Effects of Plant Growth Regulators and Nitrogen Management on Root Lodging Resistance and Grain Yield under High-Density Maize Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Equipment and Methodology
2.3.1. Stalk Traits
2.3.2. Photosynthetic Characters of Ear Leaf
2.3.3. Root Morphological Traits, Root Dry Weight, and Root Activity
2.3.4. The Bleeding Sap Rate and Endogenous Hormones Flow
2.3.5. Root Lodging Rate and Grain Yield
2.4. Statistical Methods
3. Results
3.1. Agronomic Characteristics of Maize Stalk
3.2. Photosynthetic Characteristics of Ear Leaves
3.3. The Root Characteristics of Maize
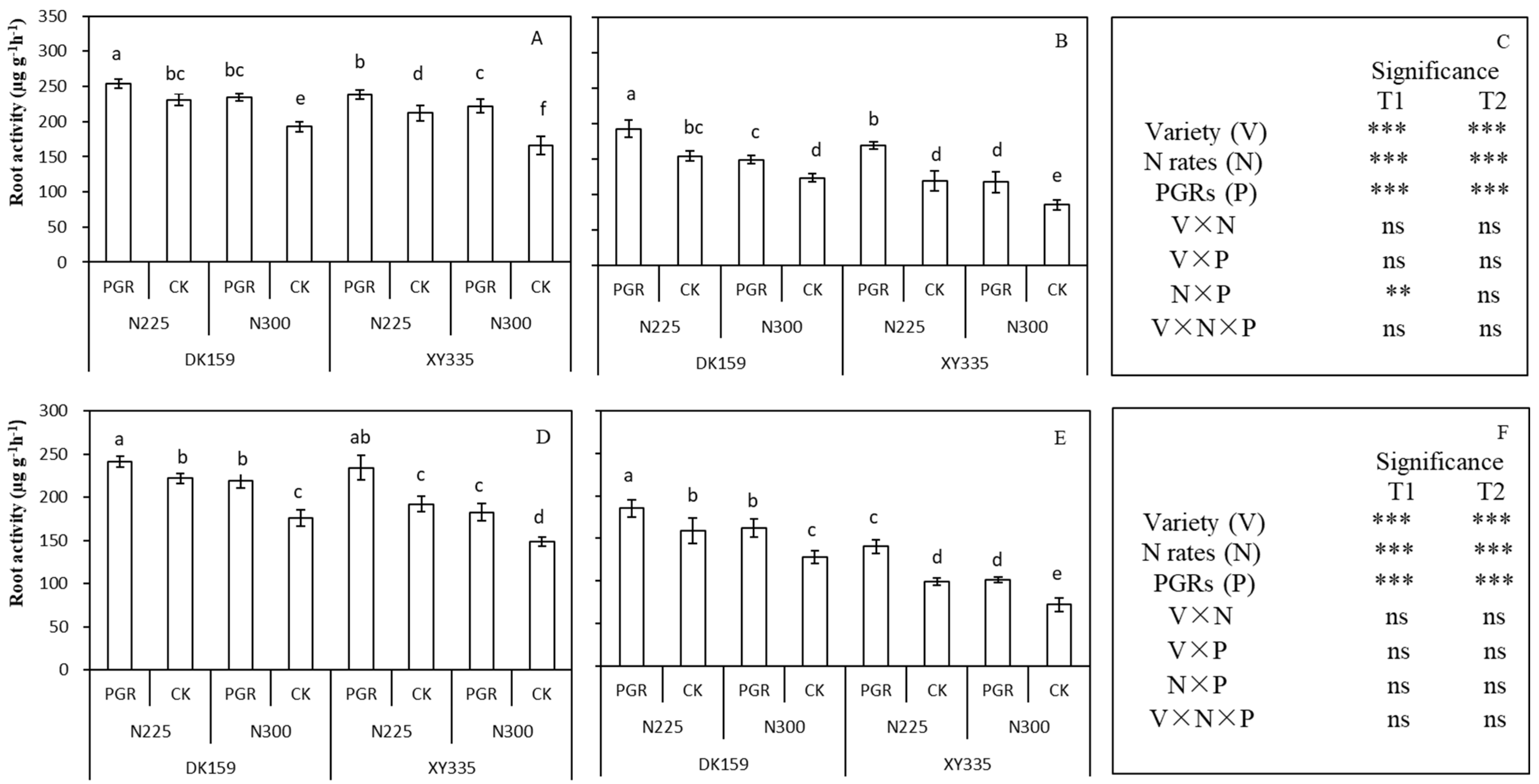
3.4. The Root Activity of Maize
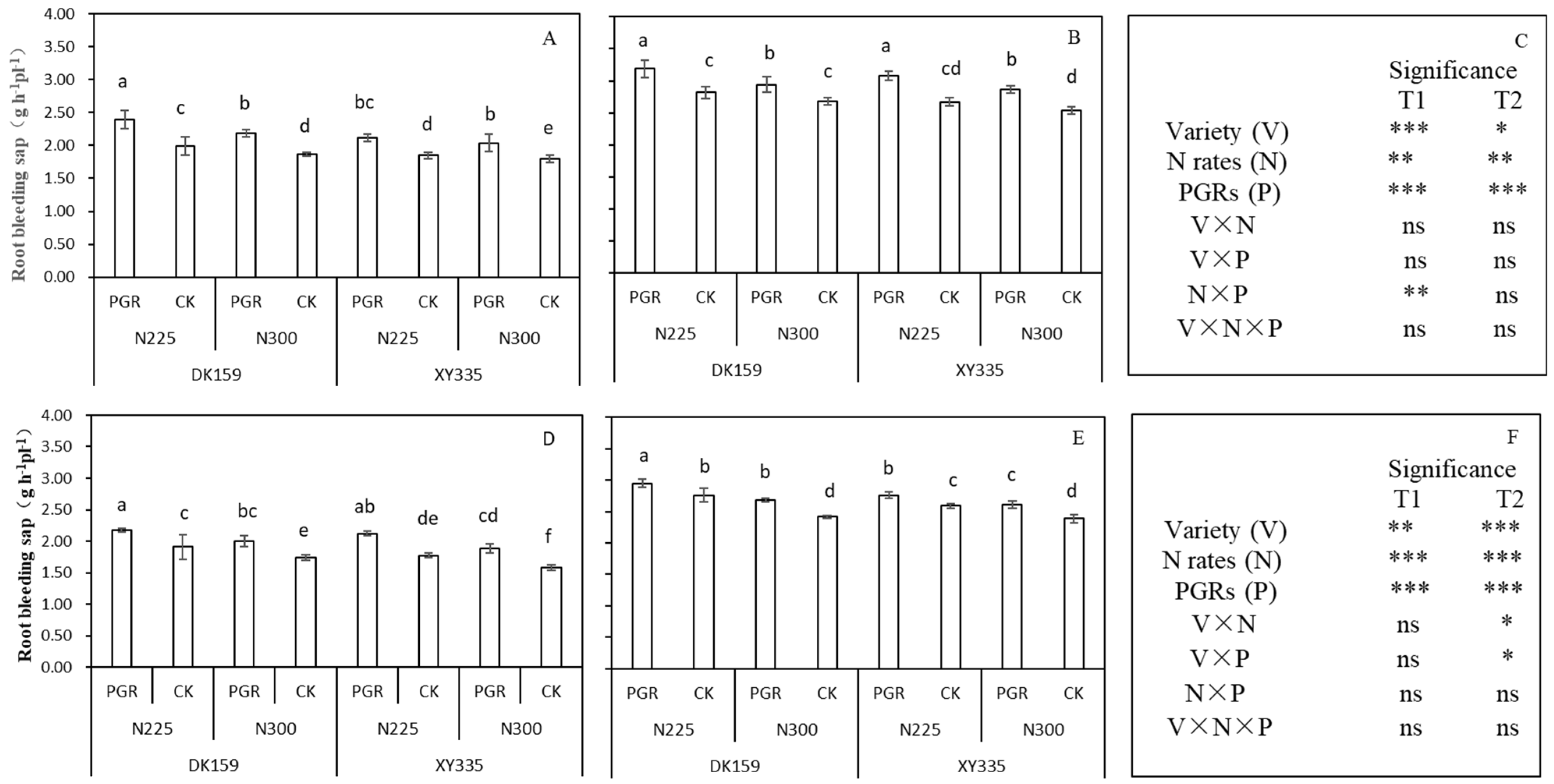
3.5. The Root Bleeding Sap
3.6. The Endogenous Hormones of Maize Root Bleeding Sap
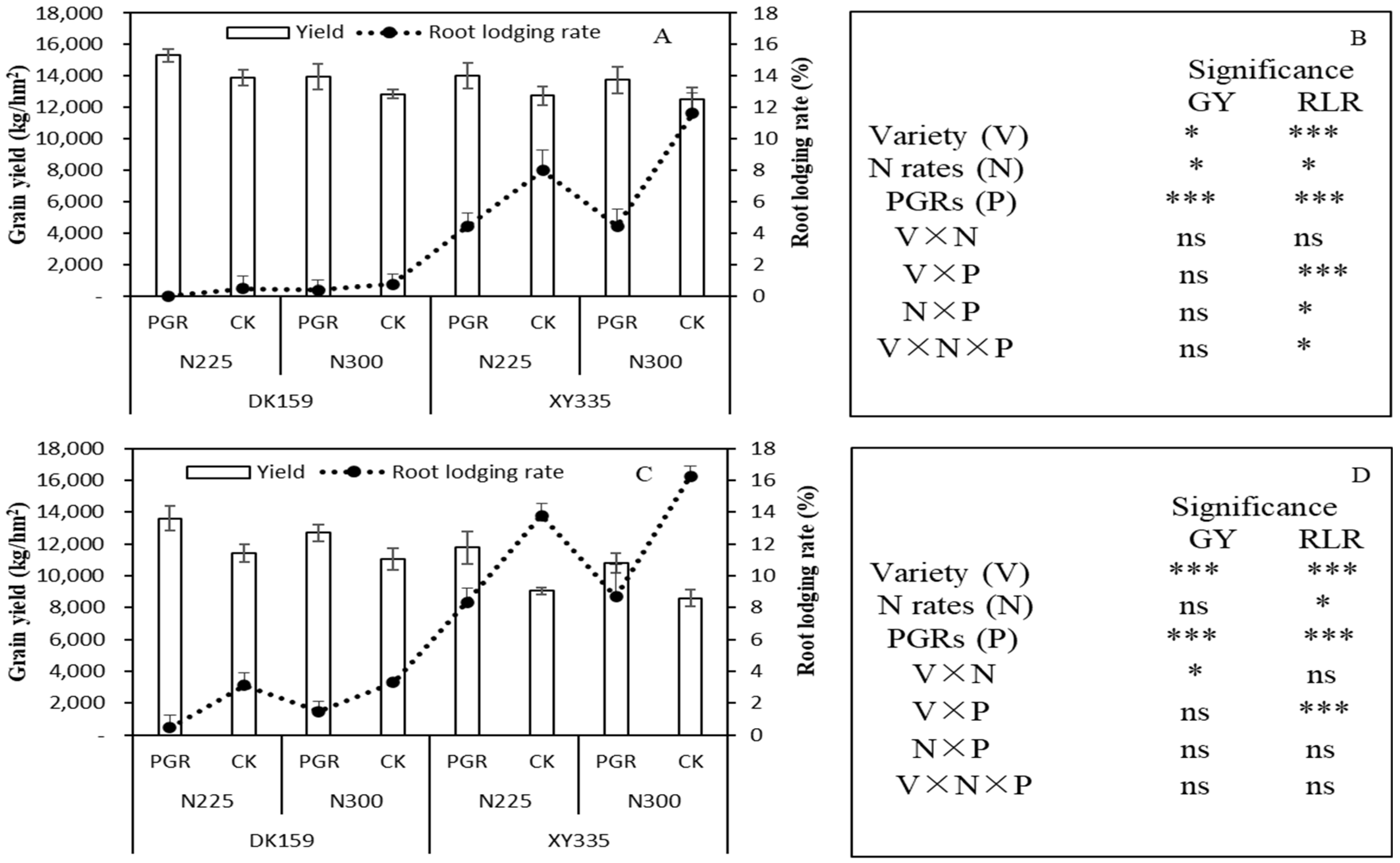
3.7. Yield and Lodging Rate
3.8. Correlation Analysis
4. Discussion
4.1. Effects of PGRs and Nitrogen Fertilizer on Root Lodging Resistance
4.2. Effects of PGRs and Nitrogen Fertilizer on Photosynthetic Traits of Plants
4.3. Effects of PGRs and Nitrogen Fertilizer on Root Traits and Root Bleeding Sap
4.4. Effects of PGRs and Nitrogen Fertilizer on Grain Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tokatlidis, I.S.; Koutroubas, S.D. A review of maize hybrids’ dependence on high plant populations and its implications for crop yield stability. Field Crops Res. 2004, 88, 103–114. [Google Scholar] [CrossRef]
- Tollenaar, M.T.; Lee, E.A. Yield potential, yield stability and stress tolerance in maize. Field Crops Res. 2002, 75, 161–169. [Google Scholar] [CrossRef]
- Yang, J.Z.; Chen, M.L.; Zhang, H.S. Meta-Analysis of the relationship between maize crop yield and plant density from 1950s to 2000s in China. Sci. Agric. Sin. 2013, 46, 3562–3570. [Google Scholar]
- Li, S.T.; Bian, D.H.; He, L.; Wang, D.M.; Zheng, X.M.; Cui, Y.H. Lodging characteristics of summer maize and chemical regulation research progresses preventing lodging in the north China plain. J. Maize Sci. 2018, 26, 95–101. [Google Scholar]
- Boomsma, C.R.; Santini, J.B.; Tollenaar, M.; Vyn, T.J. Maize morphophysiological responses to intense crowding and low nitrogen availability: An analysis and review. Agron. J. 2009, 101, 1426. [Google Scholar] [CrossRef]
- Liu, T.N.; Gu, L.M.; Dong, S.T.; Zhang, J.W.; Liu, P.; Zhao, B. Optimum leaf removal increases canopy apparent photosynthesis, 13C-photosynthate distribution and grain yield of maize crops grown at high density. Field Crops Res. 2015, 170, 32–39. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wu, Q.P.; Chen, X.C.; Chen, F.J.; Zhang, Y.J.; Li, Q.; Yuan, L.X.; Mi, G.H. Root growth and its response to increasing planting density in different maize hybrids. Plant Nutr. Fertil. Sci. 2012, 18, 52–59. [Google Scholar] [CrossRef]
- Li, N.; Huo, Z.X.; Li, J.M.; Wu, P.B.; Duan, L.S.; Li, Z.H. Effects of density on agronomic, root traits and yield of maize with different plant types. Maize Sci. 2008, 16, 98–102. [Google Scholar]
- Guo, S.L.; Chen, N.N.; Qi, J.S.; Yue, R.Q.; Han, X.H.; Yan, S.F.; Lu, C.X.; Fu, X.L.; Guo, X.H.; Tie, S.G. Study on the relationship between yield and lodging traits of maize under different planting densities. Maize Sci. 2018, 26, 71–77. [Google Scholar]
- Li, S.Y.; Ma, W.; Peng, J.Y.; Chen, Z.M. Study on yield loss of summer maize due to lodging at the big flare stage and grain filling stage. Sci. Agric. Sin. 2015, 48, 3952–3964. [Google Scholar]
- Wang, X.B.; Hou, H.P.; Zhou, B.Y.; Sun, X.F.; Ma, W.; Zhao, M. Effect of strip subsoiling on population root spatial distribution of maize under different planting densities. Asta Agron. Sin. 2014, 40, 2136–2148. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.Z.; Evers, J.; Werf, W.V.D.; Zhang, W.Q.; Duan, L.S. Maize yield and quality in response to plant densityand application of a novel plant growth regulator. Field Crops Res. 2014, 164, 82–89. [Google Scholar] [CrossRef]
- Fan, H.C.; Gu, W.R.; Yang, D.G.; Yu, J.P.; Po, L.; Zhang, Q.; Zhang, L.G.; Yang, X.H. Effect of chemical regulators on physical and chemical properties and lodging resistance of spring maize stem in Northeast China. Acta Agron. Sin. 2018, 44, 909–919. [Google Scholar] [CrossRef]
- Ahmad, I.; Kamran, M.; Ali, S.; Bilegjargal, B.; Cai, T.; Ahmad, S.; Meng, X.P.; Su, W.N.; Liu, T.N.; Han, Q.F. Uniconazole application strategies to improve lignin biosynthesis, lodging resistance and production of maize in semiarid regions. Field Crops Res. 2018, 222, 66–77. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, I.; Wang, H.; Wu, X.; Xu, J.; Liu, T.; Ding, R.; Han, Q. Mepiquat chloride application increases lodging resistance of maize by enhancing stalk physical strength and lignin biosynthesis. Field Crops Res. 2018, 224, 148–159. [Google Scholar] [CrossRef]
- Herder, G.D.; Isterdael, G.V.; Beeckman, T.; Smet, I.D. Theroots of a new green revolution. Trends Plant Sci. 2010, 15, 600–607. [Google Scholar] [CrossRef]
- Garnett, T.; Conn, V.; Kaiser, B.N. Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ. 2009, 32, 1272–1283. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, Cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Li, C.H.; Liu, K.; Zhou, S.M.; Luan, L.M. Response of photosynthesis to ecophysiobgial factors of summer maize on different fertilizer amounts. Acta Agron. Sin. 2002, 28, 265–269. [Google Scholar]
- Liu, P.; Dong, S.T.; Li, S.K.; Zhang, J.W. Efficient utilization of nitrogen in high-yielding maize. Sci. Agric. Sin. 2017, 50, 2232–2237. [Google Scholar] [CrossRef]
- Ye, D.L.; Zhang, Y.S.; Al-Kaisi, M.M. Ethephon improved stalk strength associated with summer maize adaptations to environments differing in itrogen availability in the North China Plain. J. Agric. Sci. 2016, 154, 960–977. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2005; pp. 134–140. ISBN 978-7-04-008076-6. [Google Scholar]
- Qi, Q.G.; Li, K.; Li, G.; Li, C.Y.; Cao, G.J. Effects of nitrogen nutrient level on leaf carbon metabolism of spring maize. Agric. Sci. Technol. 2010, 38, 9973–9974, 9978. [Google Scholar]
- Holanda, F.; Mengel, D.; Paula, M.; Carvaho, J.; Bertoni, J. Influence of crop rotations and tillage systems on phosphorus and potassium stratification and root distribution in the soil profile. Commun. Soil Sci. Plant Anal. 1998, 29, 2383–2394. [Google Scholar] [CrossRef]
- Zhao, S.J.; Shi, G.A.; Dong, X.C. Plant Physiology Experiment Instruction; China Agricultural Science and Technology Press: Beijing, China, 2002; pp. 30–40. ISBN 978-7-10-921288-6. [Google Scholar]
- Chen, F.J.; Mi, G.H.; Liu, J.A.; Zhang, F.S. Differences of nitrogen forms in xylem bleeding fluid of maize inbred lines and its relationship with nitrogen efficiency. Sci. Agric. Sin. 1999, 32, 43–48. [Google Scholar]
- Wang, H.; Xu, R.R.; Li, Y.; Yang, L.Y.; Shi, W.; Liu, Y.J. Enhance root-bleeding sap flow and root lodging resistance of maize under a combination of nitrogen strategies and farming practices. Agric. Water Manag. 2019, 224, 105742. [Google Scholar] [CrossRef]
- He, Z.P. Experimental Guidance for Chemical Control of Crops; Beijing Agricultural University Press: Beijing, China, 1993; pp. 10–15. ISBN 978-7-81-002841-7. [Google Scholar]
- Sezegen, B.; Carena, M.J. Divergent recurrent selection for cold tolerance in two improved maize populations. Euphytica 2009, 167, 237–244. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.G.; Zang, B.; Dong, Z.Q.; Wang, M.Y. Compensation mechanism for tapping potential of crop high yield. J. Crops 2006, 32, 1566–1577. [Google Scholar]
- Guan, J.H.; Guo, X.Y.; Liu, Y.; Liu, K.L.; Wang, J.H.; Guo, X.D. Spatial distribution dynamics of maize root dry weight under different densities. Maize Sci. 2007, 4, 105–108, 118. [Google Scholar]
- Zhang, Y.; Qin, H.D.; Wu, L.M.; Zhang, J.; Li, Z.; Huang, M.; Jiang, L.G. Growth characteristics and the effect of nitrogen application on the maize root. J. China Agric. Univ. 2014, 19, 62–70. [Google Scholar]
- Zhang, X.J.; Cai, J. Application effect of maize special regulator Yuhuangjin on summer maize. Anhui Agric. Sci. 2007, 13, 88. [Google Scholar]
- Zhang, P.; Gu, S.C.; Wang, Y.Y.; Yang, R.M.; Yan, Y.; Zhang, S.; Sheng, D.C.; Wang, P.; Huang, S. Morphological and mechanical variables associated with lodging in maize (Zea mays L.). Field Crop Res 2021, 269, 108178. [Google Scholar] [CrossRef]
- Xue, J.; Gao, S.; Fan, Y.; Li, L.; Ming, B.; Wang, K.; Xie, R.; Hou, P.; Li, S. Traits of plant morphology, stalk mechanical strength, and biomass accumulation in the selection of lodging-resistant maize cultivars. Eur. J. Agron. 2020, 117, 126073. [Google Scholar] [CrossRef]
- Lv, L.H.; Tao, H.B.; Zhang, Y.J.; Zhao, M.; Zhao, J.R.; Wang, P. Canopy structure and photosynthesis traits of summer maize under different planting densities. Acta Agron. Sin. 2008, 34, 447–455. [Google Scholar]
- Chen, Z.H.; Fan, L.Y.; Li, C.F. Study on regulation technology of spring maize density fertilizer. J. Maize Sci. 1996, 4, 57–59. [Google Scholar]
- Li, Q.; Ma, X.J.; Cheng, Q.B.; Dou, P.; Yu, D.H.; Luo, Y.H.; Yuan, J.C.; Kong, F.L. Effects of nitrogen fertilizer on post-anthesis matter production and leaf function characteristics of different maize cultivars with low nitrogen tolerance. Chin. J. Eco-Agric. 2016, 24, 17–26. [Google Scholar] [CrossRef]
- Sun, X.F.; Ding, Z.S.; Hou, H.P.; Ge, J.Z.; Tang, L.Y.; Zhao, M. Characteristics of light and matter production and changes of carbon and nitrogen content in different spring maize varieties after anthesis. Acta Agron. Sin. 2013, 39, 1284–1292. [Google Scholar] [CrossRef]
- Qi, D.L.; Wu, X.; Hu, T.T. Effects of nitrogen application on maize root growth, yield and nitrogen utilization. Sci. Agric. Sin. 2014, 47, 2804–2813. [Google Scholar] [CrossRef]
- Cheng, S.; Li, P.C.; Liu, Z.G.; Zhao, L.F.; Mi, G.H.; Yuan, L.X.; Chen, F.J. Effects of density and nitrogen fertilizer on nodal root number of maize hybrids. J. Plant Nutr. Fertil. 2016, 22, 1118–1125. [Google Scholar]
- Shi, X.D.; Liu, Y.F.; Wen, Z.Q.; Wang, W.W. Research progress of plant root bleeding sap. Anhui Agric. Sci. 2006, 34, 2043–2045. [Google Scholar]
- Li, H.L.; Sun, Y.Y.; Qu, J.L.; Wei, C.Q.; Sun, G.H.; Zhao, Y.T.; Chai, Y.S. Effects of nitrogen application rate on morphological and physiological characteristics of roots of Northeast japonica rice. Chin. J. Rice Sci. 2012, 26, 723–730. [Google Scholar] [CrossRef]
- Li, C.Z.; Li, C.J. Ridge-furrow with plastic film mulching system decreases the lodging risk for summer maize plants under different nitrogen fertilization rates and varieties in dry semi-humid areas. Field Crops Res. 2021, 263, 108056. [Google Scholar] [CrossRef]
- Wei, X.T.; Zhang, M.C.; Zhang, Y.; Li, Z.H.; Duan, L.S. Effects of ethephon on internode elongation and endogenous hormones in different genotypes of maize. Chin. J. Pestic. Sci. 2011, 13, 475–479. [Google Scholar]
- Cai, H.G.; Yuan, J.C.; Liu, J.Z.; Yan, X.G.; Zhang, H.X.; Liang, Y.; Ren, J. Nitrogen demand and optimum nitrogen application rate of spring maize under high density planting. Sci. Agric. Sin. 2017, 50, 1995–2005. [Google Scholar] [CrossRef]
- Huang, G.M.; Liu, Y.R.; Guo, Y.L.; Peng, C.X.; Tan, W.M.; Zhang, M.C.; Li, Z.H.; Zhou, Y.Y.; Duan, L.S. A novel plant growth regulator improves the grain yield of high-density maize crops by reducing stalk lodging and promoting a compact plant type. Field Crops Res. 2021, 260, 107982. [Google Scholar] [CrossRef]
- Otie, V.; Ping, A.; John, N.M. Interactive effects of plant growth regulators and nitrogen on corn growth and nitrogen use efficiency. J. Plant Nutr. 2016, 39, 597–1609. [Google Scholar] [CrossRef]


| Variety | N Rates (kg·hm−2) | Treatments | Plant Height (cm) | Ear Height (cm) | Center of Gravity Height (cm) | Ear Height Coefficient (%) |
|---|---|---|---|---|---|---|
| 2019 | ||||||
| DK159 | N225 | PGR | 280 d | 120 d | 106 e | 43.0 d |
| CK | 318 c | 152 b | 128 c | 47.8 b | ||
| N300 | PGR | 292 d | 130cd | 114 d | 44.7 c | |
| CK | 323 c | 165 ab | 137 b | 51.0 a | ||
| XY335 | N225 | PGR | 329 bc | 140 c | 129 c | 42.6 d |
| CK | 340 ab | 170 ab | 141 ab | 50.0 a | ||
| N300 | PGR | 338 ab | 148 b | 124 c | 43.8 d | |
| CK | 350 a | 177 a | 147 a | 51.4 a | ||
| Variety (V) | *** | *** | *** | ns | ||
| N rates (N) | * | * | * | ns | ||
| PGRs (P) | *** | *** | *** | *** | ||
| V × N | ns | ns | ns | ns | ||
| V × P | ** | ns | ns | ns | ||
| N × P | ns | ns | ns | ns | ||
| V × N × P | ns | ns | ns | ns | ||
| 2020 | ||||||
| DK159 | N225 | PGR | 303 e | 128 d | 104 e | 42.4 c |
| CK | 333 c | 154 ab | 138 b | 46.4 b | ||
| N300 | PGR | 311 d | 133 bc | 119 d | 42.6 c | |
| CK | 343 bc | 163 a | 141 b | 47.6 a | ||
| XY335 | N225 | PGR | 339 bc | 141 d | 126 d | 41.6 d |
| CK | 351 ab | 165 a | 140 b | 47.2 a | ||
| N300 | PGR | 353 ab | 151 cd | 135 c | 42.7 d | |
| CK | 361 a | 174 a | 156 a | 48.2 b | ||
| Variety (V) | *** | *** | *** | ns | ||
| N rates (N) | *** | * | *** | ns | ||
| PGRs (P) | *** | *** | *** | *** | ||
| V × N | ns | ns | ns | ns | ||
| V × P | *** | ns | * | ns | ||
| N × P | ns | ns | ns | ns | ||
| V × N × P | ns | ns | ns | ns |
| Variety | N Rates (kg·hm−2) | Treatments | Pn (µmol·m−2·s−1) | RuBPCase Activity (µmolprotein·h−1·mg−1) | PEPCase Activity (µmolprotein·h−1·mg−1) | |||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | |||
| 2019 | ||||||||
| DK159 | N225 | PGR | 66.4 a | 49.5 a | 169.9 a | 131.0 a | 95.0 a | 68.9 a |
| CK | 60.7 c | 46.6 b | 158.8 bc | 117.5 cd | 87.3 b | 65.9 b | ||
| N300 | PGR | 63.5 b | 43.2 c | 163.8 ab | 120.7 bc | 90.7 b | 65.8 b | |
| CK | 57.4 d | 39.2 d | 148.9 de | 115.3 de | 81.5 cd | 60.6 cd | ||
| XY335 | N225 | PGR | 61.8 bc | 47.7 ab | 153.0 cd | 123.3 b | 83.5 c | 61.0 c |
| CK | 56.9 d | 43.1 c | 143.7 ef | 118.6 cd | 75.8 ef | 55.5 e | ||
| N300 | PGR | 54.3 e | 39.5 d | 145.0 e | 112.6 e | 78.5 de | 58.2 d | |
| CK | 49.5 f | 36.1 e | 138.7 f | 107.2 f | 72.6 f | 49.7 f | ||
| Variety (V) | ** | ** | *** | ** | * | *** | ||
| N rates (N) | *** | ** | ** | *** | ** | *** | ||
| PGRs (P) | *** | *** | *** | *** | ** | *** | ||
| V × N | ns | ns | ns | ns | ns | * | ||
| V × P | * | ns | ** | ns | ns | ns | ||
| N × P | ns | ns | ns | ns | ns | ns | ||
| V × N × P | ns | ns | ns | ns | ns | ns | ||
| 2020 | ||||||||
| DK159 | N225 | PGR | 63.7 a | 44.2 a | 154.8 a | 131.9 a | 90.1 a | 59.4 a |
| CK | 56.7 cd | 39.9 b | 142.1 cd | 116.2 bc | 82. 0c | 56.9 b | ||
| N300 | PGR | 58.5 bc | 38.8 bc | 145.5 bc | 118.9 b | 86.0 b | 56.0 b | |
| CK | 52.8 e | 34.9 de | 137.3 e | 103.8 d | 75.5 d | 49.8 cd | ||
| XY335 | N225 | PGR | 59.6 b | 40.9 b | 149.1 b | 117.5 bc | 81.7 c | 52.0 c |
| CK | 54.4 de | 36.4 cd | 142.9 cd | 103.4 d | 75.0 d | 47.3 de | ||
| N300 | PGR | 52.0 e | 35.2 de | 138.9 e | 111.6 c | 77.2 d | 47.2 e | |
| CK | 45.9 f | 32.2 e | 134.8 f | 95.7 e | 72.8 e | 41.3 f | ||
| Variety (V) | *** | ** | *** | ** | *** | ** | ||
| N rates (N) | ** | ** | *** | ** | *** | ** | ||
| PGRs (P) | *** | *** | *** | *** | *** | *** | ||
| V × N | * | ns | ns | ns | ns | ns | ||
| V × P | ns | * | * | ns | ns | ns | ||
| N × P | ns | ns | ns | ns | * | ns | ||
| V × N × P | ns | ns | ns | ns | * | ns | ||
| Varieties | N Rates (kg·hm−2) | Treatments | Total Root Number | Root Length (cm) | Root Breadth Area (cm2) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|
| 2019 | ||||||
| DK159 | N225 | PGR | 59 a | 1769a | 494 a | 28.1 a |
| CK | 55 b | 1584 bc | 472 bc | 24.8 b | ||
| N300 | PGR | 56 b | 1575 bc | 482 ab | 25.0 b | |
| CK | 48 de | 1362 de | 440 de | 20.7 d | ||
| XY335 | N225 | PGR | 55 b | 1625 b | 453 cd | 25.2 b |
| CK | 50 cd | 1473 cd | 427 e | 22.9 c | ||
| N300 | PGR | 53 bc | 1397 d | 438 de | 21.6 cd | |
| CK | 46 e | 1254 e | 392 f | 18.2 e | ||
| Variety (V) | *** | *** | *** | *** | ||
| N rates (N) | *** | *** | *** | *** | ||
| PGRs (P) | *** | *** | *** | *** | ||
| V × N | ns | ns | ns | ns | ||
| V × P | ns | ns | ns | ns | ||
| N × P | ns | ns | * | ns | ||
| V × N × P | ns | ns | ns | ns | ||
| 2020 | ||||||
| DK159 | N225 | PGR | 60 a | 1688 a | 488 a | 26.6 a |
| CK | 54 bc | 1540 b | 460 b | 24.4 bc | ||
| N300 | PGR | 56 b | 1487 b | 463 b | 25.1 ab | |
| CK | 49 e | 1345 c | 429 c | 22.3 de | ||
| XY335 | N225 | PGR | 52 cd | 1535 b | 452 b | 24.9 ab |
| CK | 48 e | 1362 c | 431 c | 21.5 de | ||
| N300 | PGR | 50 de | 1376 c | 420 c | 22.5 cd | |
| CK | 43 f | 1219 d | 388 d | 20.4 e | ||
| Variety (V) | *** | *** | *** | *** | ||
| N rates (N) | *** | *** | *** | *** | ||
| PGRs (P) | *** | *** | *** | *** | ||
| V × N | ns | ns | ns | ns | ||
| V × P | ns | ns | ns | ns | ||
| N × P | ns | ns | ns | ns | ||
| V × N × P | ns | ns | ns | ns |
| Variety | N Rates (kg·hm−2) | Treatments | IAA (μL h−1pl−1) | GA (μL h−1pl−1) | CTK (μL h−1pl−1) | GA/IAA | CTK/IAA | CTK/GA |
|---|---|---|---|---|---|---|---|---|
| 2019 | ||||||||
| DK159 | N225 | PGR | 514.8 b | 198.9 d | 594.7 a | 0.39 d | 1.16 b | 3.01 b |
| CK | 291.1 c | 309.7 a | 425.2 c | 1.07 a | 1.47 a | 1.38 e | ||
| N300 | PGR | 542.3 ab | 188.1 de | 518.8 b | 0.35 d | 0.96 d | 2.79 c | |
| CK | 305.5 c | 271.8 b | 364.8 d | 0.89 b | 1.20 b | 1.34 f | ||
| XY335 | N225 | PGR | 556.9 a | 137.8 f | 548.6 b | 0.25 e | 0.99 c | 3.98 a |
| CK | 320.7 c | 292.6 ab | 399.7 c | 0.91 b | 1.25 b | 1.37 e | ||
| N300 | PGR | 538.0 ab | 162.6 ef | 516.1 b | 0.30 e | 0.96 d | 3.18 b | |
| CK | 314.8 c | 226.5 c | 328.8 e | 0.72 c | 1.04 c | 1.45 d | ||
| Variety (V) | * | *** | ** | *** | ** | *** | ||
| N rates (N) | ns | ** | *** | *** | *** | ** | ||
| PGRs (P) | *** | *** | *** | *** | *** | *** | ||
| V × N | * | ns | ns | ns | ns | ns | ||
| V × P | ns | ns | ns | ns | ns | ** | ||
| N × P | ns | *** | ns | *** | ns | ** | ||
| V × N × P | ns | * | ns | ns | ns | * | ||
| 2020 | ||||||||
| DK159 | N225 | PGR | 572.4 b | 222.9 cd | 614.9 a | 0.39 d | 1.08 b | 2.78 b |
| CK | 329.9 d | 349.5 a | 437.8 c | 1.06 a | 1.34 a | 1.25 e | ||
| N300 | PGR | 614.1 ab | 212.4 d | 536.4 b | 0.35 d | 0.87 c | 2.56 c | |
| CK | 350.8 cd | 306.3 b | 375.8 de | 0.90 b | 1.11 b | 1.23 e | ||
| XY335 | N225 | PGR | 660.9 a | 154.2 e | 567.7 b | 0.23 e | 0.86 c | 3.73 a |
| CK | 400.3 c | 330.6 ab | 411.8 cd | 0.83 b | 1.03 b | 1.25 e | ||
| N300 | PGR | 618.2 ab | 181.7 de | 533.9 b | 0.29 e | 0.86 c | 2.98 b | |
| CK | 383.5 cd | 255.6 c | 338.8 e | 0.67 c | 0.88 c | 1.33 d | ||
| Variety (V) | * | *** | * | ** | ** | * | ||
| N rates (N) | ns | ** | ** | * | * | * | ||
| PGRs (P) | *** | *** | *** | *** | ** | *** | ||
| V × N | ns | ns | ns | ns | ns | ns | ||
| V × P | ns | ns | ns | ns | ns | * | ||
| N × P | ns | ** | ns | ns | ns | ns | ||
| V × N × P | ns | ns | ns | ns | ns | ns |
| Plant Height | Ear Height | Center of Gravity Height | Ear Height Coefficient | |
|---|---|---|---|---|
| GA/IAA | 0.459 * | 0.653 ** | 0.606 ** | 0.788 ** |
| CTK/IAA | −0.051 | 0.242 | 0.263 | 0.431 * |
| CTK/GA | −0.595 ** | −0.693 ** | −0.584 ** | −0.826 ** |
| Total Root Number | Root Length | Root Breadth Area | Root Dry Weight | |
|---|---|---|---|---|
| GA/IAA | −0.277 | −0.295 | −0.265 | −0.338 |
| CTK/IAA | −0.090 | 0.167 | −0.074 | 0.116 |
| CTK/GA | 0.616 ** | 0.494 * | 0.428 * | 0.511 * |
| Pn | RuBPCase | PEPCase | Root Activity | Root Bleeding Sap | Root Lodging Rate | |
|---|---|---|---|---|---|---|
| Grain yield in 2019 | 0.846 ** | 0.894 ** | 0.869 ** | 0.924 ** | 0.953 ** | −0.669 |
| Grain yield in 2020 | 0.862 ** | 0.905 ** | 0.940 ** | 0.914 ** | 0.847 ** | −0.912 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, N.; Chen, X.; Zhao, H.; Meng, X.; Bian, S. Effects of Plant Growth Regulators and Nitrogen Management on Root Lodging Resistance and Grain Yield under High-Density Maize Crops. Agronomy 2022, 12, 2892. https://doi.org/10.3390/agronomy12112892
Sun N, Chen X, Zhao H, Meng X, Bian S. Effects of Plant Growth Regulators and Nitrogen Management on Root Lodging Resistance and Grain Yield under High-Density Maize Crops. Agronomy. 2022; 12(11):2892. https://doi.org/10.3390/agronomy12112892
Chicago/Turabian StyleSun, Ning, Xifeng Chen, Hongxiang Zhao, Xiangmeng Meng, and Shaofeng Bian. 2022. "Effects of Plant Growth Regulators and Nitrogen Management on Root Lodging Resistance and Grain Yield under High-Density Maize Crops" Agronomy 12, no. 11: 2892. https://doi.org/10.3390/agronomy12112892
APA StyleSun, N., Chen, X., Zhao, H., Meng, X., & Bian, S. (2022). Effects of Plant Growth Regulators and Nitrogen Management on Root Lodging Resistance and Grain Yield under High-Density Maize Crops. Agronomy, 12(11), 2892. https://doi.org/10.3390/agronomy12112892





