Nitrogen Application Alleviates the Adverse Effects of Defoliation Stress on Lolium perenne L. by Enhancing the Antioxidant System and Promoting Photosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture
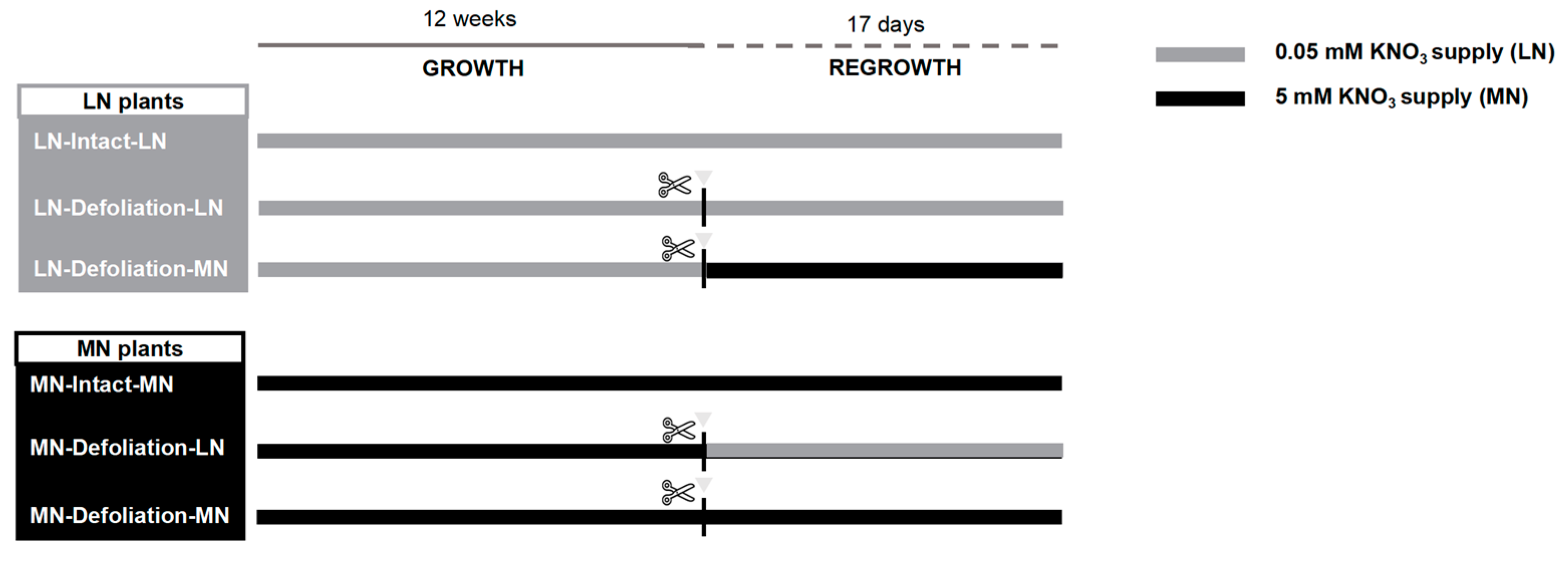
2.2. N and Defoliation Treatment
2.3. Plant Regrowth, Biomass, Nitrate Uptake Rate and Nitrogen Contents
2.4. Photosynthetic Pigments and Anthocyanin Content Measurement
2.5. Gas Exchange Measurement
2.6. Chlorophyll Fluorescence Measurement Analysis
2.7. Assays of Antioxidant Enzyme Activity and Soluble Protein
2.8. Determination of Soluble Sugar Content, Proline Content and Lipid Peroxidation
2.9. RNA Isolation and Quantitative RT-PCR
2.10. Statistical Analysis
3. Results
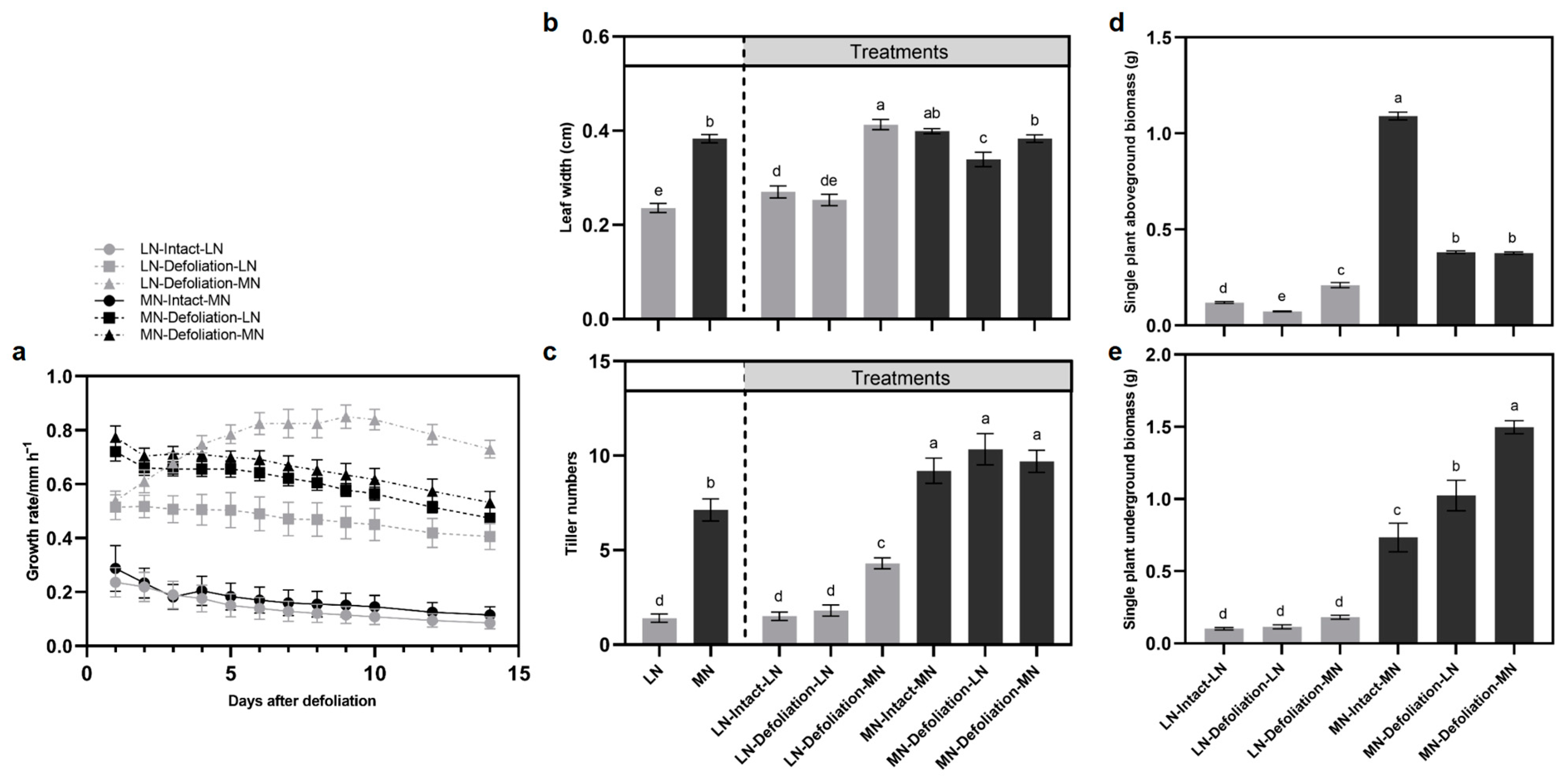
3.1. Comparison of Plant Regrowth under LN and MN Treatments
3.2. Effect of Defoliation and Nitrogen Treatments on Photosynthetic Pigments and Gas Exchange Parameters
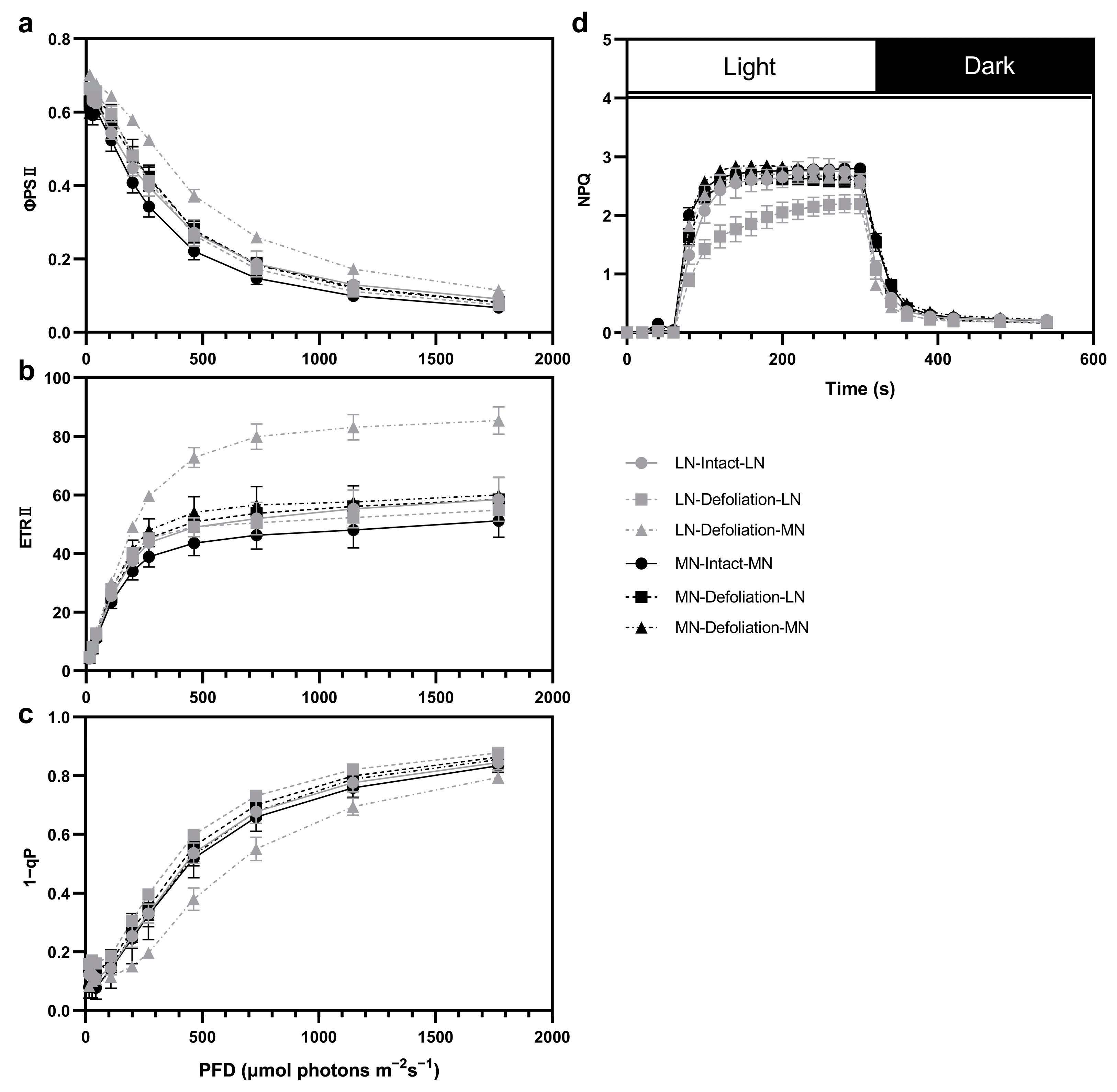
3.3. Chlorophyll Fluorescence Response under Defoliation and Nitrogen Treatments
3.4. Plant Antioxidant Responses under Defoliation and Nitrogen Treatments
3.5. Changes in Anthocyanin Content under Defoliation and Nitrogen Treatments
3.6. Response of Lipid Peroxidation and Proline Concentration to Defoliation and Differential N Supply
3.7. Effect of Defoliation and Nitrogen Treatments on the Content of Soluble Sugar and Protein in Ryegrass
3.8. Nitrate Uptake Rate and NRT Transcript Levels
4. Discussion
4.1. Nitrate Supply Significantly Affects the Establishment of Ryegrass Leaf Photosynthesis after Defoliation
4.2. Moderate Nitrate Supply Alleviates Defoliation Stress by Enhancing Antioxidant Defense System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Humphreys, M.; Feuerstein, U.; Vandewalle, M.; Baert, J. Ryegrasses. In Handbook of Plant Breeding; Beat Boller, B., Posselt, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 5, pp. 211–260. [Google Scholar] [CrossRef]
- Lestienne, F.; Thornton, B.; Gastal, F. Impact of defoliation intensity and frequency on N uptake and mobilization in Lolium perenne. J. Exp. Bot. 2006, 57, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Briske, D.; Richards, J. Physiology of plants recovering from defoliation. In Proceedings of the XVII International Grassland Congress, Palmerston North, New Zealand, 8–21 February 1993; SIR Publishing: Auckland, New Zealand, 1993; pp. 85–94. [Google Scholar]
- Zhou, R.L.; Zhao, H.L. Seasonal pattern of antioxidant enzyme system in the roots of perennial forage grasses grown in alpine habitat, related to freezing tolerance. Physiol. Plant. 2004, 121, 399–408. [Google Scholar] [CrossRef]
- Lee, J.; Puthigae, S.; Donaghy, D.; Roche, J. Impact of defoliation severity on photosynthesis, carbon metabolism and transport gene expression in perennial ryegrass. Funct. Plant Biol. 2011, 38, 808–817. [Google Scholar] [CrossRef]
- Guo, Q.; Turnbull, M.H.; Song, J.; Roche, J.; Novak, O.; Spath, J.; Jameson, P.E.; Love, J. Depletion of carbohydrate reserves limits nitrate uptake during early regrowth in Lolium perenne L. J. Exp. Bot. 2017, 68, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Turnbull, M.H.; Guo, Q.; Novak, O.; Spath, J.; Gieseg, S.P.; Jameson, P.E.; Love, J. Coordinated nitrogen and carbon remobilization for nitrate assimilation in leaf, sheath and root and associated cytokinin signals during early regrowth of Lolium perenne. Ann. Bot. 2017, 119, 1353–1364. [Google Scholar] [CrossRef]
- Morvan-Bertrand, A.; Boucaud, J.; Le Saos, J.; Prud’homme, M.P. Roles of the fructans from leaf sheaths and from the elongating leaf bases in the regrowth following defoliation of Lolium perenne L. Planta 2001, 213, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, P.; Press, C. Handbook of Plant and Crop Physiology. Phytochemistry 2002, 63, 631. [Google Scholar]
- Kim, T.-H.; Jung, W.-J.; Lee, B.-R.; Yoneyama, T.; Kim, H.-Y.; Kim, K.-Y. P effects on N uptake and remobilization during regrowth of Italian ryegrass (Lolium multiflorum). Environ. Exp. Bot. 2003, 50, 233–242. [Google Scholar] [CrossRef]
- Peng, J.; Feng, Y.; Wang, X.; Li, J.; Xu, G.; Phonenasay, S.; Luo, Q.; Han, Z.; Lu, W. Effects of nitrogen application rate on the photosynthetic pigment, leaf fluorescence characteristics, and yield of indica hybrid rice and their interrelations. Sci. Rep. 2021, 11, 7485. [Google Scholar] [CrossRef]
- Lawlor, D.W. Carbon and nitrogen assimilation in relation to yield: Mechanisms are the key to understanding production systems. J. Exp. Bot. 2002, 53, 773–787. [Google Scholar] [CrossRef]
- Allison, J.; Williams, H.; Pammenter, N. Effect of specific leaf nitrogen on photosynthesis in sugarcane. Ann. Appl. Biol. 1997, 131, 339–350. [Google Scholar] [CrossRef]
- Vos, J.; van der Putten, P.E.L.; Birch, C.J. Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). Field Crops Res. 2005, 93, 64–73. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Zhang, C.K.; Camberato, J.J.; Jiang, Y.W. Nitrogen and carbon contents, nitrogen use efficiency, and antioxidant responses of perennial ryegrass accessions to nitrogen deficiency. J. Plant Nutr. 2019, 42, 2092–2101. [Google Scholar] [CrossRef]
- Lv, R.; El-Sabagh, M.; Obitsu, T.; Sugino, T.; Kurokawa, Y.; Kawamura, K. Effects of nitrogen fertilizer and harvesting stage on photosynthetic pigments and phytol contents of Italian ryegrass silage. Anim. Sci. J. 2017, 88, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.; He, Y. Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of citrus huanglongbing. Front. Plant Sci. 2017, 8, 1509. [Google Scholar] [CrossRef]
- Humplik, J.F.; Lazar, D.; Fuerst, T.; Husickova, A.; Hybl, M.; Spichal, L. Automated integrative high-throughput phenotyping of plant shoots: A case study of the cold-tolerance of pea (Pisum sativum L.). Plant Methods 2015, 11, 20. [Google Scholar] [CrossRef]
- Tantray, A.Y.; Bashir, S.S.; Ahmad, A. Low nitrogen stress regulates chlorophyll fluorescence in coordination with photosynthesis and Rubisco efficiency of rice. Physiol. Mol. Biol. Plants 2020, 26, 83–94. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Ervin, E. Antioxidative responses in roots and shoots of creeping bentgrass under high temperature: Effects of nitrogen and cytokinin. J. Plant Physiol. 2012, 169, 492–500. [Google Scholar] [CrossRef]
- Ibrahim, M.E.H.; Zhu, X.; Zhou, G.; Ali, A.Y.A.; Ahmad, I.; Farah, G.A. Nitrogen fertilizer alleviated negative impacts of NaCl on some physiological parameters of wheat. Pak. J. Bot. 2018, 50, 2097–2104. [Google Scholar]
- Ibrahim, M.E.H.; Zhu, X.; Zhou, G.; Ali, A.Y.A.; Ahmad, I.; Elsiddig, A.M.I.; Zhu, G.; Nimir, N.E.A. Promoting salt tolerance in wheat seedlings by application of nitrogen fertilizer. Pak. J. Bot. 2019, 51, 1995–2002. [Google Scholar] [CrossRef]
- Kirova, E. Effect of nitrogen nutrition source on antioxidant defense system of soybean plants subjected to salt stress. Comptes Acad. Bulg. Sci. 2020, 73, 211–219. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Elsiddig, A.M.I.; Jiao, X.; Zhu, G.; Salih, E.G.I.; Suliman, M.S.E.S.; Elradi, S.B.M. Gibberellic acid and nitrogen efficiently protect early seedlings growth stage from salt stress damage in Sorghum. Sci. Rep. 2021, 11, 6672. [Google Scholar] [CrossRef]
- Costa Cunha de Souza, E.A.; Alvarez-Pizarro, J.C.; Lopes, L.d.S.; Miranda, R.d.S.; Gomes-Filho, E. Nitrate and ammonium nutrition modulates the photosynthetic performance and antioxidant defense in salt-stressed grass species. J. Soil Sci. Plant Nutr. 2021, 21, 3016–3029. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, Z.; Xu, Y.; Lu, H.; Ji, Z.; Zhou, G. Different types of fertilizers enhanced salt resistance of oat and associated physiological mechanisms in saline soils. Agronomy 2022, 12, 317. [Google Scholar] [CrossRef]
- Sikder, R.K.; Wang, X.; Zhang, H.; Gui, H.; Dong, Q.; Jin, D.; Song, M. Nitrogen Enhances Salt Tolerance by Modulating the Antioxidant Defense System and Osmoregulation Substance Content in Gossypium hirsutum. Plants 2020, 9, 450. [Google Scholar] [CrossRef]
- Lichtenthaler, H. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148C, 350–382. [Google Scholar] [CrossRef]
- Rabino, I.; Mancinelli, A.L. Light, temperature, and anthocyanin production. Plant Physiol. 1986, 81, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Hu, L.; Li, H.; Pang, H.; Fu, J. Responses of antioxidant gene, protein and enzymes to salinity stress in two genotypes of perennial ryegrass (Lolium perenne) differing in salt tolerance. J. Plant Physiol. 2012, 169, 146–156. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Enzymatic responses of the ascorbate-glutathione cycle to drought in sorghum and sunflower plants. Plant Sci. 1996, 113, 139–147. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ebell, L.F. Variation in total soluble sugars of conifer tissues with method of analysis. Phytochemistry 1969, 8, 227–233. [Google Scholar] [CrossRef]
- Du, H.; You, Y.; Xiong, L.Z. Measurment of proline content in rice tissue. Bio-Protocol 2018, 8, e1010146. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S. Microsomal lipid peroxidation. Method Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Hou, W.F.; Yan, J.Y.; Jakli, B.; Lu, J.W.; Ren, T.; Cong, R.H.; Li, X.K. Synergistic effects of nitrogen and potassium on quantitative limitations to photosynthesis in Rice (Oryza sativa L.). J. Agric. Food Chem. 2018, 66, 5125–5132. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, X.; Ge, Q.; Li, B.; Tong, Y.; Zhang, A.; Li, Z.; Kuang, T.; Lu, C. Tolerance of photosynthesis to photoinhibition, high temperature and drought stress in flag leaves of wheat: A comparison between a hybridization line and its parents grown under field conditions. Plant Sci. Int. J. Exp. Plant Biol. 2006, 171, 389–397. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef]
- Zhang, X.; Shangguan, Z. Effect of nitrogen fertilization on photosynthetic pigment and fluorescence characteristics in leaves of winter wheat cultivars on dryland. J. Agric. Sci. 2007, 21, 299. [Google Scholar] [CrossRef]
- Zhao, L.-S.; Li, K.; Wang, Q.-M.; Song, X.-Y.; Su, H.-N.; Xie, B.-B.; Zhang, X.-Y.; Huang, F.; Chen, X.-L.; Zhou, B.-C.; et al. Nitrogen starvation impacts the photosynthetic performance of porphyridium cruentum as revealed by chlorophyll a fluorescence. Sci. Rep. 2017, 7, 8542. [Google Scholar] [CrossRef]
- Kursar, T.A.; Alberte, R.S. Photosynthetic unit organization in a red alga: Relationships between light-harvesting pigments and reaction centers. Plant Physiol. 1983, 72, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yang, G.; Tan, C.; Zhao, C. Effects of nitrogen stress on the photosynthetic CO2 assimilation, chlorophyll fluorescence, and sugar-nitrogen ratio in corn. Sci. Rep. 2015, 5, 9311. [Google Scholar] [CrossRef]
- Liu, T.; Ren, T.; White, P.J.; Cong, R.; Lu, J. Storage nitrogen co-ordinates leaf expansion and photosynthetic capacity in winter oilseed rape. J. Exp. Bot. 2018, 69, 2995–3007. [Google Scholar] [CrossRef]
- Shen, H.; Dong, S.; Li, S.; Wang, W.; Xiao, J.; Yang, M.; Zhang, J.; Gao, X.; Xu, Y.; Zhi, Y.; et al. Effects of warming and N deposition on the physiological performances of Leymus secalinus in Alpine Meadow of Qinghai-Tibetan Plateau. Front. Plant Sci. 2019, 10, 1804. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Li, L.L.; Xie, J.H.; Fudjoe, S.K.; Zhang, R.Z.; Luo, Z.Z.; Anwar, S. Nitrogen supply affects grain yield by regulating antioxidant enzyme activity and photosynthetic capacity of maize plant in the Loess Plateau. Agronomy 2021, 11, 1094. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 00600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, Q. Growth and photosynthetic responses of two coniferous species to experimental warming and nitrogen fertilization. Can. J. For. Res. 2009, 39, 1–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, E.; Wang, C.; Yun, N. Effect of different water and nitrogen levels on chlorophyll fluorescence parameters and photosynthetic characteristics of rice. Nongye Jixie Xuebao/Trans. Chin. Soc. Agric. Machin. 2017, 48, 176–183. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. An RNA-Seq analysis of the response of photosynthetic system to low nitrogen supply in maize leaf. Int. J. Mol. Sci. 2017, 18, 2624. [Google Scholar] [CrossRef]
- Song, H.; Xu, X.; Wang, H.; Wang, H.; Tao, Y. Exogenous gamma-aminobutyric acid alleviates oxidative damage caused by aluminium and proton stresses on barley seedlings. J. Sci. Food Agric. 2010, 90, 1410–1416. [Google Scholar] [CrossRef]
- Kornyeyev, D.; Logan, B.A.; Allen, R.D.; Holaday, A.S. Effect of chloroplastic overproduction of ascorbate peroxidase on photosynthesis and photoprotection in cotton leaves subjected to low temperature photoinhibition. Plant Sci. 2003, 165, 1033–1041. [Google Scholar] [CrossRef]
- Zakari, S.A.; Asad, M.-A.-U.; Han, Z.; Zhao, Q.; Cheng, F. Relationship of nitrogen deficiency-induced leaf senescence with ROS generation and ABA concentration in rice flag leaves. J. Plant Growth Regul. 2020, 39, 1503–1517. [Google Scholar] [CrossRef]
- Foyer, C.; Lelandais, M.; Kunert, K. Photooxidative stress in plants. Physiol. Plant. 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus, and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Llerena, J.L.; Valdes, M.E.; Mancha, L.A.; Uriarte, D.; Prieto, M.D.; Espinosa, F. Effects of defoliation and water restriction on total phenols and antioxidant activities in grapes during ripening. J. Int. Sci. Vigne Vin. 2014, 48, 31–42. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef]
- Scheible, W.R.; Morcuende, R.; Czechowski, T.; Fritz, C.; Osuna, D.; Palacios-Rojas, N.; Schindelasch, D.; Thimm, O.; Udvardi, M.K.; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 2004, 136, 2483–2499. [Google Scholar] [CrossRef] [PubMed]



| Time | Treatments | Pn (µmol CO2 m−2·s−1) | Gs (mol H2O m−2·s−1) | Ci (µmol CO2 mol−1) | Tr (mmol H2O m−2·s−1) |
|---|---|---|---|---|---|
| 6 Days | LN-Intact-LN | 8.593 ± 1.032 e | 0.082 ± 0.008 c | 383.512 ± 14.214 b | 2.535 ± 0.205 c |
| LN-Defoliation-LN | 15.581 ± 1.042 d | 0.119 ± 0.015 ab | 467.214 ± 6.361 a | 3.245 ± 0.391 bc | |
| LN-Defoliation-MN | 29.605 ± 0.449 a | 0.179 ± 0.013 a | 373.478 ± 8.062 b | 4.683 ± 0.305 a | |
| MN-Intact-MN | 19.376 ± 0.690 c | 0.109 ± 0.014 c | 297.427 ± 24.895 d | 2.892 ± 0.351 bc | |
| MN-Defoliation-LN | 20.558 ± 0.872 c | 0.121 ± 0.009 bc | 354.892 ± 16.270 bc | 3.542 ± 0.445 b | |
| MN-Defoliation-MN | 23.364 ± 0.354 b | 0.140 ± 0.009 bc | 309.176 ± 32.182 cd | 3.767 ± 0.241 ab | |
| 10 Days | LN-Intact-LN | 10.553 ± 0.464 e | 0.112 ± 0.005 b | 382.759 ± 9.638 a | 2.473 ± 0.139 d |
| LN-Defoliation-LN | 14.173 ± 0.328 d | 0.120 ± 0.010 b | 324.357 ± 18.568 b | 2.671 ± 0.222 cd | |
| LN-Defoliation-MN | 27.174 ± 1.730 a | 0.180 ± 0.010 a | 301.904 ± 21.455 bc | 3.980 ± 0.198 a | |
| MN-Intact-MN | 20.137 ± 0.967 bc | 0.155 ± 0.022 ab | 311.940 ± 3.798 bc | 2.433 ± 0.120 d | |
| MN-Defoliation-LN | 18.896 ± 1.268 c | 0.147 ± 0.009 ab | 303.040 ± 5.690 bc | 2.931 ± 0.122 c | |
| MN-Defoliation-MN | 21.411 ± 0.453 b | 0.160 ± 0.003 a | 280.452 ± 7.561 c | 3.437 ± 0.093 b |
| Time | Treatments | Anthocyanin (µg 100 mg−1 FW) | Soluble Sugar (mg g−1 FW) | ||
|---|---|---|---|---|---|
| Stubble | Leaf | Stubble | Leaf | ||
| 5 Days | LN-Intact-LN | 0.443 ± 0.018 b | 0.598 ± 0.033 a | 116.554 ± 10.637 a | 106.417 ± 6.086 a |
| LN-Defoliation-LN | 0.552 ± 0.048 a | 0.327 ± 0.027 b | 43.443 ± 5.554 b | 29.299 ± 1.483 c | |
| LN-Defoliation-MN | 0.251 ± 0.048 d | 0.142 ± 0.021 c | 20.060 ± 2.809 c | 6.253 ± 0.460 d | |
| MN-Intact-MN | 0.296 ± 0.020 cd | 0.303 ± 0.038 b | 110.861 ± 3.534 a | 87.136 ± 7.238 b | |
| MN-Defoliation-LN | 0.386 ± 0.024 bc | 0.073 ± 0.008 cd | 40.908 ± 5.279 b | 12.938 ± 2.700 d | |
| MN-Defoliation-MN | 0.319 ± 0.009 cd | 0.041 ± 0.010 d | 36.851 ± 3.139 bc | 8.822 ± 1.635 d | |
| 10 Days | LN-Intact-LN | 0.682 ± 0.028 b | 0.532 ± 0.091 a | 103.230 ± 12.356 a | 77.340 ± 3.725 b |
| LN-Defoliation-LN | 0.957 ± 0.114 a | 0.362 ± 0.053 bc | 61.951 ± 6.470 b | 29.337 ± 2.549 c | |
| LN-Defoliation-MN | 0.436 ± 0.081 c | 0.148 ± 0.018 d | 24.565 ± 3.502 c | 14.254 ± 0.490 e | |
| MN-Intact-MN | 0.527 ± 0.056 bc | 0.384 ± 0.016 b | 109.709 ± 10.492 a | 94.564 ± 2.613 a | |
| MN-Defoliation-LN | 0.976 ± 0.042 a | 0.233 ± 0.007 cd | 48.273 ± 4.655 bc | 25.396 ± 1.884 cd | |
| MN-Defoliation-MN | 0.713 ± 0.079 b | 0.122 ± 0.031 d | 43.326 ± 4.146 bc | 21.544 ± 0.818 d | |
| Treatments | MDA (nmol g−1 FW) | Proline (µg g−1 FW) | Soluble Protein (mg g−1 FW) | |
|---|---|---|---|---|
| Stubble | Stubble | Stubble | Leaf | |
| LN-Intact-LN | 23.960 ± 1.351 a | 23.241 ± 1.155 c | 1.515 ± 0.122 b | 3.344 ± 0.218 c |
| LN-Defoliation-LN | 23.603 ± 2.613 a | 26.186 ± 2.143 c | 1.404 ± 0.197 b | 3.721 ± 0.311 c |
| LN-Defoliation-MN | 14.785 ± 1.081 b | 161.491 ± 12.601 a | 4.283 ± 0.424 a | 11.442 ± 0.689 a |
| MN-Intact-MN | 17.765 ± 2.985 ab | 46.084 ± 7.996 b | 1.841 ± 0.111 b | 4.017 ± 0.343 c |
| MN-Defoliation-LN | 18.755 ± 4.897 ab | 11.308 ± 0.827 c | 1.313 ± 0.132 b | 3.330 ± 0.183 c |
| MN-Defoliation-MN | 13.683 ± 1.408 b | 21.048 ± 2.928 c | 1.925 ± 0.103 b | 6.045 ± 0.274 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, H.; Yin, S.; Wang, T.; Xiong, X.; Shi, M.; Guo, Q. Nitrogen Application Alleviates the Adverse Effects of Defoliation Stress on Lolium perenne L. by Enhancing the Antioxidant System and Promoting Photosynthesis. Agronomy 2022, 12, 2902. https://doi.org/10.3390/agronomy12112902
Zuo H, Yin S, Wang T, Xiong X, Shi M, Guo Q. Nitrogen Application Alleviates the Adverse Effects of Defoliation Stress on Lolium perenne L. by Enhancing the Antioxidant System and Promoting Photosynthesis. Agronomy. 2022; 12(11):2902. https://doi.org/10.3390/agronomy12112902
Chicago/Turabian StyleZuo, Hui, Shuxia Yin, Tiemei Wang, Xinyue Xiong, Mengtong Shi, and Qianqian Guo. 2022. "Nitrogen Application Alleviates the Adverse Effects of Defoliation Stress on Lolium perenne L. by Enhancing the Antioxidant System and Promoting Photosynthesis" Agronomy 12, no. 11: 2902. https://doi.org/10.3390/agronomy12112902
APA StyleZuo, H., Yin, S., Wang, T., Xiong, X., Shi, M., & Guo, Q. (2022). Nitrogen Application Alleviates the Adverse Effects of Defoliation Stress on Lolium perenne L. by Enhancing the Antioxidant System and Promoting Photosynthesis. Agronomy, 12(11), 2902. https://doi.org/10.3390/agronomy12112902






