Seed Germination and Early Seedling Growth Responses to Drought Stress in Annual Medicago L. and Trifolium L. Forages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Germination under Increasing Water Limitation and Temperatures
2.2. Seedling Emergence under Moisture Stress
2.3. Early Growth Responses to Moisture Stress in Medicago and Trifolium Seedlings
2.4. Statistical Analyses
3. Results
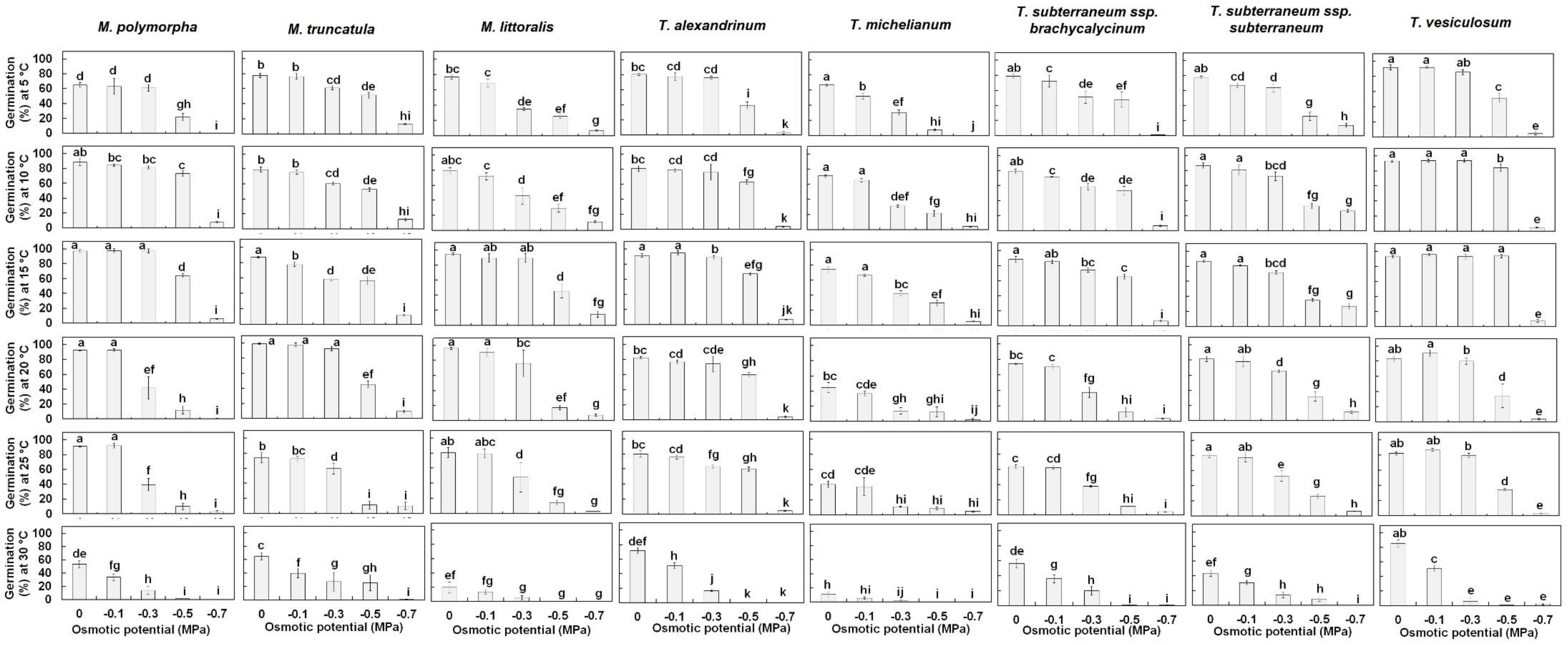
3.1. Seed Germination
3.2. Seedling Emergence
3.3. Drought Stress Resistance in the Seedling Stage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thrnton, P.K.; Herrero, M. Impacts of climate change on the livestock food supply chain: A review of the evidence. Glob. Food Secur. 2021, 28, 100488. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-U.; Seo, K.-H.; Chen, D. Climate change over the Mediterranean and current destruction of marine ecosystem. Sci. Rep. 2019, 9, 18813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddoudi, L.; Hdira, S.; Hanana, M.; Romero, I.; Haddoudi, I.; Mahjoub, A.; Jouira, H.B.; Djebali, N.; Ludidi, N.; Sanchez-Ballesta, M.T.; et al. Evaluation of the Morpho-Physiological, Biochemical and Molecular Responses of Contrasting Medicago truncatula Lines under Water Deficit Stress. Plants 2021, 10, 2114. [Google Scholar] [CrossRef] [PubMed]
- Roffe, S.J.; Fitchett, J.M.; Curtis, C.J. Determining the utility of a percentile-based wet-seasonstart- and end-date metrics across South Africa. Theor. Appl. Climatol. 2020, 140, 1331–1347. [Google Scholar] [CrossRef]
- Engelbrecht, F.A.; Monteiro, P.M. The IPCC Assessment Report Six Working Group 1 report and southern Africa: Reasons to take action. S. Afr. J. Sci. 2021, 117, 12679. [Google Scholar] [CrossRef] [PubMed]
- Van Heerden, J.M. A case study of annual legume seedling and seed populations in commercial crop-pasture systems in the agro-pastoral region of the Western Cape. Grassroots 2013, 13, 41–51. [Google Scholar]
- Swanepoel, P.A.; Tshuma, F. Soil quality effects on regeneration of annual Medicago pastures in the Swartland of South Africa. Afr. J. Range Forage Sci. 2017, 34, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Swanepoel, P.A.; Labuschagne, J.; Hardy, M. Historical development and future perspective of conservation agriculture practices in crop-pasture rotation systems in the Mediterranean region of South Africa. In Ecosystem Services and Socio-Economic Benefits of Mediterranean Grasslands; Kyriazopoulos, A., Lopez-Francos, A., Porqueddu, C., Sklavou, P., Eds.; CIHEAM: Zaragoza, Spain, 2016; pp. 75–78. [Google Scholar]
- Peoples, M.B.; Bowman, A.M.; Gault, R.R.; Herridge, D.F.; McCallum, M.H.; McCormicj, K.M.; Norton, R.M.; Rochester, I.J.; Scammell, G.J.; Schwenke, G.D. Factors regulating the contributions of fixed nitrogen by pasture and crop legumes to different farming systems of eastern Australia. Plant Soil 2001, 228, 29–41. [Google Scholar] [CrossRef]
- Peoples, M.B.; Baldock, J.A. The nitrogen dynamics of pastures: Nitrogen fixation inputs, the impact of legumes on soil nitrogen fertility, and the contributions of fixed nitrogen to Australian pastures. Aust. J. Exp. Agric. 2000, 41, 327–346. [Google Scholar] [CrossRef]
- Porqueddu, C.; Gonzales, F. Role and potential of annual legumes in Mediterranean farming systems. Pastos 2011, 36, 125–142. [Google Scholar]
- Denton, M.D.; Coventry, D.R.; Bellotti, W.D.; Howieson, J.G. Nitrogen fixation in annual Trifolium species in alkaline soils as assessed by the 15N natural abundance method. Crop Pasture Sci. 2011, 62, 712–720. [Google Scholar] [CrossRef]
- Thomas, D.T.; Flohr, B.M.; Manjardino, M.; Loi, A.; Lewellyn, R.S.; Lawes, R.A.; Norman, H.C. Selecting higher nutritive value annual pasture legumes increases the profitability of sheep production. Agric. Syst. 2021, 194, 103272. [Google Scholar] [CrossRef]
- Le Roux, D.J.; Agenbag, G.A.; Mills, L.J. Grasbeheer in peulgewasweidings: Invloed op die opbrengspotensiaal van opvolgende koringaanplanting. Appl. Plant Sci. 1995, 9, 39–42. [Google Scholar]
- Howieson, J.G.; O’Hara, G.W.; Carr, S.J. Changing roles for legumes in Mediterranean agriculture: Developments from an Australian perspective. Field Crops Res. 2000, 65, 107–122. [Google Scholar] [CrossRef]
- Ewing, M.A.; Loi, A.; McRobb, R.; Nutt, B.J. Potential new alternative annual pasture legumes for Australian Mediterranean farming systems. In Legumes for Mediterranean Forage Crops, Pastures and Alternative Uses; Sulas, L., Ed.; CIHEAM: Zaragoza, Spain, 2000; pp. 51–54, (Cahiers Options Méditerranéennes. n 45). [Google Scholar]
- Loi, A.; Howieson, J.G.; Nutt, B.J.; Carr, S.J. A second generation of annual pasture legumes and their potential for inclusion in Mediterranean-type farming systems. Aust. J. Exp. Agric. 2005, 45, 289–299. [Google Scholar] [CrossRef]
- Loi, A.; Nutt, B.J.; Revell, C.K. Domestication of new annual pasture legumes for resilient Mediterranean farming susyems. Options Mediterr. 2008, 79, 363–371. [Google Scholar]
- Knott, S.C. An analysis of the financial implications of different tillage systems within different crop rotations in the Swartland area of the Western Cape, South Africa. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2015. [Google Scholar]
- Edwards, T.; Howieson, J.; Nutt, B.; Yates, R.; O’Hara, G.; van Wyk, B.-E. A ley-farming system for marginal lands based upon a self-regenerating perennial pasture legume. Agron. Sustain. Dev. 2019, 39, 13. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.; Durand, J.-L.; Duru, M.; Gastal, F.; Hulier, B.; Litrico, I.; Louarn, G.; Mediene, S.; Moreau, D.; Valentin-Morison, M.; et al. Role of ley pastures in tomorrow’s cropping systems. A review. Agron. Sustain. Dev. 2020, 40, 17. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Comas, P. Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Glob. Chang. Biol. 2002, 8, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Elsevier: San Diego, CA, USA; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination, 2nd ed.; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Singh, P.; Ibrahim, H.M.; Flury, M.; Schillinger, W.F.; Knappenberger, T. Critical water potentials for germination of wheat cultivars in the dryland Northwest USA. Seed Sci. Res. 2013, 23, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.W.; Fan, Y.; Baskin, C.; Baskin, J.M.; Wang, Y.R. Comparison of the effects of temperature and water potential on seed germination of Fabaceae species from desert and subalpine grassland. Am. J. Bot. 2015, 102, 649–660. [Google Scholar] [CrossRef] [Green Version]
- Patané, C.; Saita, A.; Tubeileh, A.; Cosentino, S.L.; Cavallaro, V. Modeling seed germination of unprimed and primed seeds of sweet sorghum under PEG-induced water stress through the hydrotime analysis. Acta Physiol. Plant 2016, 38, 115. [Google Scholar] [CrossRef]
- Nichols, P.G.H.; Loi, A.; Nutt, B.J.; Evans, P.M.; Craig, A.D.; Pengelly, B.C.; Dear, B.S.; Lloyd, D.L.; Revell, C.K.; Nair, R.M.; et al. New annual and short-lived perennial pasture legumes for Australian agriculture—15 years of revolution. Field Crop Res. 2007, 104, 10–23. [Google Scholar] [CrossRef]
- Nichols, P.G.H.; Loi, A.; Nutt, B.J.; Snowball, R.; Revell, C.K. Domestication of new Mediterranean annual pasture legumes. In Sustainable Use of Genetic Diversity in Forage and Turf Breeding; Huyghe, C., Ed.; Springer Science + Business Media B.V.: Berlin/Heidelberg, Germany, 2010; pp. 137–141. [Google Scholar]
- Michael, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914. [Google Scholar] [CrossRef] [PubMed]
- Woodman, R.F.; Doney, R.J.; Allen, B.E. Effects of drilling depth on seedling growth of seven dryland pasture species. Proc. N. Z. Grassl. Assoc. 1990, 52, 167–170. [Google Scholar] [CrossRef]
- Wolfson, M.M.; Tainton, N.M. The morphology and physiology of the major forage plants. In Pasture Management in South Africa; Tainton, N.M., Ed.; University of Natal Press: Pietermaritzburg, South Africa, 2000. [Google Scholar]
- Van Heerden, J.M. The influence of the application of grass herbicides on the production of dryland medic and lucerne pastures in the Rûens area of the Southern Cape. J. Grassl. Soc. S. Afr. 1990, 7, 152–156. [Google Scholar] [CrossRef]
- Van Heerden, J.M.; Botha, P.R.; Tainton, N.M. A comparison of grass and grass/legume pastures under irrigation in the Outeniqua area of the Southern Cape. J. Grassl. Soc. S. Afr. 1989, 6, 220–224. [Google Scholar] [CrossRef]
- Nichol, A.M.; Edwards, G.R. Why is clover better than ryegrass? Proc. N. Z. Soc. Anim. Prod. 2011, 7, 71–78. [Google Scholar]

| N (g/kg) | P (g/kg) | K (g/kg) | Ca (g/kg) | Mg (g/kg) | Na (g/kg) | pH (Water) | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1.42 ± 0.17 | 0.06 ± 0.01 | 2.35 ± 0.06 | 2.23 ± 0.20 | 0.59 ± 0.04 | 0.02 ± 0.001 | 7.73 ± 0.14 | 69.8 ± 4.45 | 10.0 ± 0.75 | 20.2 ± 1.90 |
| Temperature Treatment | Osmotic Treatment | T. alexandrinum | T. michelianum | T. subterraneum ssp. brachycalycinum | T. subterraneum ssp. subterraneum | T. vesiculosum | M. polymorpha | M. truncatula | M. littoralis |
|---|---|---|---|---|---|---|---|---|---|
| 5 °C | 0 MPa | 80 ± 1.2 b | 67 ± 1.3 c | 67 ± 2.1 c | 65 ± 1.0 cd | 92 ± 3.3 a | 59 ± 2.7 d | 67 ± 2.5 c | 77± 2.7 b |
| −0.1 MPa | 78 ± 5.5 b | 51 ± 3.8 d | 62 ± 6.6 cd | 56 ± 2.6 cd | 92 ± 1.5 a | 57 ± 9.7 cd | 66 ± 3.2 bcd | 69 ± 5.1 bc | |
| −0.3 MPa | 76 ± 2.0 a | 30 ± 3.6 e | 43 ± 7.1 cd | 53 ± 4.4 bc | 86 ± 3.3 a | 55 ± 4.1 b | 52 ± 2.0 bc | 35 ± 1.9 de | |
| −0.5 MPa | 39 ± 4.4 b | 7 ± 1.1 d | 41 ± 8.6 b | 22 ± 4.6 c | 52 ± 5.9 a | 20 ± 4.5 cd | 44 ± 3.2 b | 25 ± 2.3 c | |
| −0.7 MPa | 3 ± 1.7 c | 0 ± 0.0 d | 1 ± 0.5 d | 12 ± 2.1 a | 5 ± 1.8 bc | 0 ± 0.0 d | 11 ± 0.9 a | 7 ± 0.8 b | |
| 10 °C | 0 MPa | 81 ± 3.5 b | 71 ± 1.8 cd | 67 ± 2.5 d | 72 ± 2.9 bcd | 93 ± 1.2 a | 80 ± 4.2 bc | 68 ± 2.9 d | 79 ± 4.0 bc |
| −0.1 MPa | 76 ± 2.8 b | 65 ± 3.4 de | 61 ± 0.5 e | 67 ± 5.3 cde | 94 ± 1.8 a | 76 ± 1.6 bc | 65 ± 2.4 de | 72± 4.7 bcd | |
| −0.3 MPa | 76 ± 10.7 b | 31 ± 2.3 d | 50 ± 3.8 c | 60 ± 4.9 bc | 94 ± 2.4 a | 73 ± 2.0 b | 52 ± 1.9 c | 46 ± 10.9 cd | |
| −0.5 MPa | 63 ± 3.0 b | 21 ± 5.4 d | 46 ± 4.6 c | 28 ± 2.6 d | 84 ± 4.9 a | 66 ± 3.3 b | 45 ± 1.9 c | 29 ± 5.2 d | |
| −0.7 MPa | 4 ± 0.6 d | 4 ± 1.1 d | 6 ± 1.1 cd | 22 ± 1.4 a | 5 ± 0.4 cd | 7 ± 0.9 c | 11 ± 1.5 b | 11 ± 1.3 b | |
| 15 °C | 0 MPa | 92 ± 2.4 ab | 74 ± 3.4 cd | 75 ± 3.4 cd | 72 ± 1.6 d | 93 ± 1.7 ab | 88 ± 1.7 b | 75 ± 0.8 c | 95 ± 1.3 a |
| −0.1 MPa | 95 ± 2.6 a | 66 ± 1.4 b | 73 ± 2.1 b | 67 ± 0.6 b | 96 ± 1.7 a | 88 ± 2.6 a | 67 ± 2.3 b | 89 ± 6.0 a | |
| −0.3 MPa | 89 ± 2.4 a | 42 ± 3.4 d | 63 ± 2.4 b | 60 ± 1.7 bc | 93 ± 3.3 a | 88 ± 2.5 a | 50 ± 1.8 cd | 89 ± 5.8 a | |
| −0.5 MPa | 67 ± 1.7 b | 30 ± 3.6 d | 56 ± 2.8 b | 29 ± 1.7 d | 94 ± 2.2 a | 58 ± 2.4 bc | 49 ± 4.3 c | 45 ± 9.3 c | |
| −0.7 MPa | 7 ± 0.5 c | 5 ± 0.4 c | 6 ± 0.9 c | 23 ± 3.1 a | 8 ± 1.7 c | 5 ± 0.7 c | 10 ± 0.8 bc | 15 ± 4.1 b | |
| 20 °C | 0 MPa | 82 ± 1.4 b | 45 ± 6.6 d | 63 ± 1.1 c | 68 ± 3.0 c | 83 ± 3.9 ab | 83 ± 0.7 ab | 90 ± 3.6 ab | 96 ± 1.5 a |
| −0.1 MPa | 77 ± 2.2 b | 37 ± 3.0 d | 60 ± 2.7 c | 65 ± 5.7 c | 91 ± 4.5 a | 83 ± 1.6 ab | 91 ± 4.5 a | 91 ± 5.1 a | |
| −0.3 MPa | 74 ± 10.2 b | 14 ± 4.3 d | 32 ± 5.8 d | 55 ± 1.7 c | 80 ± 4.5 a | 50 ± 7.2 c | 81 ± 3.2 a | 76 ± 17.5 b | |
| −0.5 MPa | 60 ± 2.9 a | 12 ± 6.8 d | 10 ± 5.3 d | 27 ± 5.0 bc | 35 ± 4.6 bc | 11 ± 4.6 d | 39 ± 3.9 b | 17 ± 2.8 c | |
| −0.7 MPa | 4 ± 1.2 bc | 2 ± 1.7 c | 3 ± 0.4 c | 10 ± 1.7 a | 4 ± 1.1 bc | 1 ± 0.3 c | 8 ± 1.6 a | 7 ± 1.7 ab | |
| 25 °C | 0 MPa | 80 ± 4.3 a | 40 ± 4.0 d | 55 ± 2.1 c | 67 ± 2.6 bc | 82 ± 2.2 a | 82 ± 0.8 a | 64 ± 6.2 c | 81 ±7.0 a |
| −0.1 MPa | 76 ± 2.6 b | 37 ± 12.0 e | 53 ± 1.8 d | 64 ± 4.8 cd | 87 ± 1.9 a | 83 ± 2.9 a | 63 ± 2.8 cd | 79 ± 5.6 b | |
| −0.3 MPa | 63 ± 2.0 ab | 11 ± 0.4 d | 32 ± 0.8 c | 44 ± 5.7 bc | 79 ± 3.0 a | 36 ± 7.5 c | 51 ± 6.2 bc | 48 ± 19.5 bc | |
| −0.5 MPa | 60 ± 2.8 a | 8 ± 1.9 d | 10 ± 0.3 d | 22 ± 2.8 c | 34 ± 1.5 b | 9 ± 3.8 d | 13 ± 4.7 d | 14 ± 3.3 cd | |
| −0.7 MPa | 5 ± 0.9 a | 4 ± 0.8 a | 4 ± 0.3 a | 5 ± 0.6 a | 3 ± 0.9 a | 3 ± 1.1 a | 9 ± 4.3 a | 2 ± 0.0 a | |
| 30 °C | 0 MPa | 72 ± 3.6 a | 11 ± 3.6 d | 48 ± 4.9 bc | 36 ± 3.4 c | 85 ± 4.6 a | 48 ± 4.9 bc | 56 ± 4.8 b | 20 ± 7.4 d |
| −0.1 MPa | 51 ± 4.2 a | 5 ± 1.5 c | 31 ± 4.4 b | 26 ± 2.2 b | 51 ± 3.8 a | 31 ± 4.4 b | 34 ± 5.8 b | 13 ± 3.3 c | |
| −0.3 MPa | 17 ± 1.3 a | 1 ± 0.8 a | 17 ± 4.9 a | 12 ± 3.4 a | 6 ± 5.4 a | 17 ± 4.9 a | 23 ± 11.9 a | 4 ± 2.0 a | |
| −0.5 MPa | 0 ± 0.0 b | 0 ± 0.0 b | 1 ± 0.5 b | 7 ± 2.4 b | 1 ± 0.3 b | 1 ± 0.3 b | 29 ± 9.5 a | 0 ± 0.0 b | |
| −0.7 MPa | 0 ± 0.0 a | 0 ± 0.0 a | 1 ± 0.5 a | 0 ± 0.0 a | 2 ± 1.2 a | 0 ± 0.0 a | 1 ± 0.5 a | 0 ± 0.0 a |
| Maximum Seedling Emergence | Days to First Seedling Emergence | Days to 100% Seedling Mortality | ||
|---|---|---|---|---|
| T. alexandrinum | Soil moisture at planting | 0.807 * | −0.848 * | 0.879 * |
| Maximum seedling emergence | −0.945 * | 0.888 * | ||
| Days to first seedling emergence | −0.933 * | |||
| T. vesiculosum | Soil moisture at planting | 0.875 * | −0.868 * | 0.861 * |
| Maximum seedling emergence | −0.955 * | 0.888 * | ||
| Days to first seedling emergence | 0.952 * | |||
| T. michelianum | Soil moisture at planting | 0.945 * | −0.836 * | 0.764 * |
| Maximum seedling emergence | −0.901 * | 0.908 * | ||
| Days to first seedling emergence | −0.787 * | |||
| T. subterraneum ssp. brachycalycinum | Soil moisture at planting | 0.853 * | −0.658 * | 0.849 * |
| Maximum seedling emergence | −0.816 * | 0.945 * | ||
| Days to first seedling emergence | −0.841 * | |||
| T. subterraneum ssp. subterraneum | Soil moisture at planting | 0.944 * | −0.792 * | 0.762 * |
| Maximum seedling emergence | −0.820 * | 0.906 * | ||
| Days to first seedling emergence | −0.785 * | |||
| M. polymorpha | Soil moisture at planting | 0.763 * | −0.892 * | 0.877 * |
| Maximum seedling emergence | −0.910 * | 0.930 * | ||
| Days to first seedling emergence | −0.968 * | |||
| M. truncatula | Soil moisture at planting | 0.813 * | −0.775 * | 0.821 * |
| Maximum seedling emergence | −0.844 * | 0.905 * | ||
| Days to first seedling emergence | −0.871 * | |||
| M. littoralis | Soil moisture at planting | 0.946 * | −0.725 * | 0.844 * |
| Maximum seedling emergence | −0.781 * | 0.798 * | ||
| Days to first seedling emergence | −0.847 * |
| Seedling Emergence (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Soil Moisture | Days after Planting | |||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| T. alexandrinum | 100% | 20 ± 2 d | 60 ± 0 hi | 81 ± 3 k | 97 ± 2 l | 98 ± 1 l | 98 ± 1 l | 98 ± 1 l | 98 ± 1 l | 90 ± 3 l | 57 ± 4 hi | 24 ± 3 de | 8 ± 2 bc | 7 ± 3 b | 0 ± 0 a |
| 70% | 16 ± 3 cd | 53 ± 2 h | 65 ± 5 ij | 96 ± 2 l | 98 ± 1 l | 98 ± 1 l | 72 ± 3 j | 53 ± 2 h | 22 ± 1 de | 9 ± 1 bc | 5 ± 3 b | 5 ± 3 b | 1 ± 1 a | 0 ± 0 a | |
| 50% | 0 ± 0 a | 7 ± 1 ab | 18 ± 1 d | 36 ± 7 fg | 54 ± 6 h | 65 ± 6 ij | 64 ± 12 ij | 39 ± 4 g | 29 ± 3 ef | 6 ± 1 b | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| 30% | 0 ± 0 a | 0 ± 0 a | 1 ± 1 a | 23 ± 10 de | 17 ± 12 cd | 1 ± 1 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| T. vesiculosum | 100% | 26 ± 4 efg | 55 ± 2 jk | 74 ± 3 l | 88 ± 8 mn | 95 ± 3 n | 96 ± 3 n | 96 ± 3 n | 94 ± 5 n | 79 ± 7 lm | 50 ± 5 j | 18 ± 1 cde | 7 ± 2 b | 5 ± 3 b | 0 ± 0 a |
| 70% | 26 ± 4 efg | 36 ± 3 hi | 40 ± 5 i | 74 ± 7 l | 92 ± 5 n | 93 ± 6 n | 52 ± 5 j | 49 ± 5 j | 18 ± 1 cde | 9 ± 3 b | 4 ± 3 ab | 4 ± 3 ab | 1 ± 1 a | 0 ± 0 a | |
| 50% | 0 ± 0 a | 8 ± 2 b | 21 ± 4 cde | 55 ± 7 jk | 56 ± 7 jk | 64 ± 6 k | 58 ± 7 jk | 34 ± 2 ghi | 28 ± 7 fgh | 12 ± 3 bc | 1 ± 1 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| 30% | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 24 ± 4 def | 16 ± 3 cd | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| T. michelianum | 100% | 12 ± 2 c | 24 ± 3 d | 34 ± 4 fg | 55 ± 5 jk | 75 ± 2 l | 75 ± 2 l | 75 ± 2 l | 75 ± 2 l | 51 ± 6 ijk | 32 ± 2 rf | 12 ± 0 c | 5 ± 3 bc | 5 ± 3 bc | 0 ± 0 a |
| 70% | 8 ± 2 bc | 21 ± 1 d | 27 ± 5 def | 59 ± 7 k | 68 ± 8 l | 76 ± 2 l | 53 ± 7 ijk | 51 ± 5 ijk | 20 ± 2 d | 9 ± 1 bc | 4 ± 2 ab | 4 ± 2 ab | 1 ± 1 a | 0 ± 0 a | |
| 50% | 0 ± 0 a | 2 ± 1 a | 6 ± 3 bc | 41 ± 2 gh | 47 ± 2 hi | 56 ± 3 jk | 49 ± 5 ij | 26 ± 1 de | 11 ± 2 c | 6 ± 1 bc | 1 ± 1 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| 30% | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 12 ± 0 c | 10 ± 6 bc | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| T. subterraneum ssp. brachycalycinum | 100% | 1 ± 1 a | 11 ± 2 bc | 43 ± 2 g | 80 ± 2 ij | 97 ± 2 l | 97 ± 2 l | 97 ± 2 l | 93 ± 2 kl | 83 ± 6 j | 47 ± 4 g | 15 ± 1 cde | 9 ± 3 bc | 4 ± 4 ab | 0 ± 0 a |
| 70% | 17 ± 3 cde | 31 ± 7 f | 49 ± 6 g | 73 ± 11 i | 84 ± 7 jk | 85 ± 8 jk | 72 ± 6 i | 42 ± 1 g | 24 ± 2 ef | 17 ± 3 cde | 12 ± 3 cd | 5 ± 3 ab | 1 ± 1 a | 0 ± 0 a | |
| 50% | 0 ± 0 a | 6 ± 2 ab | 21 ± 6 de | 44 ± 5 g | 49 ± 7 g | 60 ± 9 h | 71 ± 6 i | 42 ± 7 g | 22 ± 1 def | 11 ± 2 bc | 1 ± 1 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| 30% | 0 ± 0 a | 0 ± 0 a | 2 ± 1 ab | 3 ± 1 ab | 5 ± 2 ab | 2 ± 2 ab | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| T. subterraneum ssp. subterraneum | 100% | 8 ± 4 bc | 16 ± 3 d | 51 ± 5 hi | 62 ± 7 k | 93 ± 3 l | 59 ± 2 jk | 59 ± 2 jk | 59 ± 2 jk | 41 ± 1 g | 28 ± 0 f | 5 ± 1 b | 3 ± 2 ab | 2 ± 2 a | 0 ± 0 a |
| 70% | 5 ± 3 b | 25 ± 5 ef | 46 ± 5 gh | 53 ± 6 hij | 57 ± 6 ijk | 60 ± 5 jk | 47 ± 4 gh | 32 ± 3 f | 18 ± 2 de | 6 ± 1 bc | 6 ± 1 bc | 3 ± 2 ab | 1 ± 1 a | 0 ± 0 a | |
| 50% | 0 ± 0 a | 4 ± 2 ab | 13 ± 4 cd | 47 ± 1 gh | 50 ± 1 hi | 57 ± 2 ijk | 57 ± 2 ijk | 42 ± 1 g | 32 ± 3 f | 12 ± 2 cd | 2 ± 1 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| 30% | 0 ± 0 a | 0 ± 0 a | 3 ± 1 ab | 5 ± 4 ab | 5 ± 1 ab | 3 ± 1 ab | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| M. polymorpha | 100% | 10 ± 3 bc | 22 ± 3 def | 69 ± 3 l | 80 ± 4 m | 97 ± 2 n | 97 ± 2 n | 97 ± 2 n | 97 ± 2 n | 81 ± 8 m | 44 ± 3 h | 16 ± 4 cd | 16 ± 4 cd | 2 ± 1 a | 0 ± 0 a |
| 70% | 21 ± 5 def | 52 ± 4 i | 72 ± 10 l | 92 ± 3 n | 97 ± 2 n | 97 ± 2 n | 54 ± 2 ij | 31 ± 3 g | 18 ± 3 de | 8 ± 2 b | 4 ± 0 ab | 3 ± 1 a | 1 ± 1 a | 0 ± 0 a | |
| 50% | 0 ± 0 a | 9 ± 1 bc | 31 ± 3 g | 64 ± 2 kl | 70 ± 1 l | 87 ± 3 mn | 87 ± 3 mn | 60 ± 6 jk | 28 ± 5 fg | 10 ± 1 bc | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| 30% | 0 ± 0 a | 0 ± 0 a | 19 ± 1 de | 25 ± 4 efg | 25 ± 3 efg | 19 ± 1 de | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| M. truncatula | 100% | 4 ± 2 a | 23 ± 4 cd | 54 ± 3 gh | 64 ± 4 hi | 84 ± 5 l | 86 ± 3 l | 86 ± 3 l | 83 ± 4 kl | 60 ± 6 ghi | 32 ± 2 de | 6 ± 2 ab | 6 ± 1 ab | 1 ± 1 a | 0 ± 0 a |
| 70% | 15 ± 4 bc | 40 ± 8 ef | 50 ± 8 fg | 69 ± 3 ij | 74 ± 3 jk | 76 ± 2 jk | 50 ± 3 fg | 33 ± 2 de | 17 ± 1 bc | 9 ± 1 b | 2 ± 1 a | 2 ± 1 a | 1 ± 1 a | 0 ± 0 a | |
| 50% | 0 ± 0 a | 8 ± 2 ab | 28 ± 3 d | 56 ± 10 gh | 63 ± 9 hi | 73 ± 10 jk | 73 ± 10 jk | 59 ± 8 gh | 40 ± 3 ef | 17 ± 2 bc | 2 ± 1 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| 30% | 0 ± 0 a | 0 ± 0 a | 1 ± 1 a | 8 ± 2 ab | 9 ± 4 ab | 1 ± 1 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| M. littoralis | 100% | 4 ± 3 a | 14 ± 2 bc | 34 ± 8 f | 52 ± 5 hi | 72 ± 4 k | 75 ± 4 k | 75 ± 4 k | 75 ± 4 k | 64 ± 4 jk | 25 ± 5 e | 8 ± 2 bc | 8 ± 2 bc | 2 ± 1 a | 0 ± 0 a |
| 70% | 10 ± 7 bc | 19 ± 5 de | 34 ± 1 f | 45 ± 1 gh | 58 ± 2 ij | 45 ± 2 gh | 26 ± 4 e | 22 ± 1 de | 11 ± 1 bc | 6 ± 1 ab | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| 50% | 0 ± 0 a | 4 ± 2 ab | 16 ± 4 cd | 16 ± 4 cd | 19 ± 5 de | 24 ± 6 d | 24 ± 6 d | 43 ± 2 g | 23 ± 4 de | 6 ± 3 ab | 2 ± 1 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| 30% | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 18 ± 14 cd | 8 ± 4 bc | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, F.; Masemola, L.; Britz, E.; Ngcobo, N.; Modiba, S.; Cyster, L.; Samuels, I.; Cupido, C.; Raitt, L. Seed Germination and Early Seedling Growth Responses to Drought Stress in Annual Medicago L. and Trifolium L. Forages. Agronomy 2022, 12, 2960. https://doi.org/10.3390/agronomy12122960
Müller F, Masemola L, Britz E, Ngcobo N, Modiba S, Cyster L, Samuels I, Cupido C, Raitt L. Seed Germination and Early Seedling Growth Responses to Drought Stress in Annual Medicago L. and Trifolium L. Forages. Agronomy. 2022; 12(12):2960. https://doi.org/10.3390/agronomy12122960
Chicago/Turabian StyleMüller, Francuois, Letty Masemola, Ethan Britz, Nothando Ngcobo, Stephen Modiba, Lilburne Cyster, Igshaan Samuels, Clement Cupido, and Lincoln Raitt. 2022. "Seed Germination and Early Seedling Growth Responses to Drought Stress in Annual Medicago L. and Trifolium L. Forages" Agronomy 12, no. 12: 2960. https://doi.org/10.3390/agronomy12122960
APA StyleMüller, F., Masemola, L., Britz, E., Ngcobo, N., Modiba, S., Cyster, L., Samuels, I., Cupido, C., & Raitt, L. (2022). Seed Germination and Early Seedling Growth Responses to Drought Stress in Annual Medicago L. and Trifolium L. Forages. Agronomy, 12(12), 2960. https://doi.org/10.3390/agronomy12122960





