Non-Thermal Plasma as an Alternative to Enhance the Early Growth Structures in Lentil Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lentil Seeds for Experimental Tests
- Experimental test 1: Potable water (PW).
- Experimental test 2: Activation of potable water by non-thermal plasma (ANTP-PW) for subsequent irrigation of lentils.
- Experimental test 3: Direct application of non-thermal plasma on lentils irrigated previously with potable water (DNTP-PW).
- Experimental test 4: Wastewater from poultry farming according to the physical and microbiological conditions generated within 24 h. Water could contain wastes from chicken feed, which has 12.0% raw protein, 3.5% crude fiber, 12.0% moisture, and pH = 6.4 because of a mixture of ground cereals such as sorghum, corn, and wheat (FW).
- Experimental test 5: Activation of wastewater from poultry farming by non-thermal plasma (ANTP-FW) for watering the lentils afterward.
- Experimental test 6: Direct application of non-thermal plasma in lentils irrigated previously with wastewater from poultry farming (DNTP-FW).
- Experimental test 7: Rainwater, harvested in the rainy season, 30 min after the rain started (RW).
- Experimental test 8: Activation of rainwater by non-thermal plasma (ANTP-RW).
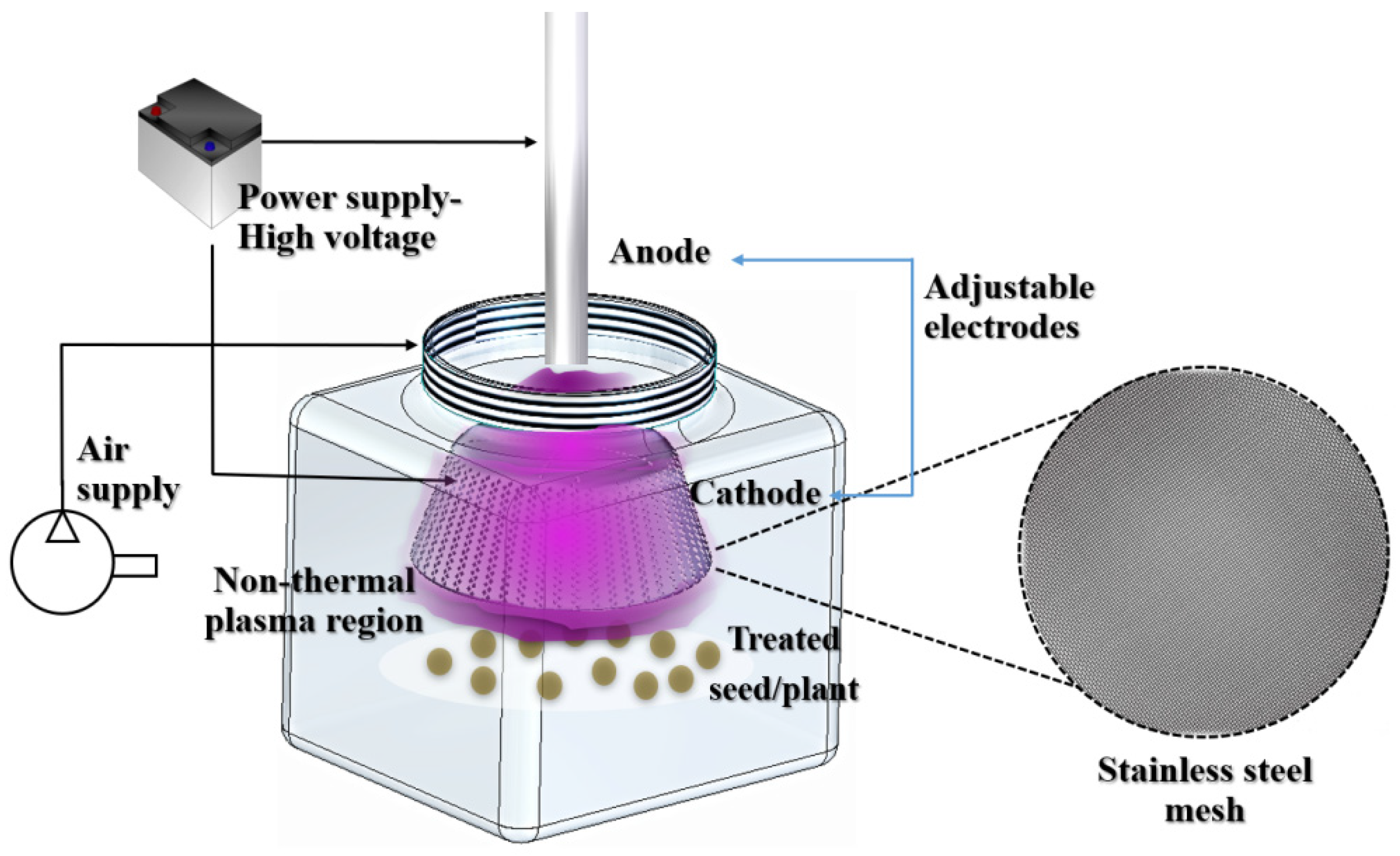
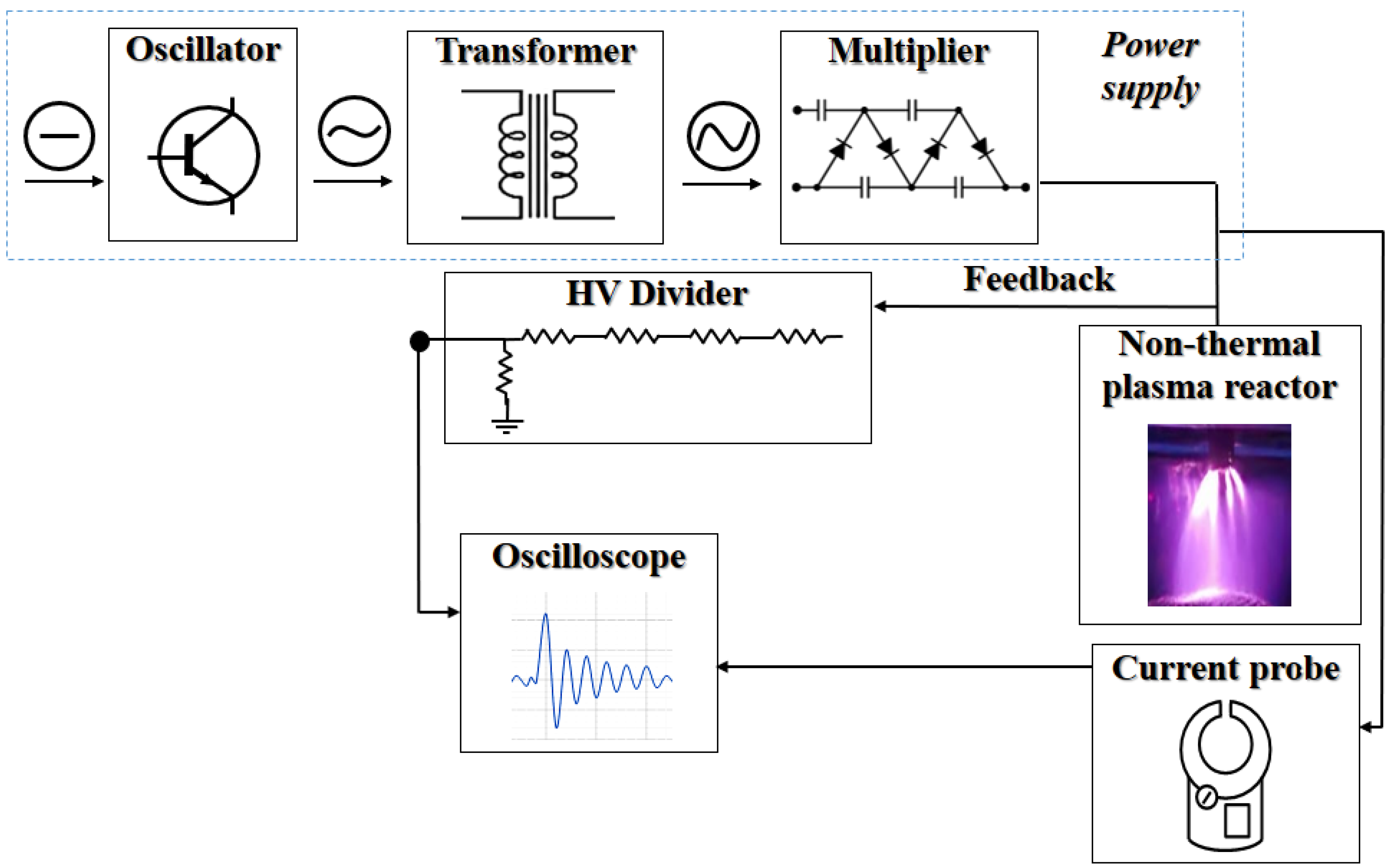
2.2. Configuration of the Non-Thermal Plasma Reactor
2.3. Activation of Hydric Supply by NTP and Irrigation Stage
2.4. Direct Application of NTP
2.5. Measurement of Structures in Lentil Plants
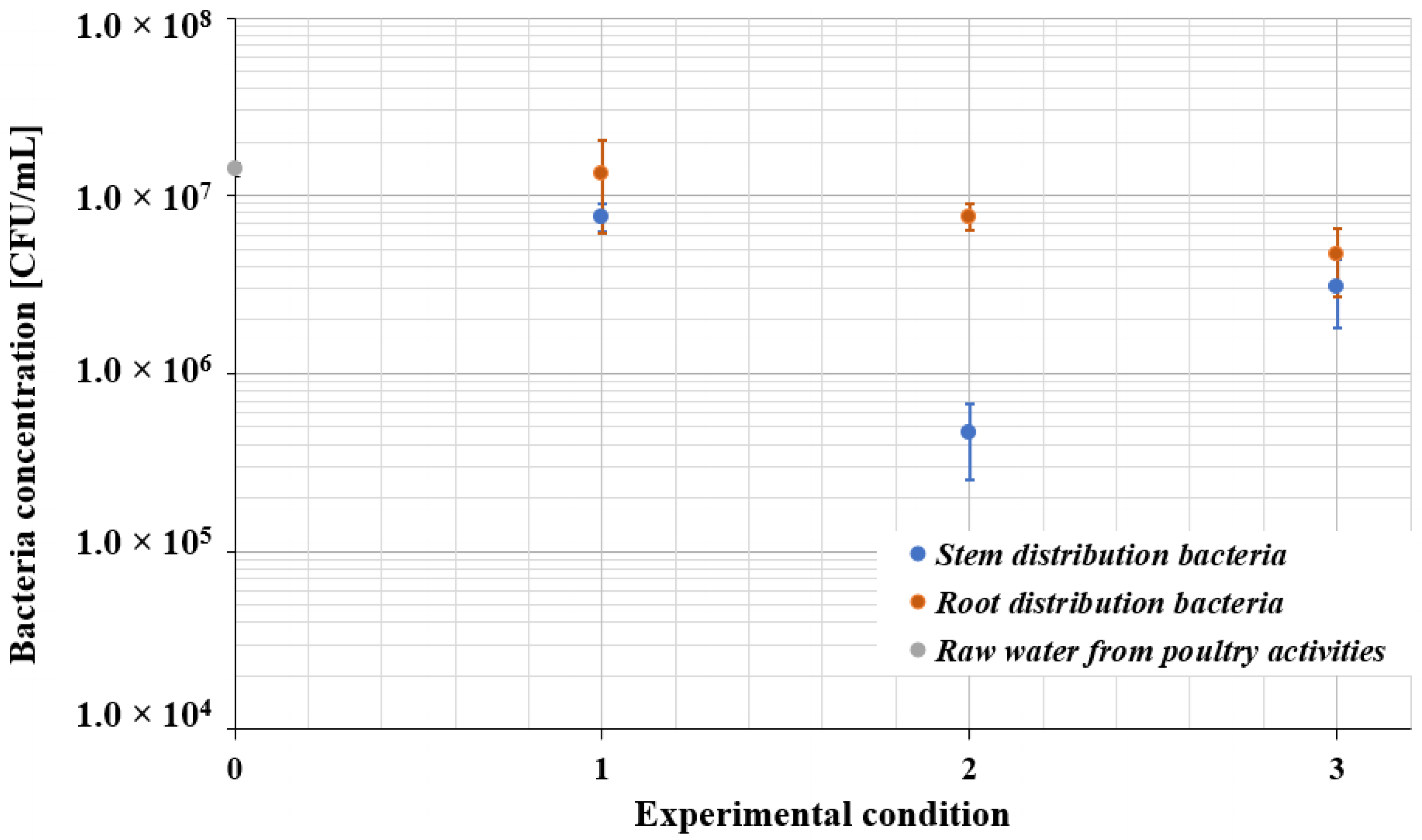
2.6. Microbiological Analysis of Water from Poultry Farming
2.7. Microbiological Analysis for Internalization Bacteria
3. Results
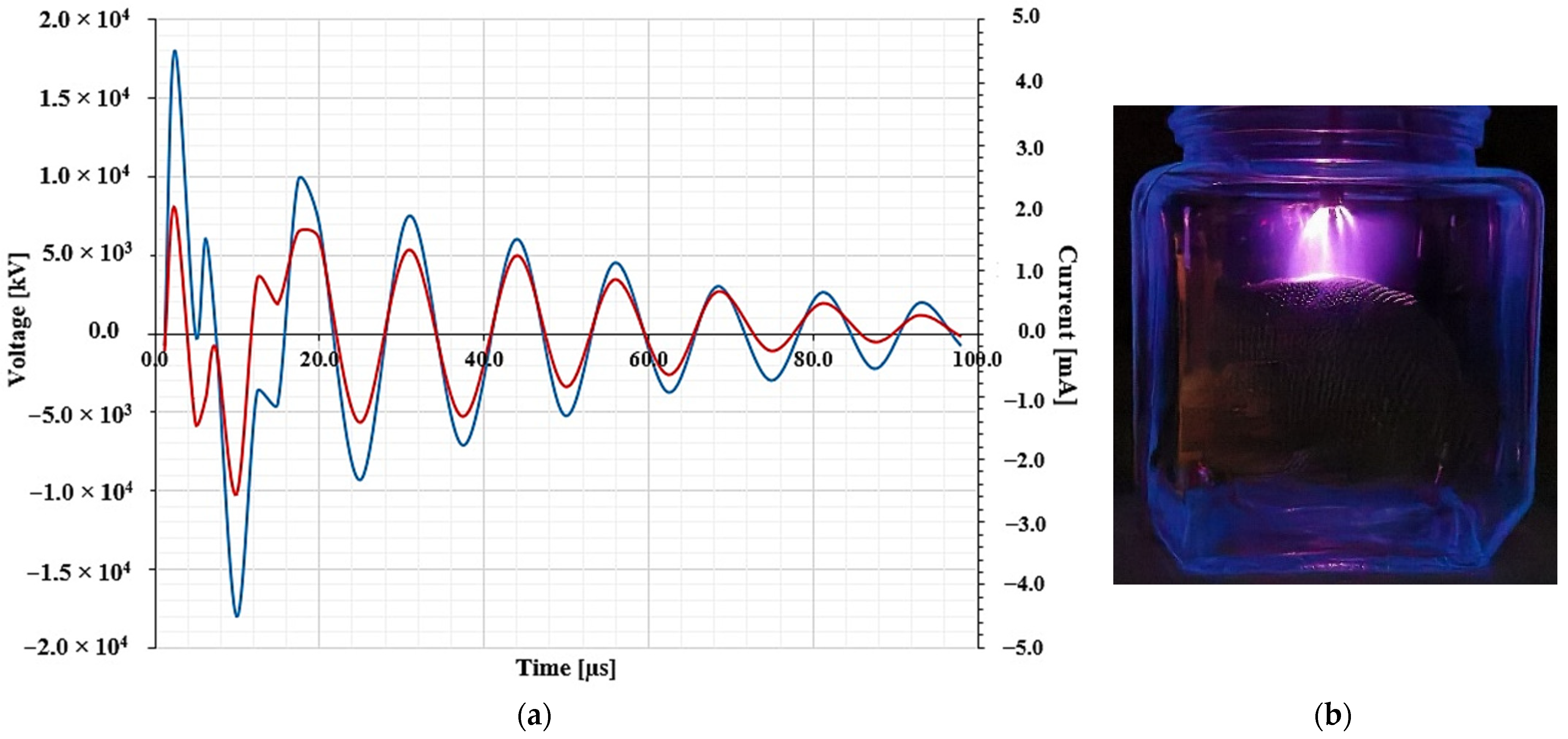
- The experimental conditions established in the tests of this work allowed the identification of effects generated in different hydric supplies by the application of NTP. Figure 3a shows the voltage and current waveforms detected in corona discharge with the injection of air (Figure 3b), in the early development of structures of lentils.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Sharma, P. Agricultural production, marketing and food security in India: A peep into progress. Productivity 2017, 58, 155–165. [Google Scholar]
- Praburaj, M.L. Role of agriculture in the economic development of a country. Shanlax Int. J. Commer. 2018, 6, 1–5. [Google Scholar]
- Eini-Zinab, H.; Edalati, S.; Sobhani, S.R.; Kezabi, M.F.; Hosseini, S. Undernourishment trends and determinants: An ecological study of 76 countries. Public Health 2020, 186, 230–239. [Google Scholar]
- Pawlask, K.; Kolodziejczak, M. The role of agriculture in ensuring food security in developing countries: Considerations in the context of the problem of sustainable food production. Sustainability 2020, 12, 5488. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017; pp. 4–7. [Google Scholar]
- Olanipekun, I.O.; Olasehinde-Williams, G.O.; Alao, R.O. Agriculture and environmental degradation in Africa: The role of income. Sci. Total Environ. 2019, 692, 60–67. [Google Scholar]
- Rizou, M.; Galanakis, I.M.; Aldawoud, T.M.S.; Galanakis, C.M. Safety of foods, food supply chain and environment within the COVID-19 pandemic. Trends Food Sci. Technol. 2020, 102, 293–299. [Google Scholar]
- World Economic Forum. The Global Risks Report 2021, 16th ed.; World Economic Forum: Geneva, Switzerland, 2021; pp. 86–89. [Google Scholar]
- Berardy, A.; Chester, M. Climate change vulnerability in the food, energy, and water nexus: Concerns for agricultural production in Arizona and its urban export supply. Environ. Res. Lett. 2017, 12, 035004. [Google Scholar]
- Myers, S.S.; Matthew, R.S.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate Change and Global Food Systems: Potential Impacts on Food Security and Undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar]
- Bierbaum, R.M.; Holdren, J.P.; MacCracken, M.C.; Moss, R.H.; Raven, P.H. Confronting Climate Change: Avoiding the Unmanageable and Managing the Unavoidable; UN Foundation-Sigma XI: Research Triangle Park, NC, USA, 2007. [Google Scholar]
- Benka-Coker, W.; Hoskovec, L.; Severson, R.; Balmes, J.; Wilson, A.; Magzamen, S. The joint effect of ambient air pollution and agricultural pesticide exposures on lung function among children with asthma. Environ. Res. 2020, 190, 109903. [Google Scholar]
- Srivastav, A.L. Chemical fertilizers and pesticides: Role in groundwater contamination. In Agrochemicals Detection, Treatment and Remediation; Butterworth-Heinemann: Oxford, UK, 2020; pp. 143–159. [Google Scholar]
- Burke, I.C.; Thomas, W.E.; Spears, J.F.; Wilcut, J.W. Influence of environmental factors on after-ripened crowfootgrass (Dactyloctenium aegyptium) seed germination. Weed Sci. 2003, 51, 342–347. [Google Scholar]
- Ziuzina, D.; Misra, N.N. Cold plasma for food safety. In Cold Plasma in Food and Agriculture; Fundamentals and Applications; Academic Press: Cambridge, MA, USA, 2016; pp. 223–252. [Google Scholar]
- Mahajan, G.; Mutti, N.K.; Jha, P.; Walsh, M.; Chauhan, B.S. Evaluation of dormancy breaking methods for enhanced germination in four biotypes of Brassica tournefortii. Sci. Rep. 2018, 8, 17103. [Google Scholar]
- Martínez-Piernas, A.B.; Plaza-Bolaños, P.; Fernández-Ibánez, P.; Agüera, A. Organic microcontaminants in tomato crops irrigated with reclaimed water grown under field conditions: Occurrence, uptake, and health risk assessment. J. Agric. Food Chem. 2019, 67, 6930–6939. [Google Scholar]
- Zhang, W.; Mace, W.J.; Matthew, C.; Card, S.D. The impact of endophyte infection, seed aging, and imbibition on selected sugar metabolite concentrations in seed. J. Agric. Food Chem. 2019, 67, 6921–6929. [Google Scholar]
- Moisan, M.; Barbeau, J.; Crevier, M.-C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma sterilization: Methods and mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar]
- Schlüter, O.; Ehlbeck, J.; Hertel, C.; Habermeyer, M.; Roth, A.; Engel, K.-H.; Holzhauser, T.; Knorr, D.; Eisenbrand, G. Opinion on the use of plasma processes for treatment of foods. Mol. Nut. Food Res. 2013, 57, 920–927. [Google Scholar]
- Randeniya, L.K.; Groot, G.J.J.B. Non-thermal plasma treatment agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: A review. Plasma Proc. Polym. 2015, 12, 608–623. [Google Scholar]
- Nucifera, N.; Jabbar-Kanie, M.A.; Hayu-Pratiwi, S.; Pratiwi, R.; Pruto, S.P.; Nur, M. Corona discharge plasma technology to accelerate the growth of black soybean plants. J. Nat. Sci. Res. 2016, 6, 35–40. [Google Scholar]
- Zhou, R.; Li, J.; Zhou, R.; Zhang, X.; Yang, S. Atmospheric-pressure plasma treated water for seed germination and seedling growth of mung bean and its sterilization effect on mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 53, 36–44. [Google Scholar]
- Khamsen, N.; Onwimol, D.; Teerakawanich, N.; Dechanupaprittha, S.; Kanokbannakorn, W.; Hongesombut, K.; Srisonphan, S. Rice (Oryza sativa L.) seed sterilization and germination enhancement via atmospheric hybrid non-thermal discharge plasma. Appl. Mat. Interfaces 2016, 8, 19268–19275. [Google Scholar]
- Puligundla, P.; Kim, J.W.; Mok, C. Effect of corona discharge plasma jet treatment on decontamination and sprouting of rapeseed (Brassica napus L.) seeds. Food Cont. 2017, 71, 376–382. [Google Scholar]
- Ikeura, H.; Goto, T.; Tamaki, M. Effects of adding a chelator after ozone microbubble generation on nutrient composition, medium sterility, and plant growth. Water Air Soil Poll. 2018, 229, 1. [Google Scholar]
- Šerá, B.; Šerý, M. Non-thermal plasma treatment as a new biotechnology in relation to seeds, dry fruits, and grains. Plasma Sci. Technol. 2018, 20, 044012. [Google Scholar]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňaková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of cold plasma atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar]
- Zhang, S.; Rousseau, A.; Dufour, T. Promoting lentil germination and stem growth by plasma activated tap water, demineralized water and liquid fertilizer. RSC Adv. 2017, 7, 31244–31251. [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). Zoonotic Diseases. 2017. Available online: https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html (accessed on 26 August 2022).
- Hirneisen, K.A.; Sharma, M.; Kniel, K.E. Human enteric pathogen internalization by root uptake into food crops. Foodborne Pathog. Dis. 2012, 9, 396–405. [Google Scholar]
- Wright, K.M.; Chapman, S.; MGeachy, K.; Humphris, S.; Campbell, E.; Toth, I.K.; Holden, N.J. The endophylic lifestyle of Escherichia coli O156:H7: Quantification and internal localization in roots. Phytopathology 2013, 103, 333–340. [Google Scholar]
- Smith, B.N.; Harris, L.C.; McCarlie, V.W.; Stradling, D.L.; Thygerson, T.; Walker, J.; Criddle, R.S.; Hansen, L.D. Time, plant growth, respiration, and temperature. In Handbook of Plant and Crop Physiology; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Wu, W.; Ma, B.-L.; Whalen, J.K. Chapter Three-enhancing rapeseed tolerance to heat and drought stresses in a changing climate: Perspectives for stress adaptation from root system architecture. Adv. Agron. 2018, 151, 87–157. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Part 1 ¿Qué son las Legumbres? In Legumbres, Semillas Nutritivas para un Futuro Sostenible; Departamento de Comunicación Corporative de la FAO: Rome, Italy, 2016; pp. 13–25. [Google Scholar]
- Hoigné, J. The chemistry of ozone in water. In Process Technologies for Water Treatment; Springer: Boston, MA, USA, 1988; pp. 121–141. [Google Scholar]
- Langlais, B.; Reckhow, D.A.; Brink, D.R. Ozone in Water Treatment, Application and Engineering; American Water Association, Lewis Publishers: Boca Raton, FL, USA, 1991. [Google Scholar]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Amaeze, N.; Richardson, K.; Mackay, W.; Rateb, M.E.; Yaseen, M. Stabilisation of ozone in water for microbial disinfection. Environments 2022, 9, 45. [Google Scholar]
- Gao, X.; Zhang, A.; Héroux, P.; Sand, W.; Sun, Z.; Zhan, J.; Wang, C.; Hao, S.; Li, Z.; Li, Z.; et al. Effect of dielectric barrier discharge cold plasma on pea seed growth. J. Agric. Food Chem. 2019, 67, 10813–10822. [Google Scholar]
- Riley, J. Presentation of statistical analyses. Exp. Agric. 2001, 37, 115–123. [Google Scholar]
- Boyer, J.S. Biochemical and biophysical aspects of water deficits and the predisposition to disease. Annu. Rev. Phytopathol. 1995, 33, 251–274. [Google Scholar]
- McElrone, A.J.; Sherald, J.L.; Forseth, I.N. Effects of water stress on symptomatology and growth of Parthenocissus quinquefolia infected by Xylella fastidiosa. Plant Dis. 2001, 85, 1160–1164. [Google Scholar]
- Uyttendaele, M.; Jaykus, L.-A.; Amoah, P.; Chiodini, A.; Cunliffe, D.; Jacxsens, L.; Holvoet, K.; Korsten, L.; Lau, M.; McClure, P.; et al. Microbial hazards in irrigation water: Standards, norms, and testing to manage use of water in fresh produce primary production. Compr. Rev. Food Sci. Food Saf. 2015, 14, 336–356. [Google Scholar]
- Elad, Y.; Pertot, I. Climate change impacts on plant pathogens and plant diseases. J. Crop Improv. 2017, 28, 99–139. [Google Scholar]
- Šerá, B.; Scholtz, V.; Jirešová, J.; Khun, J.; Julák, J.; Šerý, M. Effects of non-thermal plasma treatment on seed germination and early growth of leguminous plants: A review. Plant 2021, 10, 1616. [Google Scholar]
- Mitra, A.; Li, Y.F.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioprocess Technol. 2014, 7, 645–653. [Google Scholar]
- Perez-Piza, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non-thermal plasmas. Sci. Rep. 2020, 10, 4917. [Google Scholar]
- Dechorgnat, J.; Nguyen, C.T.; Armengaud, P.; Jossier, M.; Diatloff, E.; Filleur, S.; Daniel-Vedele, F. From the soil to the seeds: The long journey of nitrate in plants. J. Exp. Bot. 2011, 62, 1349–1359. [Google Scholar]
- Messiga, A.J.; Nyamaizi, S.; Yu, S.; Dorais, M. Blueberry yield and soil mineral nitrogen response to nitrogen fertilizer and nitrification inhibitors under drip-fertigation systems. Agronomy 2021, 11, 2144. [Google Scholar]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sour Sci. Technol. 2014, 23, 015019. [Google Scholar]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.K.; Bazaka, K. Plasma activated water (PAW): Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar]
- Song, J.; Yang, J.; Jeong, B.R. Growth, quality, and nitrogen assimilation in response to high ammonium or nitrate supply in cabbage (Brassica campestris L.) and lettuce (Lactuca sativa L.). Agronomy 2021, 11, 2556. [Google Scholar]
- Shapira, Y.; Bormashenko, E.; Drori, E. Pre-germination plasma treatment of seeds does not alter cotyledon DNA structure, nor phenotype and phenology of tomato and pepper plants. Biochem. Biophys. Res. Commun. 2019, 519, 512–517. [Google Scholar]
- Pignata, C.; D’Angelo, D.; Fea, E.; Gilli, G. A review on microbiological decontamination of fresh produce with non-thermal plasma. J. Appl. Microbiol. 2017, 122, 1438–1455. [Google Scholar]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K.K. Effects of atmospheric-pressure N2, He, air, and O2 microplasmas on mung bean seed germination and seedling growth. Sci. Rep. 2016, 6, 32603. [Google Scholar]
- Tomekova, J.; Kyzek, S.; Medvecka, V.; Galova, E.; Zahoranova, A. Influence of cold atmospheric pressure plasma on pea seeds: DNA damage of seedlings and optical diagnostics of plasma. Plasma Chem. Plasma Proc. 2020, 40, 1571–1584. [Google Scholar]
- Ali, S.; Sethy, B.K.; Parandiyal, A.K.; Kumar, A.; Singh, R.K.; Somasundaram, J.; Mina, B.L. Long-term effects of rainwater conservation measure on improving yield, runoff use efficiency and soil properties of horti-pastoral system on the degraded ravine lands of India. Agric. Water Manag. 2020, 233, 106068. [Google Scholar]
- Mouli, P.C.; Mohan, S.V.; Reddy, S.J. Rainwater chemistry at a regional representative urban site: Influence of terrestrial sources on ionic composition. Atmosph. Environ. 2005, 39, 999–1008. [Google Scholar]
- Hazmi, A.; Rosadi, M.Y.; Desmiarti, R.; Li, F. Effect of radio-frequency treatment on the changes of dissolved organic matter in rainwater. Water 2022, 14, 111. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Nitrate and Nitrite; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2017. [Google Scholar]
- He, Y.; Sang, W.; Lu, W.; Zhang, W.; Zhan, C.; Jia, D. Recent advances of emerging organic pollutants degradation in environment by non-thermal plasma technology: A Review. Water 2022, 14, 1351. [Google Scholar]
- Xing, Y.; Zhang, W.; Su, W.; Zhang, H.; Wang, J.; Zhang, H.; Guo, Z.; Jia, H. The bibliometric analysis and review of the application of plasma in the field of VOCs. Catalysts 2022, 12, 173. [Google Scholar]
- Rodríguez-Méndez, B.G.; Hernández-Arias, A.N.; Gutiérrez-León, D.G.; López-Callejas, R.; Mercado-Cabrera, A.; Jaramillo-Sierra, B.; Peña-Eguiluz, R.; Valencia-Alvarado, R.; Alcántara-Díaz, D. Effect of voltage and oxygen on inactivation of E. coli and S. Typhi using pulsed dielectric barrier discharge. Bioelectrochemistry 2021, 141, 107879. [Google Scholar]
- Jiang, Y.; Sokorai, K.; Pyrgiotakis, G.; Demokritou, P.; Li, X.; Mukhopadhyay, S.; Jinb, T.; Fan, X. Cold plasma-activated hydrogen peroxide aerosol inactivates Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria innocua and maintains quality of grape tomato, spinach and cantaloupe. Int. J. Food Microbiol. 2017, 249, 53–60. [Google Scholar]
- Svubova, R.; Kyzek, S.; Medvecka, V.; Slovakova, L.; Galova, E.; Zahoranova, A. Novel insight at the effect of cold atmospheric pressure plasma on the activity of enzymes essential for the germination of Pea (Pisum sativum L. cv. Prophet) seeds. Plasma Chem. Plasma Process. 2020, 40, 1221–1240. [Google Scholar]
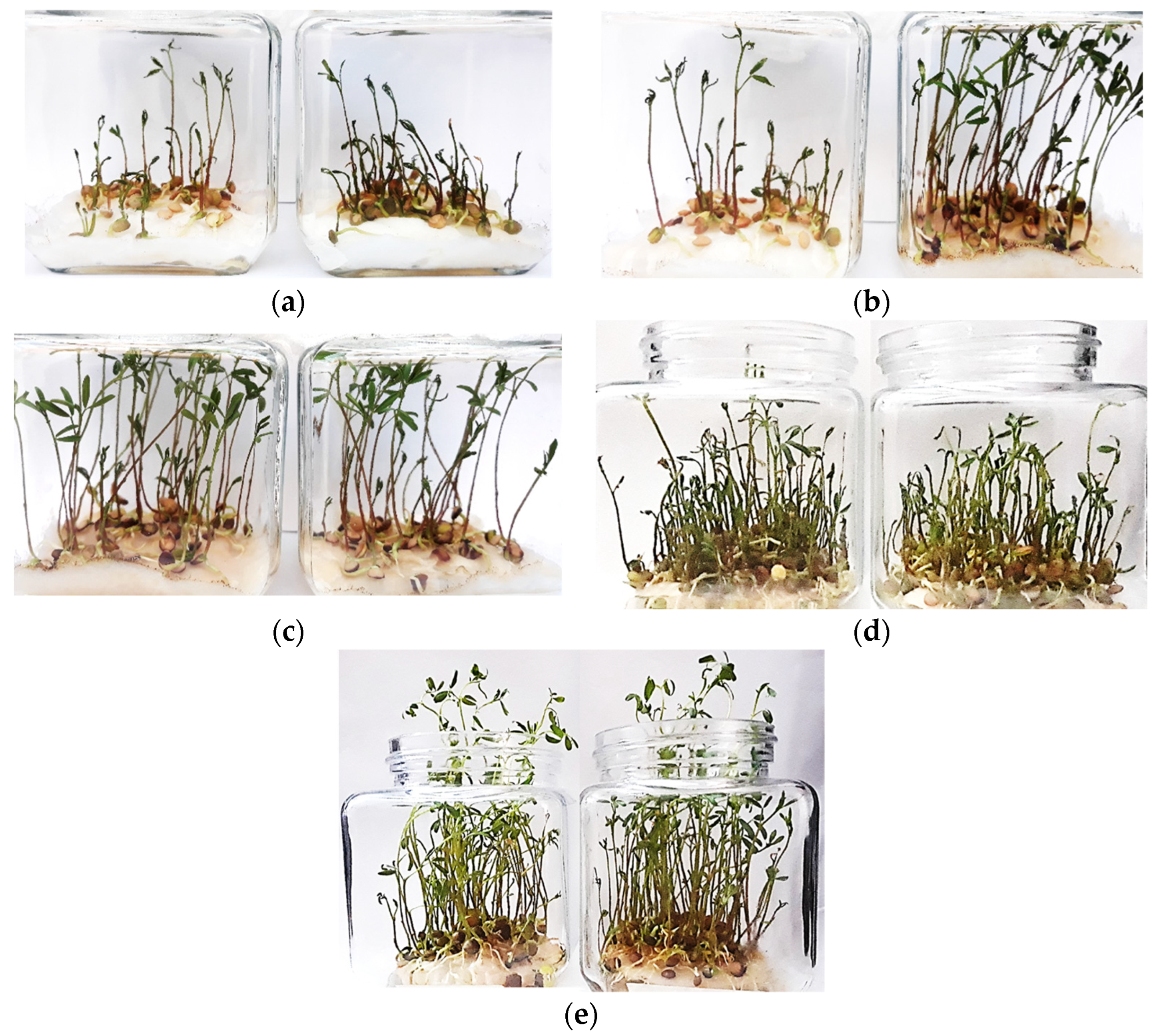

| Experimental Test | Mean Value Length of the Root [mm] |
|---|---|
| PW | 33.96 with C.I. (32.43, 35.50) |
| ANTP-PW | 49.06 with C.I. (46.19, 51.93) |
| DNTP-PW | 59.39 with C.I. (55.50, 62.73) |
| FW | 43.37 with C.I. (41.40, 45.34) |
| ANTP-FW | 62.59 with C.I. (59.99, 65.19) |
| DNTP-FW | 52.28 with C.I. (50.34, 54.22) |
| RW | 65.12 with C.I. (62.48, 67.77) |
| ANTP-RW | 42.80 with C.I. (39.90, 45.69) |
| Experimental Test | Mean Value Thickness of the Root [mm] |
|---|---|
| PW | 1.00 with C.I. (0.92, 1.08) |
| ANTP-PW | 1.38 with C.I. (1.23, 1.53) |
| DNTP-PW | 1.43 with C.I. (1.32, 1.55) |
| FW | 1.02 with C.I. (0.95, 1.08) |
| ANTP-FW | 1.43 with C.I. (1.16, 1.71) |
| DNTP-FW | 1.03 with C.I. (0.91, 1.15) |
| RW | 1.41 with C.I. (1.29, 1.52) |
| ANTP-RW | 1.42 with C.I. (1.28, 1.55) |
| Experimental Test | Mean Value Height of the Stem [mm] |
|---|---|
| PW | 31.83 with C.I. (29.96, 33.70) |
| ANTP-PW | 49.08 with C.I. (46.34, 51.81) |
| DNTP-PW | 39.03 with C.I. (35.53, 42.53) |
| FW | 88.68 with C.I. (86.10, 91.27) |
| ANTP-FW | 102.40 with C.I. (98.65, 106.16) |
| DNTP-FW | 100.64 with C.I. (97.96, 103.31) |
| RW | 45.87 with C.I. (43.64, 48.09) |
| ANTP-RW | 63.75 with C.I. (60.17, 67.34) |
| Experimental Test | Mean Value Thickness of the Stem [mm] |
|---|---|
| PW | 0.95 with C.I. (0.89, 1.01) |
| ANTP-PW | 1.07 with C.I. (0.94, 1.20) |
| DNTP-PW | 0.99 with C.I. (0.88, 1.09) |
| FW | 0.95 with C.I. (0.87, 1.03) |
| ANTP-FW | 0.98 with C.I. (0.85, 1.11) |
| DNTP-FW | 0.96 with C.I. (0.89, 1.04) |
| RW | 1.00 with C.I. (0.95, 1.06) |
| ANTP-RW | 1.03 with C.I. (0.94, 1.12) |
| Experimental Test | Mean Value Number of Leaves |
|---|---|
| PW | 0.67 with C.I. (0.22, 1.12) |
| ANTP-PW | 5.00 with C.I. (3.76, 6.24) |
| DNTP-PW | 4.55 with C.I. (4.08, 5.01) |
| FW | 7.50 with C.I. (6.22, 8.78) |
| ANTP-FW | 9.45 with C.I. (7.32, 11.59) |
| DNTP-FW | 6.17 with C.I. (5.12, 7.18) |
| RW | 5.25 with C.I. (4.26, 6.24) |
| ANTP-RW | 3.67 with C.I. (2.89, 4.44) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-León, D.G.; Serrano-Ramírez, T.; López-Callejas, R.; Rodríguez-Méndez, B.G. Non-Thermal Plasma as an Alternative to Enhance the Early Growth Structures in Lentil Plants. Agronomy 2022, 12, 2920. https://doi.org/10.3390/agronomy12122920
Gutiérrez-León DG, Serrano-Ramírez T, López-Callejas R, Rodríguez-Méndez BG. Non-Thermal Plasma as an Alternative to Enhance the Early Growth Structures in Lentil Plants. Agronomy. 2022; 12(12):2920. https://doi.org/10.3390/agronomy12122920
Chicago/Turabian StyleGutiérrez-León, Diana Guadalupe, Tomás Serrano-Ramírez, Régulo López-Callejas, and Benjamín Gonzalo Rodríguez-Méndez. 2022. "Non-Thermal Plasma as an Alternative to Enhance the Early Growth Structures in Lentil Plants" Agronomy 12, no. 12: 2920. https://doi.org/10.3390/agronomy12122920
APA StyleGutiérrez-León, D. G., Serrano-Ramírez, T., López-Callejas, R., & Rodríguez-Méndez, B. G. (2022). Non-Thermal Plasma as an Alternative to Enhance the Early Growth Structures in Lentil Plants. Agronomy, 12(12), 2920. https://doi.org/10.3390/agronomy12122920






