Chemical Composition and Insecticidal Properties of Origanum vulgare (Lamiaceae) Essential Oil against the Stored Product Beetle, Sitophilus granarius
Abstract
1. Introduction
2. Materials and Methods
2.1. Weevils
2.2. Essential Oil
2.3. Gas Chromatography-Flame Ionization Detector (GC–FID) Analysis
2.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.5. Dose–Mortality Relationship
2.6. Time–Mortality Relationship
2.7. Behavioral Response
2.8. Respiration Rate
2.9. Statistical Analysis
3. Results
3.1. Chemical O. vulgare EO Characterization
3.2. Dose–Mortality Relationship
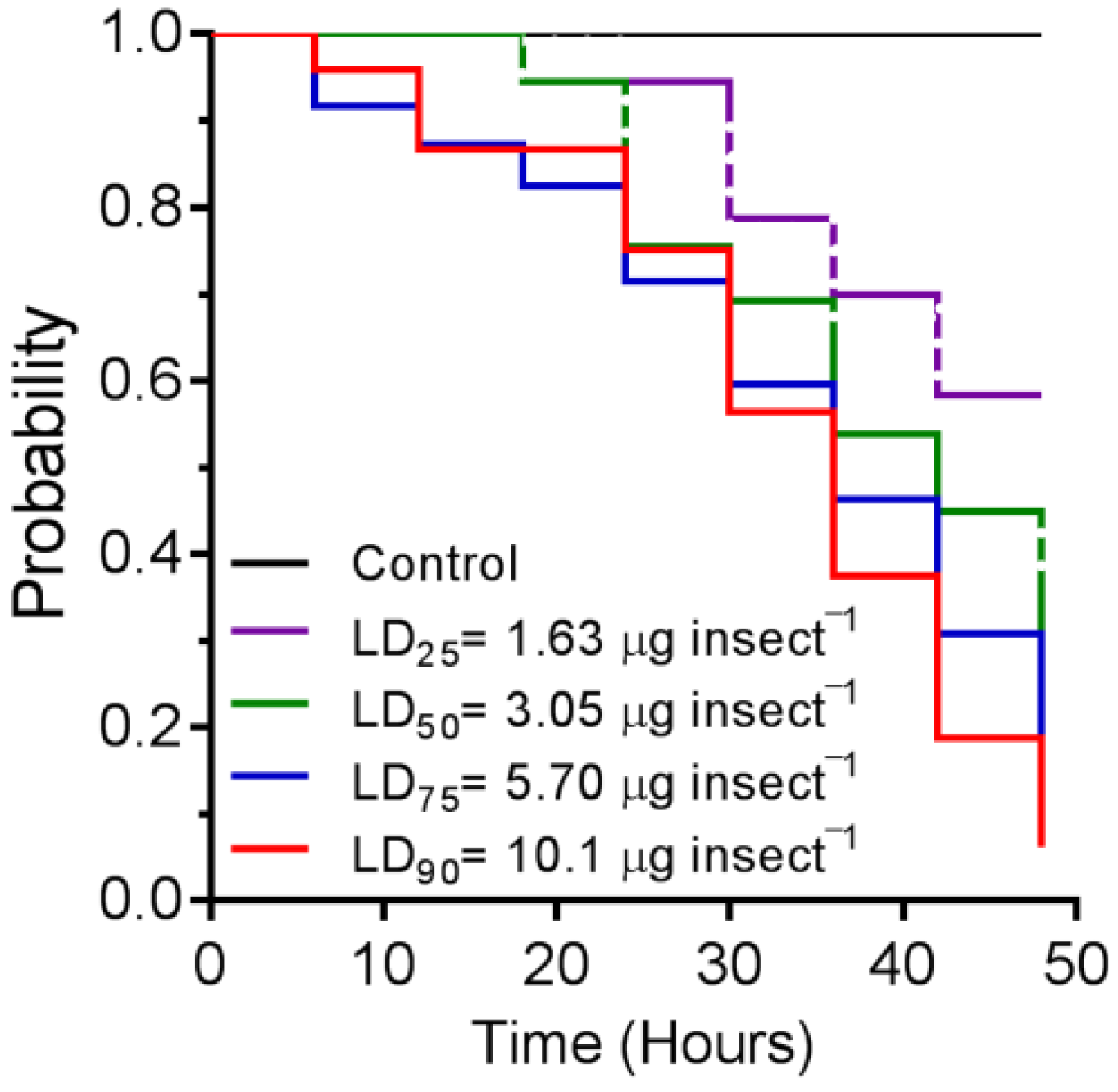
3.3. Time–Mortality Relationship
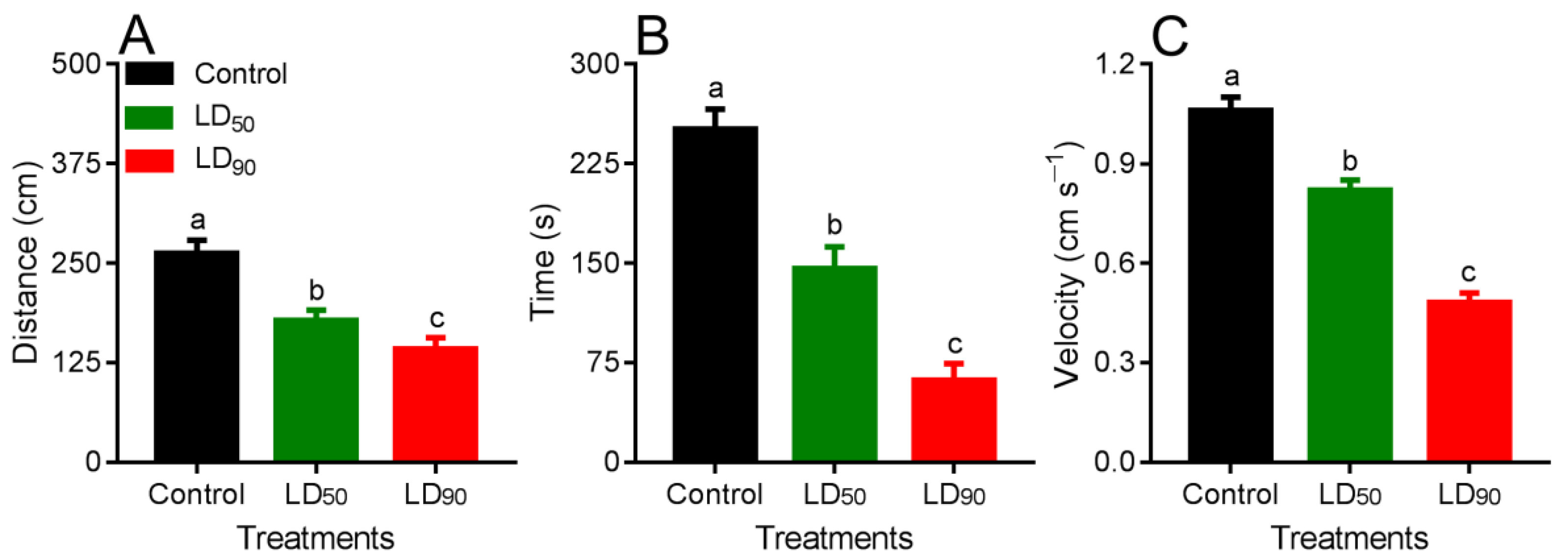
3.4. Behavioral Response
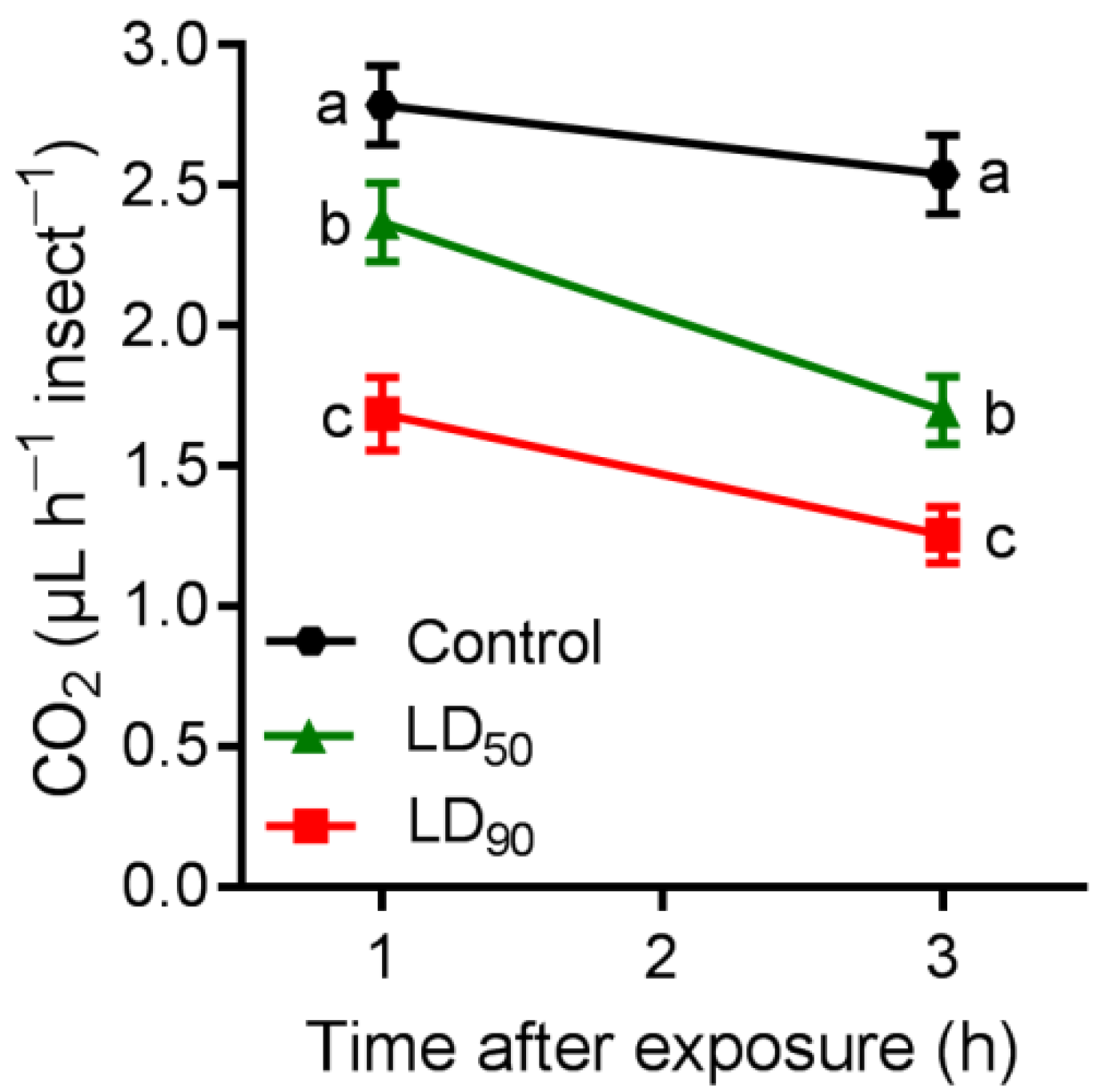
3.5. Respiration Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flinn, P.W.; Hagstrum, D.W.; Reed, C.; Phillips, T.W. United States Department of Agriculture-Agricultural Research Service stored-grain area-wide integrated pest management program. Pest Manag. Sci. 2003, 59, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Karunakaran, C.; Jayas, D.S.; White, N.D.G. Detection techniques for stored-product insects in grain. Food Control 2007, 18, 157–162. [Google Scholar] [CrossRef]
- Nesci, A.; Barra, P.; Etcheverry, M. Integrated management of insect vectors of Aspergillus flavus in stored maize, using synthetic antioxidants and natural phytochemicals. J. Stored Prod. Res. 2011, 47, 231–237. [Google Scholar] [CrossRef]
- Boyer, S.; Zhang, H.; Lempérière, G. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res. 2012, 102, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Hallman, G.; Panneton, B.; Fleurat-Lessard, F. Management of agricultural insects with physical control methods. Annu. Rev. Entomol. 2003, 48, 261–281. [Google Scholar] [CrossRef]
- Mohammed, M.E.; El-Shafie, H.A.; Alhajhoj, M.R. Design and efficacy evaluation of a modern automated controlled atmosphere system for pest management in stored dates. J. Stored Prod. Res. 2020, 89, 101719. [Google Scholar] [CrossRef]
- Morrison, W.R., III; Scully, E.D.; Campbell, J.F. Towards developing areawide semiochemical-mediated, behaviorally-based integrated pest management programs for stored product insects. Pest Manag. Sci. 2021, 77, 2667–2682. [Google Scholar] [CrossRef]
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Sousa, A.; Faroni, L.; Pimentel, M.; Guedes, R. Developmental and population growth rates of phosphine-resistant and susceptible populations of stored product insect pests. J. Stored Prod. Res. 2009, 45, 241–246. [Google Scholar] [CrossRef]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Campos, J.M.; da Silva Rolim, G.; Martínez, L.C.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Terpenoid constituents of cinnamon and clove essential oils cause toxic effects and behavior repellency response on granary weevil, Sitophilus granarius. Ecotox. Environ. Saf. 2018, 156, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Cossolin, J.F.S.; Pereira, M.J.; Martínez, L.C.; Turchen, L.M.; Fiaz, M.; Bozdoğan, H.; Serrão, J.E. Cytotoxicity of Piper aduncum (Piperaceae) essential oil in brown stink bug Euchistus heros (Heteroptera: Pentatomidae). Ecotoxicology 2019, 28, 63–770. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Rolim, G.D.S.; Wilcken, C.F.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Acute toxicity and sublethal effects of lemongrass essential oil and their components against the granary weevil, Sitophilus granarius. Insects 2020, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Brügger, B.P.; Plata-Rueda, A.; Wilcken, C.F.; de Souza, L.S.A.; Serrão, J.E.; Carvalho, A.G.; Zanuncio, J.C.; Martínez, L.C. Exposure to lemongrass essential oil and its components causes behavior and respiratory disturbs in Anticarsia gemmatalis. Int. J. Pest Manag. 2021, 1–11. [Google Scholar] [CrossRef]
- Zanuncio, J.C.; Mourão, S.A.; Martínez, L.C.; Wilcken, C.F.; Ramalho, F.S.; Plata-Rueda, A.; Soares, M.A.; Serrão, J.E. Toxic effects of the neem oil (Azadirachta indica) formulation on the stink bug predator, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 2016, 6, 30261. [Google Scholar] [CrossRef]
- Amaral, K.D.; Martínez, L.C.; Lima, M.A.P.; Serrão, J.E.; Della Lucia, T.M.C. Azadirachtin impairs egg production in Atta sexdens leaf-cutting ant queens. Environ. Pollut. 2018, 24, 809–814. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Colares, H.C.; Campos, J.M.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Toxic effects of two essential oils and their constituents on the mealworm beetle, Tenebrio molitor. Bull. Entomol. Res. 2018, 108, 716–725. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Martínez, L.C.; Rolim, G.D.S.; Coelho, R.P.; Santos, M.H.; Tavares, W.de.S.; Zanuncio, J.C.; Serrão, J.E. Insecticidal and repellent activities of Cymbopogon citratus (Poaceae) essential oil and its terpenoids (citral and geranyl acetate) against Ulomoides dermestoides. Crop Prot. 2020, 137, 105299. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Sattelle, D.B. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAA receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Braz. J. Pharmacol. 2003, 140, 1363–1372. [Google Scholar] [CrossRef]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Fiaz, M.; Martínez, L.C.; da Silva Costa, M.; Plata-Rueda, A.; Gonçalves, W.G.; Sant’Ana, A.E.G.; Zanuncio, J.C.; Serrão, J.E. Squamocin induce histological and ultrastructural changes in the midgut cells of Anticarsia gemmatalis (Lepidoptera: Noctuidae). Ecotox. Environ. Saf. 2018, 156, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farder-Gomes, C.; Saravanan, M.; Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Azadirachtin-based biopesticide affects the respiration and digestion in Anticarsia gemmatalis caterpillars. Toxin Rev. 2022, 41, 466–475. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Origanum vulgare essential oil against Tenebrio molitor (Coleoptera: Tenebrionidae): Composition, insecticidal activity, and behavioral response. Plants 2021, 10, 2513. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Nerio, L.S.; Stashenko, E.E. Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. Pest Manag. Sci. 2010, 66, 664–668. [Google Scholar] [CrossRef]
- Basile, S.; Badalamenti, N.; Riccobono, O.; Guarino, S.; Ilardi, V.; Bruno, M.; Peri, E. Chemical composition and evaluation of insecticidal activity of Calendula incana subsp. maritima and Laserpitium siler subsp. siculum essential oils against stored products pests. Molecules 2022, 27, 588. [Google Scholar]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalli, N.; Skourti, A.; Karagianni, E.S.; Nika, E.P.; Kontodimas, D.C.; Cappellacci, L.; Petrelli, R.; Cianfaglione, K.; et al. Effectiveness of eight essential oils against two key stored-product beetles, Prostephanus truncatus (Horn) and Trogoderma granarium Everts. Food Chem. Toxicol. 2020, 139, 111255. [Google Scholar] [CrossRef]
- Jayaram, C.S.; Chauhan, N.; Dolma, S.K.; Reddy, S.G.E. Chemical Composition and Insecticidal Activities of Essential Oils against the Pulse Beetle. Molecules 2022, 27, 568. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Martínez, L.C.; Dos Santos, M.H.; Fernandes, F.L.; Wilcken, C.F.; Soares, M.A.; Serrão, J.E.; Zanuncio, J.C. Insecticidal activity of garlic essential oil and their constituents against the mealworm beetle, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Sci. Rep. 2017, 7, 46406. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Bioactivity of six plant extracts on adults of Demotispa neivai (Coleoptera: Chrysomelidae). J. Insect Sci. 2015, 15, 34. [Google Scholar] [CrossRef]
- Brügger, B.P.; Martínez, L.C.; Plata-Rueda, A.; Castro, B.M.D.C.; Soares, M.A.; Wilcken, C.F.; Carvalho, A.G.; Serrão, J.E.; Zanuncio, J.C. Bioactivity of the Cymbopogon citratus (Poaceae) essential oil and its terpenoid constituents on the predatory bug, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 2019, 9, 8358. [Google Scholar] [CrossRef] [PubMed]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health D 2015, 26, 23289. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimand, F.A.; Al-Ebady, N. Evaluation of antifungal activity of some plant extracts and their applicability in extending the shelf life of stored tomato fruits. J. Food Process Technol. 2014, 5, 340. [Google Scholar]
- Kim, S.-I.; Yoon, J.-S.; Jung, J.W.; Hong, K.-B.; Ahn, Y.-J.; Kwon, H.K. Toxicity and repellency of origanum essential oil and its components against Tribolium castaneum (Coleoptera: Tenebrionidae) adults. J. Asia-Pac. Entomol. 2010, 13, 369–373. [Google Scholar] [CrossRef]
- Schillaci, D.; Napoli, E.M.; Cusimano, M.G.; Vitale, M.; Ruberto, A. Origanum vulgare subsp. hirtum essential oil prevented biofilm formation and showed antibacterial activity against planktonic and sessile bacterial cells. J. Food Prot. 2013, 76, 1747–1752. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Stamopoulos, D.C. Repellent, toxic and reproduction inhibitory effects of essential oil vapours on Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2002, 38, 117–128. [Google Scholar] [CrossRef]
- Szczepanik, M.; Walczak, M.; Zawitowska, B.; Michalska-Sionkowska, M.; Szumny, A.; Wawrzeńczyk, C.; Brzezinska, M.S. Chemical composition, antimicromicrobial activity and insecticidal activity against the lesser mealworm Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) of Origanum vulgare L. ssp. hirtum (Link) and Artemisia dracunculus L. essential oils. J. Sci. Food Agric. 2018, 98, 767–774. [Google Scholar]
- Papanikolaou, N.E.; Kavallieratos, N.G.; Iliopoulos, V.; Evergetis, E.; Skourti, A.; Nika, E.P.; Haroutounian, S.A. Essential oil coating: Mediterranean culinary plants as grain protectants against larvae and adults of Tribolium castaneum and Trogoderma granarium. Insects 2022, 13, 165. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Figiel, A.; Szumny, A.; Gutierrez-Ortiz, A.; Carbonell-Barrachina, A.A. Composition of oregano essential oil (Origanum vulgare) as affected by drying method. J. Food Eng. 2010, 98, 240–247. [Google Scholar] [CrossRef]
- La Pergola, A.; Restuccia, C.; Napoli, E.; Bella, S.; Brighina, S.; Russo, A.; Suma, P. Commercial and wild Sicilian Origanum vulgare essential oils: Chemical composition, antimicrobial activity and repellent effects. J. Essent. Oil Res. 2017, 29, 451–460. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Martínez, L.C.; Da Silva, B.K.R.; Zanuncio, J.C.; Fernandes, M.E.de.S.; Guedes, R.N.C.; Fernandes, F.L. Exposure to cyantraniliprole causes mortality and disturbs behavioral and respiratory response in the coffee berry borer (Hypothenemus hampei). Pest Manag. Sci. 2019, 75, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.M.; Martínez, L.C.; Plata-Rueda, A.; Serrão, J.E.; Zanuncio, J.C. Respiration, predatory and prey consumption by Podisus nigrispinus (Heteroptera: Pentatomidae) nymphs exposed some to insecticides. Chemosphere 2020, 261, 127720. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Pereira, A.E.; de Morais Ribeiro, L.N.; Fernandes, F.O.; Gonçalves, K.C.; Polanczyk, R.A.; Pasquoto-Stigliani, T.; Lima, R.; et al. Carvacrol and linalool co-loaded in β-cyclodextrin-grafted chitosan nanoparticles as sustainable biopesticide aiming pest control. Sci. Rep. 2018, 8, 7623. [Google Scholar] [CrossRef] [PubMed]
- Li, A.S.; Iijima, A.; Huang, J.; Li, Q.X.; Chen, Y. Putative mode of action of the monoterpenoids linalool, methyl eugenol, estragole, and citronellal on ligand-gated ion channels. Engineering 2020, 6, 541–545. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Ibanez, S.; Gallet, C.; Després, L. Plant insecticidal toxins in ecological networks. Toxins 2012, 4, 228–243. [Google Scholar] [CrossRef]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Synergistic interactions among the major constituents of lemongrass essential oil against larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni. J. Pest Sci. 2017, 90, 735–744. [Google Scholar] [CrossRef]
- Aslan, I.; Çalmaşur, Ö.; Sahin, F.; Çağlar, Ö. Insecticidal effects of essential plant oils against Ephestia kuehniella (Zell.), Lasioderma serricorne (F.) and Sitophilus granarius (L.). J. Plant Dis. Prot. 2005, 112, 257–267. [Google Scholar]
- Bertoli, A.; Conti, B.; Mazzoni, V.; Meini, L.; Pistelli, L. Volatile chemical composition and bioactivity of six essential oils against the stored food insect Sitophilus zeamais Motsch. (Coleoptera Dryophthoridae). Nat. Prod. Res. 2012, 26, 2063–2071. [Google Scholar] [PubMed]
- Xie, Y.; Huang, Q.; Rao, Y.; Hong, L.; Zhang, D. Efficacy of Origanum vulgare essential oil and carvacrol against the housefly, Musca domestica L. (Diptera: Muscidae). Environ. Sci. Pollut. Res. 2019, 26, 23824–23831. [Google Scholar] [CrossRef] [PubMed]
- Werdin González, J.O.; Gutiérrez, M.M.; Murray, A.P.; Ferrero, A.A. Composition and biological activity of essential oils from Labiatae against Nezara viridula (Hemiptera: Pentatomidae) soybean pest. Pest Manag. Sci. 2011, 67, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Sendi, J.J.; Moharramipourb, S.; Zibaee, A. Evaluation of Origanum vulgare L. essential oil as a source of toxicant and an inhibitor of physiological parameters in diamondback moth, Plutella xylustella L. (Lepidoptera: Pyralidae). J. Saud. Soc. Agr. Sci. 2017, 16, 184–190. [Google Scholar] [CrossRef]
- Baricevic, D.; Milevoj, L.; Borstnik, J. Insecticidal effect of oregano (Origanum vulgare L. ssp. hirtum Ietswaart) on the dry bean weevil (Acanthoscelides obtectus Say). Int. J. Hortic. Sci. 2001, 7, 84–88. [Google Scholar] [CrossRef]
- Alkan, M. Chemical composition and insecticidal potential of different Origanum spp. (Lamiaceae) essential oils against four stored product pests. Turk J. Entomol. 2020, 44, 149–163. [Google Scholar] [CrossRef]
- Castro, B.M.C.; Martínez, L.; Plata-Rueda, A.; Soares, M.A.; Wilcken, C.F.; Zanuncio, A.J.V.; Fiaz, M.; Zanuncio, J.C.; Serrão, J.E. Exposure to chlorantraniliprole reduces locomotion, respiration, and causes histological changes in the midgut of velvetbean caterpillar Anticarsia gemmatalis (Lepidoptera: Noctuidae). Chemosphere 2021, 263, 128008. [Google Scholar] [CrossRef]
- Vinha, G.L.; Plata-Rueda, A.; Soares, M.A.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Deltamethrin–mediated effects on locomotion, respiration, feeding and histological changes in the midgut of Spodoptera frugiperda caterpillars. Insects 2021, 12, 483. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Menezes, C.H.M.; Cunha, W.S.; Alvarenga, T.M.; Barbosa, B.F.; Zanuncio, J.C.; Martínez, L.C.; Serrão, J.E. Side-effects caused by chlorpyrifos in the velvetbean caterpillar Anticarsia gemmatalis (Lepidoptera: Noctuidae). Chemosphere 2020, 259, 127530. [Google Scholar] [CrossRef]
- Fiaz, M.; Martínez, L.C.; Plata-Rueda, A.; Cossolin, J.F.S.; Serra, R.S.; Martins, G.F.; Serrão, J.E. Behavioral and ultrastructural effects of novaluron on Aedes aegypti larvae. Infect. Genet. Evol. 2021, 93, 104974. [Google Scholar] [CrossRef]
- Lima, B.S.A.; Martínez, L.C.; Plata-Rueda, A.; dos Santos, M.H.; de Oliveira, E.E.; Zanuncio, J.C.; Serrão, J.E. Interaction between predatory and phytophagous stink bugs promoted by secretion of scent glands. Chemoecology 2021, 31, 209–219. [Google Scholar] [CrossRef]
- Price, D.N.; Berry, M.S. Comparison of effects of octopamine and insecticidal essential oils on activity in the nerve cord, foregut, and dorsal unpaired median neurons of cockroaches. J. Insect Physiol. 2006, 52, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Fiaz, M.; Brügger, B.P.; Cañas, V.; Coelho, R.P.; Zanuncio, J.C.; Martínez, L.C.; Serrão, J.E. Lemongrass essential oil and its components cause effects on survival, locomotion, ingestion, and histological changes of the midgut in Anticarsia gemmatalis caterpillars. Toxin Rev. 2022, 41, 208–217. [Google Scholar] [CrossRef]
| Peaks | Compounds | Composition (%) |
|---|---|---|
| 1 | α-thujene | 1.45 ± 0.01 |
| 2 | α-pinene | 2.74 ± 0.05 |
| 3 | Camphene | 1.99 ± 0.07 |
| 4 | β-pinene | 1.75 ± 0.02 |
| 5 | β-myrcene | 1.19 ± 0.01 |
| 6 | α-phellandrene | 1.91 ± 0.03 |
| 7 | α-terpinene | 1.25 ± 0.01 |
| 8 | p-cymene | 11.5 ± 0.11 |
| 9 | Eucalyptol | 2.98 ± 0.08 |
| 10 | γ-terpinene | 7.09 ± 0.15 |
| 11 | Cis-sabinene hydrate | 1.49 ± 0.01 |
| 12 | Terpinolene | 1.18 ± 0.01 |
| 13 | Linalool | 9.53 ± 0.22 |
| 14 | Camphor | 1.79 ± 0.01 |
| 15 | Borneol | 1.37 ± 0.01 |
| 16 | Terpinen-4-ol | 1.89 ± 0.02 |
| 17 | α-terpineol | 1.59 ± 0.01 |
| 18 | Thymol methyl ether | 1.91 ± 0.03 |
| 19 | Carvacrol methyl ether | 1.63 ± 0.01 |
| 20 | Cuminaldehyde | 1.22 ± 0.01 |
| 21 | Thymol | 7.51 ± 0.08 |
| 22 | Carvacrol | 25.4 ± 0.13 |
| 23 | Aromandrene | 1.39 ± 0.01 |
| 24 | β-bisabolene | 1.74 ± 0.01 |
| 25 | Caryophyllene oxide | 4.78 ± 0.09 |
| N° Insects | Lethal Doses | Estimated Dose (µg Insect−1) | 95% Confidence Interval (µg Insect−1) | χ2 (p-Value) |
|---|---|---|---|---|
| 150 | LD25 | 1.632 | 1.311–1.957 | 6.89 (0.14) |
| 150 | LD50 | 3.053 | 2.576–3.645 | |
| 150 | LD75 | 5.709 | 4.691–7.331 | |
| 150 | LD90 | 10.02 | 7.748–14.27 |
| ANOVA Table | SS | DF | MS | F (DFn, DFd) | p-Value |
|---|---|---|---|---|---|
| Treatments | 46.52 | 2 | 23.26 | F (2,48) = 2.57 | <0.0086 |
| Time | 138.1 | 1 | 138.1 | F (1,48) = 15.2 | <0.0001 |
| Treatments × time | 108.6 | 2 | 54.28 | F (2,48) = 6.01 | <0.0004 |
| Residual | 433.7 | 48 | 9.036 | ||
| Total | 727 | 53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plata-Rueda, A.; Santos, M.H.D.; Serrão, J.E.; Martínez, L.C. Chemical Composition and Insecticidal Properties of Origanum vulgare (Lamiaceae) Essential Oil against the Stored Product Beetle, Sitophilus granarius. Agronomy 2022, 12, 2204. https://doi.org/10.3390/agronomy12092204
Plata-Rueda A, Santos MHD, Serrão JE, Martínez LC. Chemical Composition and Insecticidal Properties of Origanum vulgare (Lamiaceae) Essential Oil against the Stored Product Beetle, Sitophilus granarius. Agronomy. 2022; 12(9):2204. https://doi.org/10.3390/agronomy12092204
Chicago/Turabian StylePlata-Rueda, Angelica, Marcelo Henrique Dos Santos, José Eduardo Serrão, and Luis Carlos Martínez. 2022. "Chemical Composition and Insecticidal Properties of Origanum vulgare (Lamiaceae) Essential Oil against the Stored Product Beetle, Sitophilus granarius" Agronomy 12, no. 9: 2204. https://doi.org/10.3390/agronomy12092204
APA StylePlata-Rueda, A., Santos, M. H. D., Serrão, J. E., & Martínez, L. C. (2022). Chemical Composition and Insecticidal Properties of Origanum vulgare (Lamiaceae) Essential Oil against the Stored Product Beetle, Sitophilus granarius. Agronomy, 12(9), 2204. https://doi.org/10.3390/agronomy12092204








