Photoperiodic Regulation of Tuber Enlargement in Water Yam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Photoperiod Treatments and Measurement of Tuber Traits
2.2.1. Experiment 1: Effects of Different Daylength Conditions on Tuber Enlargement
2.2.2. Experiment 2: Changes in Tuber Growth and FT-like Gene Expression under SD and LD
2.2.3. Experiment 3: Effects of Night-Break Treatments on Tuber Enlargement
2.3. RNA Extraction and Quantitative Real-Time PCR
3. Results and Discussion
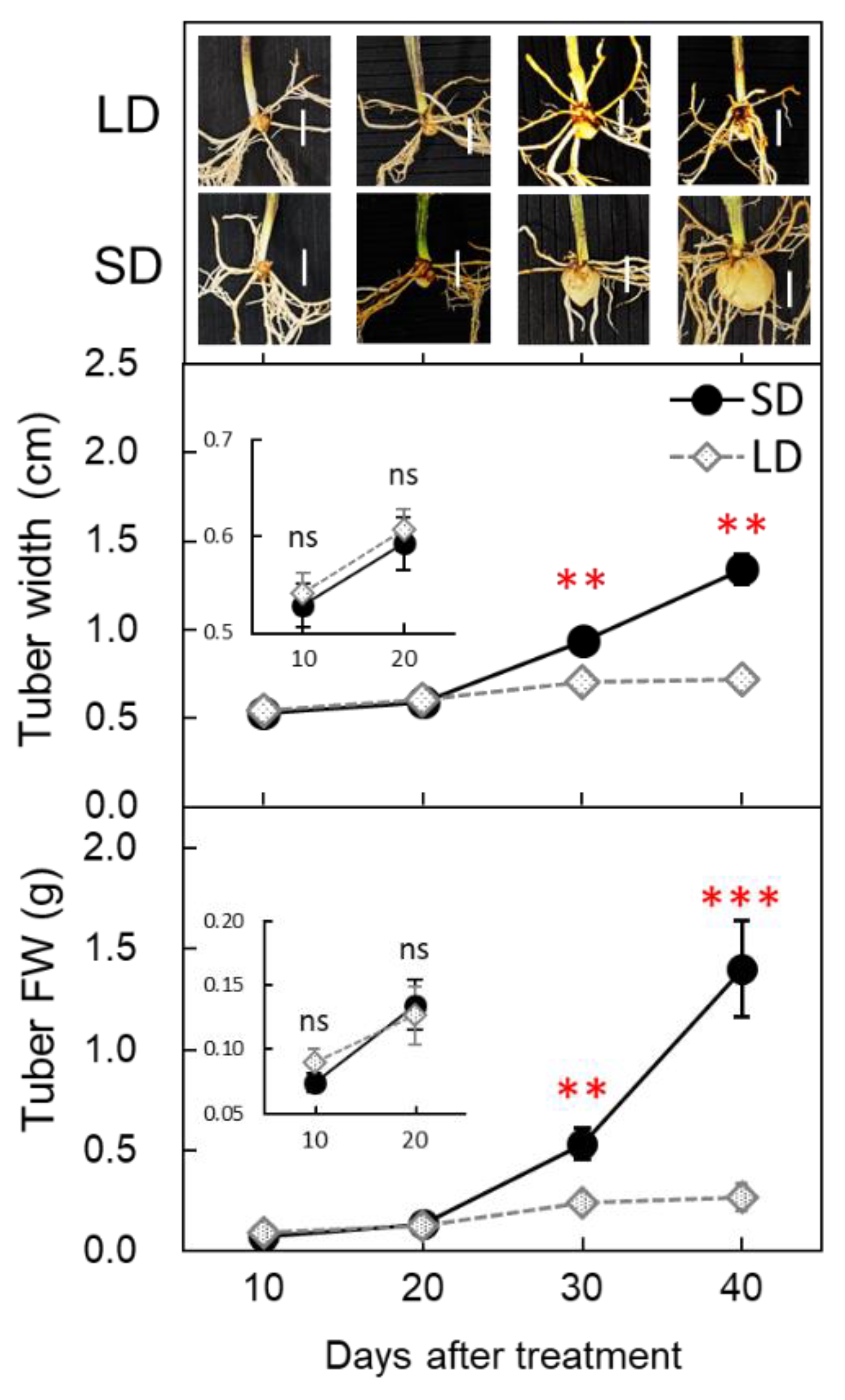
3.1. Photoperiod Response of Tuber Enlargement
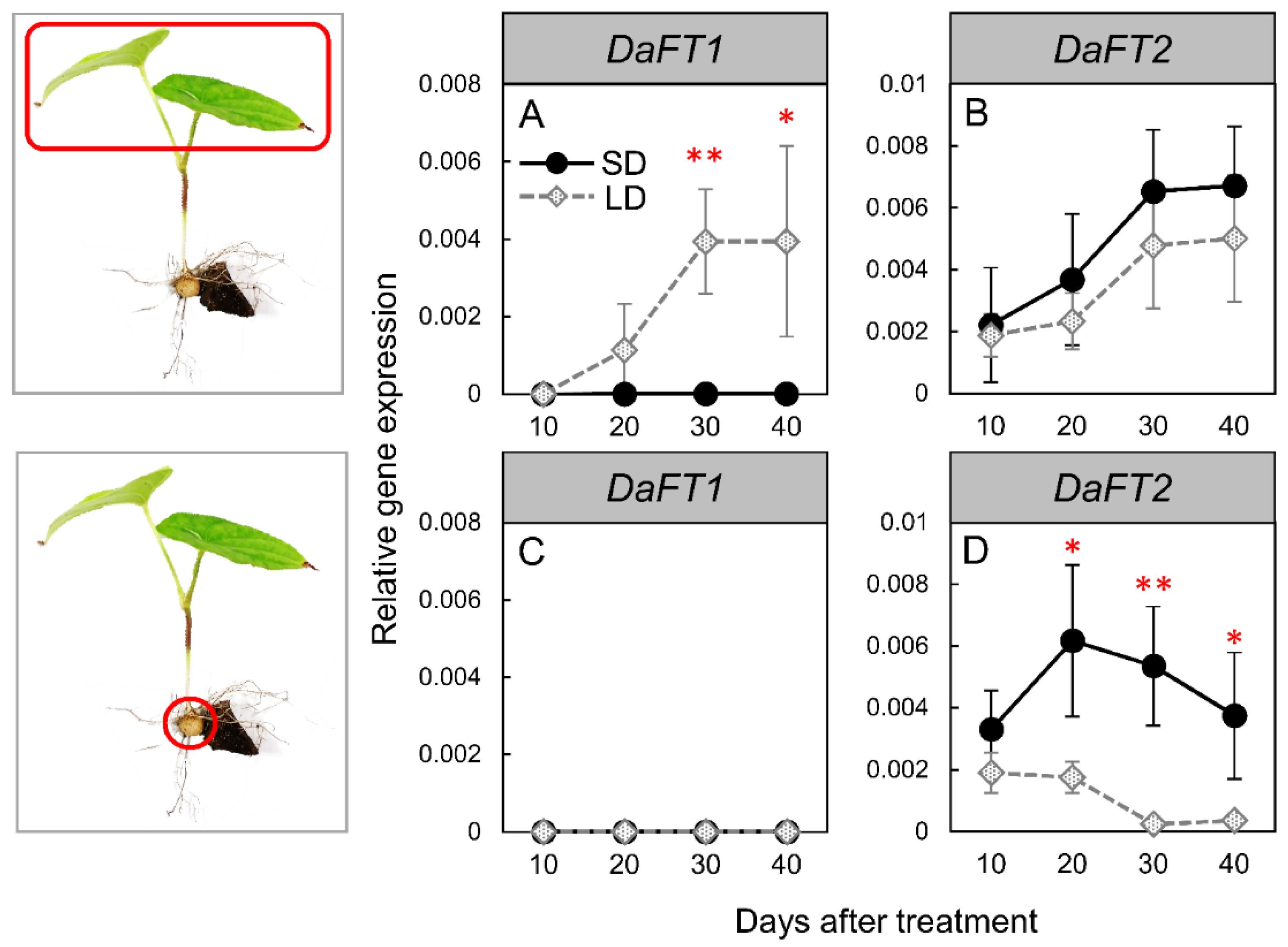
3.2. Changes in FT-like Gene Expression under SD and LD
3.3. Effects of Night-Break Treatments on Tuber Enlargement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharjee, R.; Gedil, M.; Sartie, A.; Otoo, E.; Dumet, D.; Kikuno, H.; Kumar, P.L.; Asiedu, R. Dioscorea. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- FAOSTAT. 2022. Available online: https://www.fao.org/faostat/en/#data/QV (accessed on 20 November 2022).
- Takada, K.; Kikuno, H.; Babil, P.; Irie, K.; Shiwachi, H. Water yam (Dioscorea alata L.) is able to grow in low fertile soil conditions. Trop. Agric. Dev. 2017, 61, 8–14. [Google Scholar]
- Matsumoto, R.; Ishikawa, H.; Asfaw, A.; Asiedu, R. Low soil nutrient tolerance and mineral fertilizer response in white guinea yam (Dioscorea rotundata) genotypes. Front. Plant Sci. 2021, 12, 629762. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea Genus (Yam) An appraisal of nutritional and therapeutic potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef]
- Padhan, B.; Panda, D. Potential of neglected and underutilized yams (Dioscorea spp.) for improving nutritional security and health benefits. Front. Pharmacol. 2020, 11, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coursey, D.G. Yams; Longman: London, UK, 1967; pp. 68–107. [Google Scholar]
- Abang, M.M.; Winter, S.; Mignouna, H.D.; Green, K.R.; Asiedu, R. Molecular taxonomic, epidemiological and population genetic approaches to understanding yam anthracnose disease. Afr. J. Biotechnol. 2003, 2, 486–496. [Google Scholar]
- Olorunda, S.O.; Macklon, E.S. Effects of storage and chilling temperature on low absorption salt retention capacity and respiratory pattern in yam tubers. J. Sci. Agric. 1976, 27, 405–412. [Google Scholar] [CrossRef]
- Garner, W.W.; Allard, H.A. Further studies in photoperiodism. The response of the plant to relative length of day and night. J. Agr. Res. 1923, 23, 871–920. [Google Scholar]
- Shiwachi, H.; Onjo, M.; Hayashi, M. Photoperiodic response of water yam (Dioscorea alata L.) Chinese yam (D. opposita Thunb.) and Jinen-jo (D. japonica Thunb.). Jpn. J. Trop. Agric. 2000, 44, 107–114. [Google Scholar]
- Shiwachi, H.; Ayankanmi, T.; Asiedu, R. Effect of daylength on the development of tubers in yams Dioscorea spp. Trop. Sci. 2002, 42, 162–170. [Google Scholar]
- Hayashi, M.; Ishihata, K. Studies on the development and thickening growth of tuber in yam, Dioscorea spp. 1. Some characteristics of the development of cv. Solo yam, D. alata. Jpn. J. Trop. Agric. 1990, 34, 151–155. [Google Scholar]
- Jean, M.; Cappadocia, M. In vitro tuberization in Dioscorea alata L. ‘Brazo fuerte’ and ‘Florido’ and D. abyssinica Hoch. Plant Cell Tissue Organ Cult. 1991, 26, 147–152. [Google Scholar] [CrossRef]
- Hayashi, M.; Ishihata, K. Studies on the development and the thickening growth of tubers in yams, Dioscorea spp. 2. Effect of photoperiod and temperature on the growth and enlargment of the tubers. Jpn. J. Trop. Agric. 1991, 35, 79–83. [Google Scholar]
- Vaillant, V.; Bade, P.; Constant, C. Photoperiod affects the growth and development of yam plantlets obtained by in vitro propagation. Biol. Plant. 2005, 49, 355–359. [Google Scholar] [CrossRef]
- Navarro, C.; Abelenda, J.A.; Cuéllar, C.; Tamaki, S.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Baldwin, S.; Kenel, F.; McCallum, J.; Macknight, R. FLOWERING LOCUS T genes control onion bulb formation and flowering. Nat. Commun. 2013, 4, 2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Nasim, Z.; Susila, H.; Ahn, J.H. Evolution and functional diversification of FLOWERING LOCUS T/TERMINAL FLOWER 1 family genes in plants. Semin. Cell Dev. Biol. 2021, 109, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Manjul, A.S.; Raigond, P.; Singh, B.; Siddappa, S.; Bhardwaj, V.; Kawar, P.G.; Patil, V.U.; Kardile, H.B. Key players associated with tuberization in potato: Potential candidates for genetic engineering. Crit. Rev. Biotechnol. 2017, 37, 942–957. [Google Scholar] [CrossRef]
- Tamiru, M.; Natsume, S.; Takagi, H.; White, B.; Yaegashi, H.; Shimizu, M.; Yoshida, K.; Uemura, A.; Oikawa, K.; Abe, A.; et al. Genome sequencing of the staple food crop white Guinea yam enables the development of a molecular marker for sex determination. BMC Biol. 2017, 15, 86. [Google Scholar] [CrossRef] [Green Version]
- Bredeson, J.V.; Lyons, J.B.; Oniyinde, I.O.; Okereke, N.R.; Kolade, O.; Nnabue, I.; Nwadili, C.O.; Hribová, E.; Parker, M.; Nwogha, J.; et al. Chromosome evolution and the genetic basis of agronomically important traits in greater yam. Nat. Commun. 2021, 13, 2001. [Google Scholar] [CrossRef]
- Barman, P.; Choudhary, A.K.; Geeta, R. A modified protocol yields high-quality RNA from highly mucilaginous Dioscorea tubers. 3 Biotech 2017, 7, 150. [Google Scholar] [CrossRef] [Green Version]
- Abelenda, J.A.; Cruz-Oró, E.; Franco-Zorrilla, J.M.; Prat, S. Potato StCONSTANS-like1 suppresses storage organ formation by directly activating the FT-like StSP5G repressor. Curr. Biol. 2016, 26, 872–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, B.; Vince-Prue, D. Photoperiodism in Plants; Academic Press: London, UK, 1997. [Google Scholar]
- Masuda, J.; Ozaki, Y.; Okubo, H. Rhizome transition to storage organ is under phytochrome control in lotus (Nelumbo nucifera). Planta 2007, 226, 909–915. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamaoka, N.; Nabeshima, M.; Moriyama, T.; Kozawa, Y.; Ishibashi, Y. Photoperiodic Regulation of Tuber Enlargement in Water Yam. Agronomy 2022, 12, 2939. https://doi.org/10.3390/agronomy12122939
Hamaoka N, Nabeshima M, Moriyama T, Kozawa Y, Ishibashi Y. Photoperiodic Regulation of Tuber Enlargement in Water Yam. Agronomy. 2022; 12(12):2939. https://doi.org/10.3390/agronomy12122939
Chicago/Turabian StyleHamaoka, Norimitsu, Misato Nabeshima, Takahito Moriyama, Yudai Kozawa, and Yushi Ishibashi. 2022. "Photoperiodic Regulation of Tuber Enlargement in Water Yam" Agronomy 12, no. 12: 2939. https://doi.org/10.3390/agronomy12122939
APA StyleHamaoka, N., Nabeshima, M., Moriyama, T., Kozawa, Y., & Ishibashi, Y. (2022). Photoperiodic Regulation of Tuber Enlargement in Water Yam. Agronomy, 12(12), 2939. https://doi.org/10.3390/agronomy12122939





