A Comparative Evaluation of Aquaponic and Soil Systems on Yield and Antioxidant Levels in Basil, an Important Food Plant in Lamiaceae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Location
2.2. Seed Collection and Germination
2.3. Experimental Setup
2.4. Fish Selection and Growth Conditions
2.5. Plant Growth Conditions and Parameters Analyzed
2.6. Estimation of Photosynthetic Pigments
2.7. Biochemical Analysis
2.8. Non-Enzymatic Antioxidant Contents
2.9. Antioxidant Enzymes
2.10. Microelements and Macroelements
2.11. Statistical Analysis
3. Results
3.1. Morphological Parameters
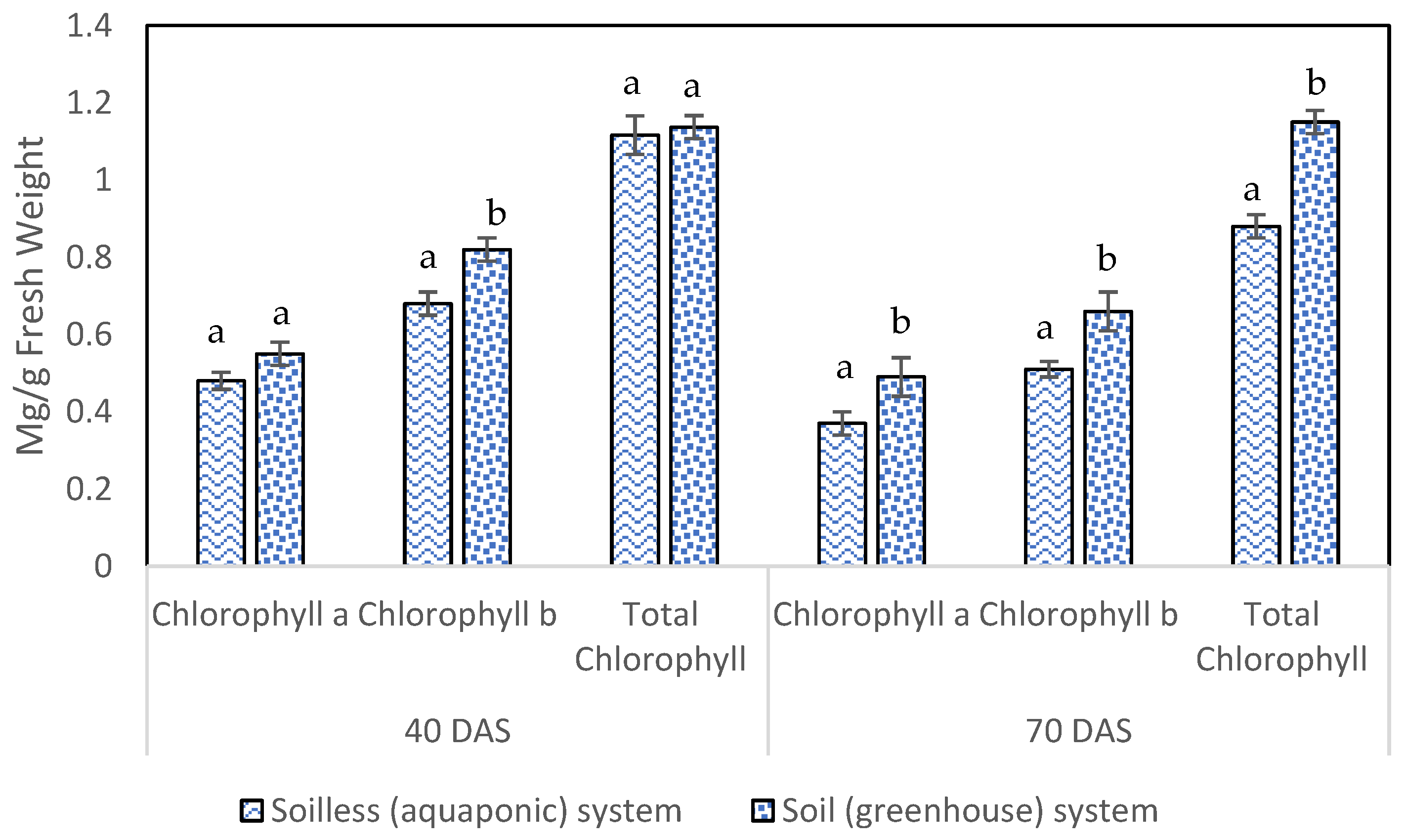
3.2. Photosynthetic Pigments
3.3. Biochemical Parameters
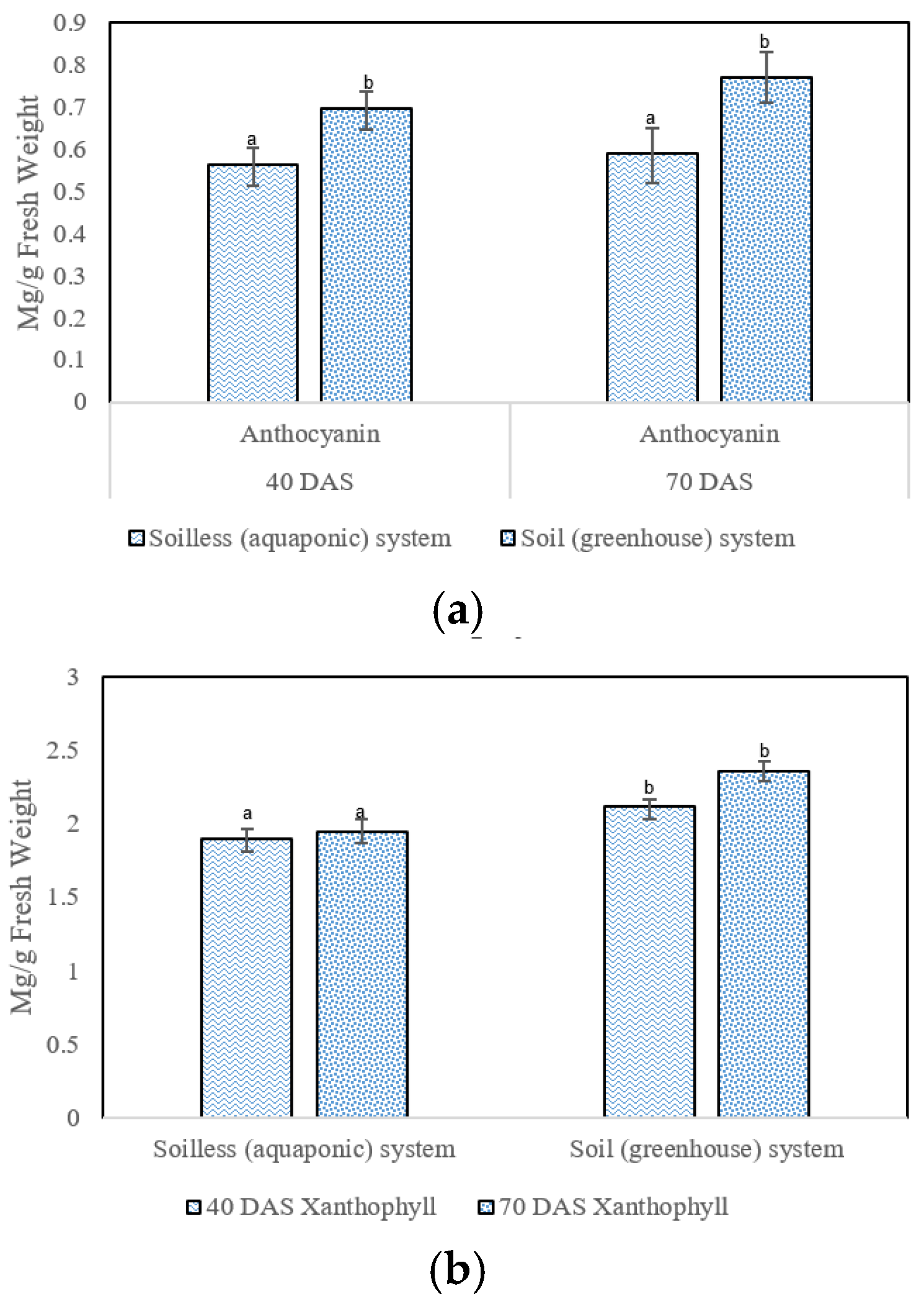
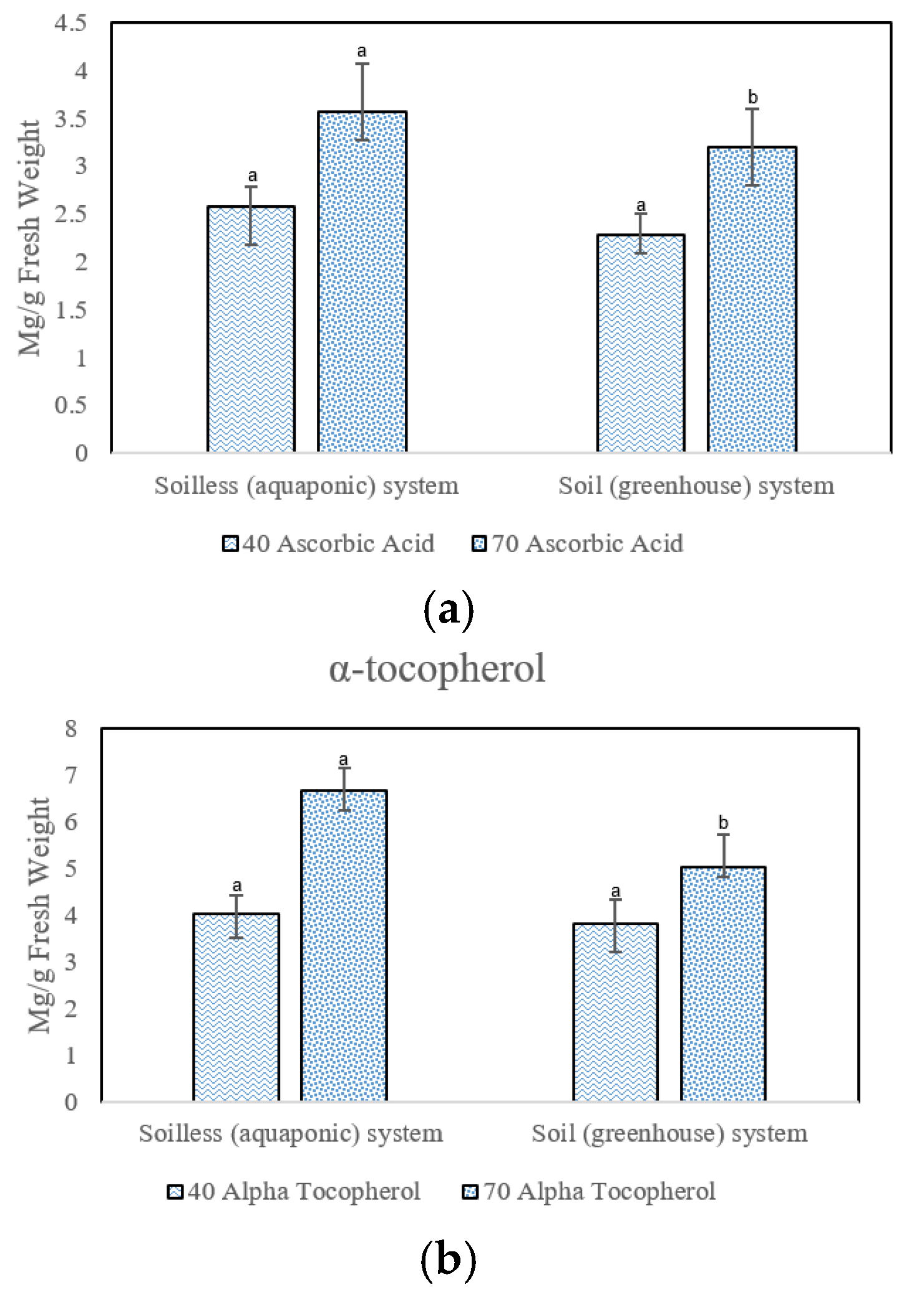
3.4. Antioxidants
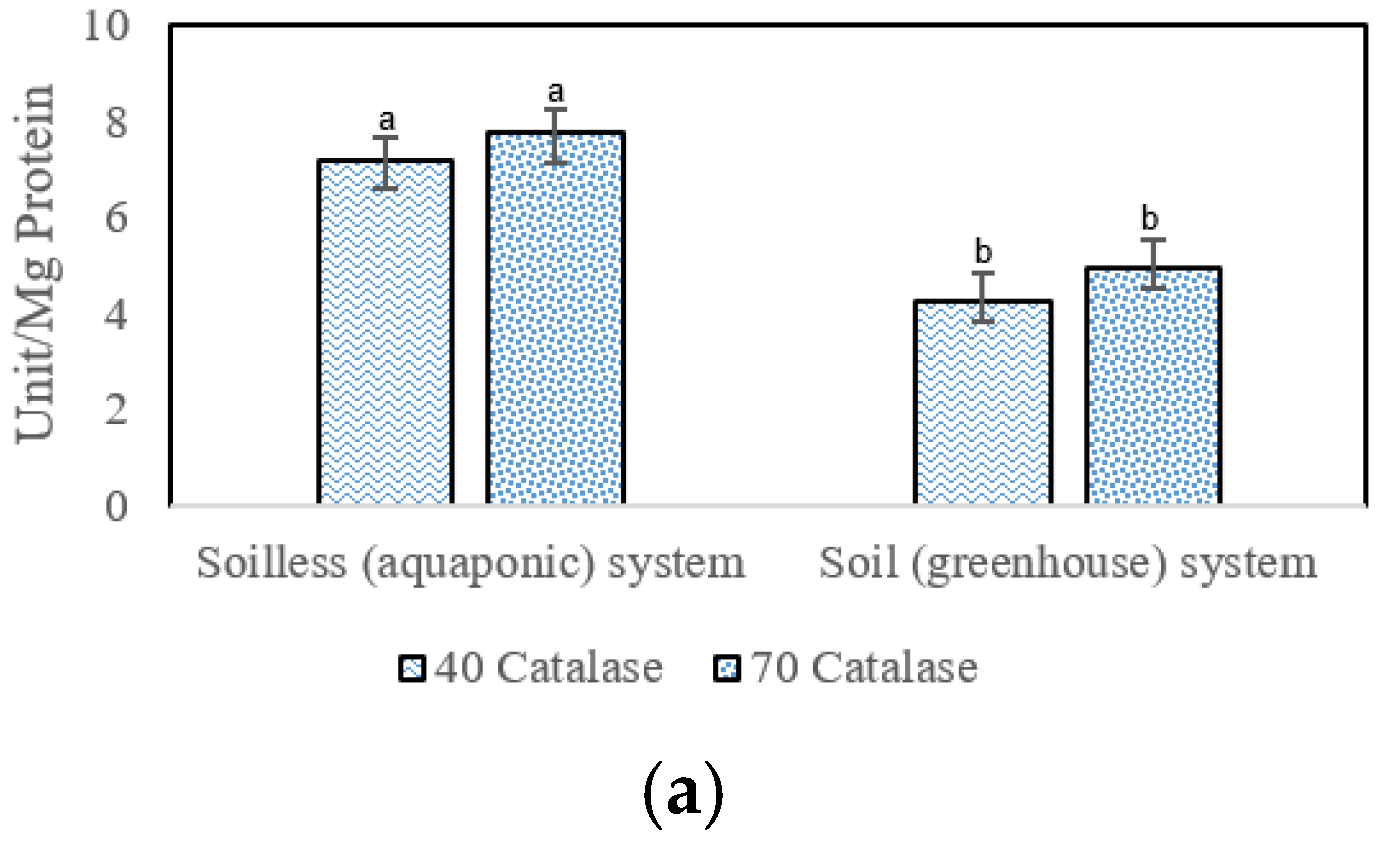
3.5. Antioxidant Enzyme Activities
3.6. Macronutrients and Micronutrients
3.7. Water Parameters
4. Discussion
4.1. Plant Growth Parameters
4.2. Photosynthetic Pigment Contents
4.3. Biochemical Analysis
4.4. Non-Enzymatic Antioxidant Contents
4.5. Antioxidant Enzymes
4.6. Microelements and Macroelements
4.7. Water Quality Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blidariu, F.; Grozea, A. Increasing the economic efficiency and sustainability of indoor fish farming by means of aquaponics-review. Anim. Sci. Biotechnol. 2011, 44, 1–8. [Google Scholar]
- Yildiz, H.Y.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish Welfare in Aquaponic Systems: Its Relation to Water Quality with an Emphasis on Feed and Faeces—A Review. Water 2017, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.M.; Akram, M.T.; Janke, R.; Qadri, R.W.K.; Al-Sadi, A.M.; Farooque, A.A. Urban Horticulture for Food Secure Cities through and beyond COVID-19. Sustainability 2020, 12, 9592. [Google Scholar] [CrossRef]
- Al Shamsi, K.B.; Compagnoni, A.; Timpanaro, G.; Cosentino, S.L.; Guarnaccia, P. A Sustainable Organic Production Model for “Food Sovereignty” in the United Arab Emirates and Sicily-Italy. Sustainability 2018, 10, 620. [Google Scholar] [CrossRef] [Green Version]
- Joyce, A.; Timmons, M.; Goddek, S.; Pentz, T. Bacterial Relationships in Aquaponics: New Research Directions. In Aquaponics Food Production Systems; Springer: Cham, Switzerland, 2019; pp. 145–161. [Google Scholar]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of Sustainable and Commercial Aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Liang, S.; Wang, J.; Yan, R. Attempts to improve nitrogen utilization efficiency of aquaponics through nitrifies addition and filler gradation. Environ. Sci. Pollut. Res. 2016, 23, 6671–6679. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges–A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Pinho, S.M.; David, L.H.; Garcia, F.; Keesman, K.J.; Portella, M.C.; Goddek, S. South American fish species suitable for aquaponics: A review. Aquac. Int. 2021, 29, 1427–1449. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Chien, Y.-H. Effects of feeding frequency and photoperiod on water quality and crop production in a tilapia–water spinach raft aquaponics system. Int. Biodeterior. Biodegrad. 2013, 85, 693–700. [Google Scholar] [CrossRef]
- Maucieri, C.; Forchino, A.A.; Nicoletto, C.; Junge, R.; Pastres, R.; Sambo, P.; Borin, M. Life cycle assessment of a micro aquaponic system for educational purposes built using recovered material. J. Clean. Prod. 2018, 172, 3119–3127. [Google Scholar] [CrossRef]
- Hu, F.; Li, Y.; Wang, Q.; Zhu, B.; Wu, S.; Wang, Y.; Zeng, W.; Yin, J.; Liu, C.; Bergmann, S.M.; et al. Immersion immunization of koi (Cyprinus carpio) against cyprinid herpesvirus 3 (CyHV-3) with carbon nanotube-loaded DNA vaccine. Aquaculture 2021, 539, 736644. [Google Scholar] [CrossRef]
- Adamek, M.; Oschilewski, A.; Wohlsein, P.; Jung-Schroers, V.; Teitge, F.; Dawson, A.; Gela, D.; Piackova, V.; Kocour, M.; Adamek, J.; et al. Experimental infections of different carp strains with the carp edema virus (CEV) give insights into the infection biology of the virus and indicate possible solutions to problems caused by koi sleepy disease (KSD) in carp aquaculture. Vet. Res. 2017, 48, 12. [Google Scholar] [CrossRef] [Green Version]
- Mokat, D.N.; Kharat, T.D. Essential Oil Composition in Leaves of Ocimum Species Found in Western Maharashtra, India. J. Essent. Oil Bear. Plants 2022, 25, 1–8. [Google Scholar] [CrossRef]
- Knaus, U.; Pribbernow, M.; Xu, L.; Appelbaum, S.; Palm, H.W. Basil (Ocimum basilicum) cultivation in decoupled aquaponics with three hydro-components (grow pipes, raft, gravel) and African catfish (Clarias gariepinus) production in Northern Germany. Sustainability 2020, 12, 8745. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Mochona, B.; Qi, X. Biosynthetic factories of essential oils: The aromatic plants. Nat. Prod. Chem. Res. 2016, 4, 227. [Google Scholar] [CrossRef] [Green Version]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2021, 275, 109733. [Google Scholar] [CrossRef]
- Pasch, J.; Ratajczak, B.; Appelbaum, S.; Palm, H.W.; Knaus, U. Growth of basil (Ocimum basilicum) in DRF, raft, and grow pipes with effluents of African catfish (Clarias gariepinus) in decoupled aquaponics. AgriEngineering 2021, 3, 92–109. [Google Scholar] [CrossRef]
- Babmann, B.; Harbach, H.; Weißbach, S.; Palm, H.W. Effect of plant density in coupled aquaponics on the welfare status of African catfish, Clarias gariepinus. J. World Aquac. Soc. 2020, 51, 183–199. [Google Scholar] [CrossRef]
- Hundley, G.C.; Navarro, F.K.S.P.; Filho, O.P.R.; Navarro, R.D. Integration of Nile tilapia (Oreochromis niloticus L.) production Origanum majorana L. and Ocimum basilicum L. using aquaponics technology. Acta Sci. Technol. 2018, 40, 35460. [Google Scholar] [CrossRef] [Green Version]
- Obirikorang, K.A.; Sekey, W.; Gyampoh, B.A.; Ashiagbor, G.; Asante, W. Aquaponics for Improved Food Security in Africa: A Review. Front. Sustain. Food Syst. 2021, 5, 705549. [Google Scholar] [CrossRef]
- Pandey, J.; Verma, R.K.; Singh, S. Suitability of aromatic plants for phytoremediation of heavy metal contaminated areas: A review. Int. J. Phytoremed. 2019, 21, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Bahl, J.R.; Singh, A.K.; Lal, R.K.; Gupta, A.K. High-yielding improved varieties of medicinal and aromatic crops for enhanced income. In New Age Herbals; Springer: Singapore, 2018; pp. 247–265. [Google Scholar]
- Yang, T.; Kim, H.-J. Comparisons of nitrogen and phosphorus mass balance for tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. J. Clean. Prod. 2020, 274, 122619. [Google Scholar] [CrossRef]
- Tolay, I. The impact of different Zinc (Zn) levels on growth and nutrient uptake of Basil (Ocimum basilicum L.) grown under salinity stress. PLoS ONE 2021, 16, e0246493. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.E.; Morales, M.R.; Phippen, W.B.; Vieira, R.F.; Hao, Z. Basil: A source of aroma compounds and a popular culinary and ornamental herb. Perspect. New Crops New Uses 1999, 16, 499–505. [Google Scholar]
- Quagrainie, K.K.; Flores, R.M.V.; Kim, H.-J.; McClain, V. Economic analysis of aquaponics and hydroponics production in the U.S. Midwest. J. Appl. Aquac. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Danner, R.I.; Mankasingh, U.; Anamthawat-Jonsson, K.; Thorarinsdottir, R.I. Designing Aquaponic Production Systems towards Integration into Greenhouse Farming. Water 2019, 11, 2123. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Thompson, S.; Glaser, M. Global Aquaculture Productivity, Environmental Sustainability, and Climate Change Adaptability. Environ. Manag. 2019, 63, 159–172. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Beggs, C.J.; Wellmann, E. Analysis of light-controlled anthocyanin formation in coleoptiles of Zea mays L.: The role of UV-B, blue, red and far-red light. Photochem. Photobiol. 1985, 41, 481–486. [Google Scholar] [CrossRef]
- Neogy, M.; Datta, J.K.; Mukherji, S.; Roy, A.K. Effect of aluminium on pigment content, Hill activity and seed yield in mungbean. Indian J. Plant Physiol. 2001, 6, 381–385. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo-Enzymology; Kalyani Publications: New Delhi, India, 1980. [Google Scholar]
- Omaye, S.T.; Turnbull, J.D.; Sauberlich, H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. In Methods in Enzymology; Academic Press: Amsterdam, The Netherlands, 1979; Volume 62, pp. 3–11. [Google Scholar]
- Baker, H.; Frank, O.; Deangelis, B.; Feingold, S. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr. Rep. Int. 1980, 21, 531–536. [Google Scholar]
- Hwang, S.-Y.; Lin, H.-W.; Chern, R.-H.; Lo, H.F.; Li, L. Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regul. 1999, 27, 167–172. [Google Scholar] [CrossRef]
- Chandlee, J.M.; Scandalios, J.G. Analysis of variants affecting the catalase developmental program in maize scutellum. Theor. Appl. Genet. 1984, 69, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.B.; Khan, P.A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- USEPA United States Environmental Protection Agency. Revision 5.4 Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma–Mass Spectrometry; Method 200.8; USEPA United States Environmental Protection Agency: Washington, DC, USA, 1994.
- Juliani, H.R.; Simon, J.E. Antioxidant activity of basil. In Trends in New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 2002; Volume 575. [Google Scholar]
- AlShrouf, A. Hydroponics, aeroponic and aquaponic as compared with conventional farming. Am. Acad. Sci. Res. J. Eng. Technol. Sci. 2017, 27, 247–255. [Google Scholar]
- MangMang, J.S.; Deaker, R.; Rogers, G. Inoculation effect of Azospirillum brasilense on basil grown under aquaponics production system. Org. Agric. 2016, 6, 65–74. [Google Scholar] [CrossRef]
- Roosta, H.R. Comparison of the vegetative growth, eco-physiological characteristics and mineral nutrient content of basil plants in different irrigation ratios of hydroponic: Aquaponic solutions. J. Plant Nutr. 2014, 37, 1782–1803. [Google Scholar] [CrossRef]
- Rodgers, D.; Won, E.; Timmons, M.B.; Mattson, N. Complementary Nutrients in Decoupled Aquaponics Enhance Basil Performance. Horticulturae 2022, 8, 111. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Nutrient management regime affects water quality, crop growth, and nitrogen use efficiency of aquaponic systems. Sci. Hortic. 2019, 256, 108619. [Google Scholar] [CrossRef]
- Mullis, D.; Reyes, J. The overall effects of soilless agricultural system on basil (Ocimum basilicum L.). Ccamlr Sci. 2019, 26, 300–307. [Google Scholar]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Bailey, D.S. Basil Performance Evaluation in Aquaponics. HortTechnology 2019, 29, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Ghandar, A.; Ahmed, A.; Zulfiqar, S.; Hua, Z.; Hanai, M.; Theodoropoulos, G. A decision support system for urban agriculture using digital twin: A case study with aquaponics. IEEE Access 2021, 9, 35691–35708. [Google Scholar] [CrossRef]
- Liebler, D.C.; Kling, D.S.; Reed, D.J. Antioxidant protection of phospholipid bilayers by alpha-tocopherol. Control of alpha-tocopherol status and lipid peroxidation by ascorbic acid and glutathione. J. Biol. Chem. 1986, 261, 12114–12119. [Google Scholar] [CrossRef]
- Yiu, J.-C.; Juang, L.-D.; Fang, D.Y.-T.; Liu, C.-W.; Wu, S.-J. Exogenous putrescine reduces flooding-induced oxidative damage by increasing the antioxidant properties of Welsh onion. Sci. Hortic. 2009, 120, 306–314. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Q.; Zhao, Q.; Li, X. Cold shock treatment with oxalic acid could alleviate chilling injury in green bell pepper by enhancing antioxidant enzyme activity and regulating proline metabolism. Sci. Hortic. 2022, 295, 110783. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.-Y.; Muhammad, I.; Chi, Y.-X.; Zeeshan, M.; Nasar, J.; Zhou, X.-B. Interactive Effects of Melatonin and Nitrogen Improve Drought Tolerance of Maize Seedlings by Regulating Growth and Physiochemical Attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef]
- Al Shamsi, S.R.H.A.; Rabert, G.A.; Kurup, S.S.; Alyafei, M.A.M.; Jaleel, A. Biochemical Changes and Antioxidant Variations in Date Palm (Phoenix dactylifera L.) Varieties during Flower Induction and Development. Plants 2021, 10, 2550. [Google Scholar] [CrossRef] [PubMed]
- Rakocy, J.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic production of tilapia and basil: Comparing a batch and staggered cropping system. In South Pacific Soilless Culture Conference-SPSCC 648; International Society for Horticultural Science: Leuven, Belgium, 2003; pp. 63–69. [Google Scholar]
- Roosta, H.R.; Hamidpour, M. Effects of foliar application of some macro-and micro-nutrients on tomato plants in aquaponic and hydroponic systems. Sci. Hortic. 2011, 129, 396–402. [Google Scholar] [CrossRef]
- Heise, J.; Müller, H.; Probst, A.J.; Meckenstock, R.U. Ammonium Removal in Aquaponics Indicates Participation of Comammox Nitrospira. Curr. Microbiol. 2021, 78, 894–903. [Google Scholar] [CrossRef] [PubMed]


| Growth Parameters | DAS | Soilless (aquaponic) System | Soil (Greenhouse) System |
|---|---|---|---|
| Total plant height (cm) | 40 | 69.42 ± 6.22 a | 65.55 ± 6.11 b |
| 70 | 98.05 ± 4.35 a | 84.21 ± 9.55 b | |
| Root length (cm) | 40 | 27.98 ± 4.15 a | 23.21 ± 2.11 b |
| 70 | 59.21 ± 3.98 a | 54.36 ± 5.23 b | |
| Leaves (No.) | 40 | 107.00 ± 20.19 a | 99.88 ± 10.03 b |
| 70 | 174 ± 12.55 a | 148 ± 15.19 b | |
| Leaf area (cm)2 | 40 | 3.78 ± 0.22 a | 3.08 ± 0.70 a |
| 70 | 3.74 ± 0.31 a | 3.66 ± 0.38 a | |
| Total fresh weight (g) | 40 | 47.80 ± 5.31 a | 45.89 ± 3.33 b |
| 70 | 84.31 ± 5.89 a | 79.25 ± 6.31 b | |
| Total dry weight (g) | 40 | 4.96 ± 0.51 a | 3.77 ± 0.19 b |
| 70 | 7.11 ± 0.22 a | 6.33 ± 0.35 b | |
| Root dry weight (g) | 40 | 0.77 ± 0.03 a | 0.44 ± 0.01 b |
| 70 | 1.2 ± 00.02 a | 0.99 ± 0.022 b |
| Biochemical Parameters | DAS | Soilless (aquaponic) System | Soil (Greenhouse) System |
|---|---|---|---|
| Protein (µg mL−1) | 40 | 36.25 ± 12.7 a | 29.03 ± 8.62 b |
| 70 | 36.90 ± 12.8 a | 30.00 ± 8.7 b | |
| Total phenol (mg GAEq g−1 DW) | 40 | 8.84 ± 2.8 a | 7.01 ± 2.0 b |
| 70 | 10.11 ± 3.6 a | 9.98 ± 2.9 b |
| Nutrients | Growth Systems | |
|---|---|---|
| Macronutrients (%) | Aquaponics | Soil |
| Nitrogen (N) | 6.32 ± 0.09 a | 4.89 ± 0.11 b |
| Phosphorus (P) | 1.70 ± 0.05 a | 0.99 ± 0.0 b |
| Potassium (K) | 0.69 ± 0.02 a | 0.71 ± 0.02 a |
| Magnesium (Mg) | 0.59 ± 0.01 a | 0.38 ± 0.0 b |
| Calcium (Ca) | 2.81 ± 0.11 a | 2.87 ± 0.13 a |
| Sulphur (S) | 0.29 ± 0.01 a | 0.29 ± 0.01 a |
| Micronutrients (mg kg−1) | ||
| Boron (B) | 41.9 ± 1.70 a | 34.9 ± 1.10 b |
| Copper (Cu) | 13.4 ± 0.86 a | 13.8 ± 0.64 a |
| Iron (Fe) | 95.2 ± 4.10 a | 97.2 ± 2.70 b |
| Manganese (Mn) | 99.8 ± 10.50 a | 87.7 ± 2.40 b |
| Sodium (Na) | 88.6 ± 13.50 a | 74.0 ± 6.10 b |
| Zinc (Zn) | 51.1 ± 5.30 a | 61.1 ± 1.80 b |
| Analysis Intervals | Temperature (°C) | Dissolved Oxygen (mg/L) | pH | TDS (ppm) | EC (mS/cm) | Ammonia (mg/L) | Nitrate (mg/L) | Nitrite (mg/L) |
|---|---|---|---|---|---|---|---|---|
| Day 1 | 24.01 ± 0.03 | 5.01 ± 0.03 | 6.85 ± 0.03 | 310 ± 1.100 | 0.62 ± 0.04 | 0.10 ± 0.03 | 5.81 ± 0.03 | 0.30 ± 0.02 |
| Day 10 | 25.09 ± 0.03 | 5.22 ± 0.04 | 6.99 ± 0.03 | 380.11 ± 1.24 | 0.76 ± 0.04 | 0.10 ± 0.03 | 6.09 ± 0.04 | 0.10 ± 0.04 |
| Day 20 | 24.02 ± 0.03 | 5.10 ± 0.04 | 6.87 ± 0.02 | 350.21 ± 0.81 | 0.70 ± 0.04 | 0.12 ± 0.03 | 11.99 ± 0.07 | 0.08 ± 0.04 |
| Day 30 | 25.02 ± 0.02 | 5.03 ± 0.03 | 6.42 ± 0.04 | 390.10 ± 1.32 | 0.78 ± 0.02 | 0.11 ± 0.02 | 17.22 ± 0.06 | 0.10 ± 0.03 |
| Day 40 | 25.24 ± 0.03 | 5.10 ± 0.03 | 6.99 ± 0.04 | 410.41 ± 1.05 | 0.82 ± 0.03 | 0.60 ± 0.02 | 18.79 ± 0.09 | 0.11 ± 0.03 |
| Day 50 | 24.84 ± 0.02 | 5.20 ± 0.03 | 7.01 ± 0.05 | 420.47 ± 1.06 | 0.84 ± 0.04 | 0.61 ± 0.02 | 17.79 ± 0.09 | 0.12 ± 0.03 |
| Day 60 | 26.01 ± 0.04 | 5.11 ± 0.03 | 6.91 ± 0.04 | 420.61 ± 1.15 | 0.84 ± 0.03 | 0.71 ± 0.03 | 14.11 ± 0.06 | 0.12 ± 0.03 |
| Day 70 | 24.21 ± 0.03 | 5.10 ± 0.03 | 6.98 ± 0.05 | 410.11 ± 1.05 | 0.82 ± 0.02 | 0.62 ± 0.02 | 18.25 ± 0.08 | 0.14 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albadwawi, M.A.O.K.; Ahmed, Z.F.R.; Kurup, S.S.; Alyafei, M.A.; Jaleel, A. A Comparative Evaluation of Aquaponic and Soil Systems on Yield and Antioxidant Levels in Basil, an Important Food Plant in Lamiaceae. Agronomy 2022, 12, 3007. https://doi.org/10.3390/agronomy12123007
Albadwawi MAOK, Ahmed ZFR, Kurup SS, Alyafei MA, Jaleel A. A Comparative Evaluation of Aquaponic and Soil Systems on Yield and Antioxidant Levels in Basil, an Important Food Plant in Lamiaceae. Agronomy. 2022; 12(12):3007. https://doi.org/10.3390/agronomy12123007
Chicago/Turabian StyleAlbadwawi, Maryam A. O. K., Zienab F. R. Ahmed, Shyam S. Kurup, Mohammed A. Alyafei, and Abdul Jaleel. 2022. "A Comparative Evaluation of Aquaponic and Soil Systems on Yield and Antioxidant Levels in Basil, an Important Food Plant in Lamiaceae" Agronomy 12, no. 12: 3007. https://doi.org/10.3390/agronomy12123007
APA StyleAlbadwawi, M. A. O. K., Ahmed, Z. F. R., Kurup, S. S., Alyafei, M. A., & Jaleel, A. (2022). A Comparative Evaluation of Aquaponic and Soil Systems on Yield and Antioxidant Levels in Basil, an Important Food Plant in Lamiaceae. Agronomy, 12(12), 3007. https://doi.org/10.3390/agronomy12123007






