Value) |
|---|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | |||||||
| Coarse sand (g kg−1) | 625.4 | 623.4 | ns (ns) | 631.0 | 633.0 | ns (ns) | 628.0 | 629.0 | ns (ns) |
| Fine sand (g kg−1) | 216.0 | 218.0 | ns (ns) | 211.2 | 207.7 | ns (ns) | 211.2 | 209.6 | ns (ns) |
| Silt (g kg−1) | 63.0 | 63.0 | ns (ns) | 60.8 | 62.6 | ns (ns) | 61.8 | 63.5 | ns (ns) |
| Clay (g kg−1) | 95.6 | 95.6 | ns (ns) | 97.0 | 96.7 | ns (ns) | 99.0 | 97.9 | ns (ns) |
| Textural class | LS | LS | LS | LS | LS | LS | |||
| Bulk density (Mg m−3) | 1.38 | 1.39 | ns (ns) | 1.42 | 1.41 | ns (ns) | 1.45 | 1.45 | ns (ns) |
| ϴ (m3 m−3) | 0.19 | 0.32 | 0.02 (0.01) | 0.14 | 0.27 | 0.02 (0.01) | 0.18 | 0.36 | 0.01 (0.001) |
| SLCHcrop | TY | RHW | Sand | Silt | Clay | BD | Moisture | RHW/TY | AGMDM | |
|---|---|---|---|---|---|---|---|---|---|---|
| Staking | ||||||||||
| SLCHspec | 0.94 *** | 0.58 * | 0.96 *** | −0.10 | 0.12 | 0.09 | −0.42 * | 0.92 *** | 0.65 * | 0.46 * |
| SLCHcrop | 0.55 * | 0.91 *** | −0.17 | 0.08 | 0.12 | −0.11 | 0.84 *** | 0.61 * | 0.43 * | |
| TY | 0.91 *** | −0.30 | 0.02 | 0.29 | −0.55 * | 0.54 * | 0.64 * | 0.74 ** | ||
| RHW | 0.11 | −0.42 * | 0.15 | −0.47 * | 0.88 *** | 0.89 *** | 0.88 *** | |||
| Sand | −0.38 * | −0.72 ** | −0.05 | −0.25 | −0.01 | −0.21 | ||||
| Silt | −0.36 | 0.01 | 0.10 | 0.12 | 0.37 | |||||
| Clay | 0.05 | 0.18 | −0.06 | 0.42 | ||||||
| BD | −0.05 | −0.45 * | −0.11 | |||||||
| Moisture | 0.76 ** | 0.74 ** | ||||||||
| RHW/TY | 0.58 * | |||||||||
| Un-staking | ||||||||||
| SLCHspec | 0.95 *** | 0.76 ** | 0.96 *** | 0.09 | 0.02 | 0.11 | −0.16 | 0.70 ** | 0.59 * | 0.42 * |
| SLCHcrop | 0.86 *** | 0.88 *** | −0.14 | 0.09 | 0.10 | −0.12 | 0.89 *** | 0.58 * | 0.41 * | |
| TY | 0.55 * | −0.23 | 0.11 | 0.19 | −0.28 | 0.75 ** | 0.60 * | 0.63 * | ||
| RHW | −0.10 | −0.12 | 0.19 | −0.27 | 0.78 ** | 0.81 ** | 0.75 ** | |||
| Sand | −0.48 * | −0.79 ** | −0.20 | −0.05 | −0.22 | −0.05 | ||||
| Silt | −0.15 | 0.27 | 0.03 | 0.02 | 0.01 | |||||
| Clay | 0.04 | 0.04 | 0.22 | 0.23 | ||||||
| BD | −0.03 | 0.47 * | −0.39 | |||||||
| Moisture | 0.71 ** | 0.52 * | ||||||||
| RHW/TY | 0.48 * | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshunsanya, S.; Yu, H.; Onunka, C.; Samson, V.; Odebode, A.; Sebiotimo, S.; Xue, T. Yam Staking Reduces Soil Loss Due to Crop Harvesting under Agronomic Management System: Environmental Effect of Soil Carbon Loss. Agronomy 2022, 12, 3024. https://doi.org/10.3390/agronomy12123024
Oshunsanya S, Yu H, Onunka C, Samson V, Odebode A, Sebiotimo S, Xue T. Yam Staking Reduces Soil Loss Due to Crop Harvesting under Agronomic Management System: Environmental Effect of Soil Carbon Loss. Agronomy. 2022; 12(12):3024. https://doi.org/10.3390/agronomy12123024
Chicago/Turabian StyleOshunsanya, Suarau, Hanqing Yu, Chibuzo Onunka, Victor Samson, Ayodeji Odebode, Shamsideen Sebiotimo, and Tingting Xue. 2022. "Yam Staking Reduces Soil Loss Due to Crop Harvesting under Agronomic Management System: Environmental Effect of Soil Carbon Loss" Agronomy 12, no. 12: 3024. https://doi.org/10.3390/agronomy12123024
APA StyleOshunsanya, S., Yu, H., Onunka, C., Samson, V., Odebode, A., Sebiotimo, S., & Xue, T. (2022). Yam Staking Reduces Soil Loss Due to Crop Harvesting under Agronomic Management System: Environmental Effect of Soil Carbon Loss. Agronomy, 12(12), 3024. https://doi.org/10.3390/agronomy12123024






