Application of Spent Sun Mushroom Substrate in Substitution of Synthetic Fertilizers at Maize Topdressing
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Site
2.2. Spent Mushroom Substrate (SMS) and Experimental Soil
2.3. Experimental Design
2.4. Biometric Parameters
2.5. Soil and Leaf Characteristics
2.6. Statical Analysis
3. Results
3.1. Analysis of Variance
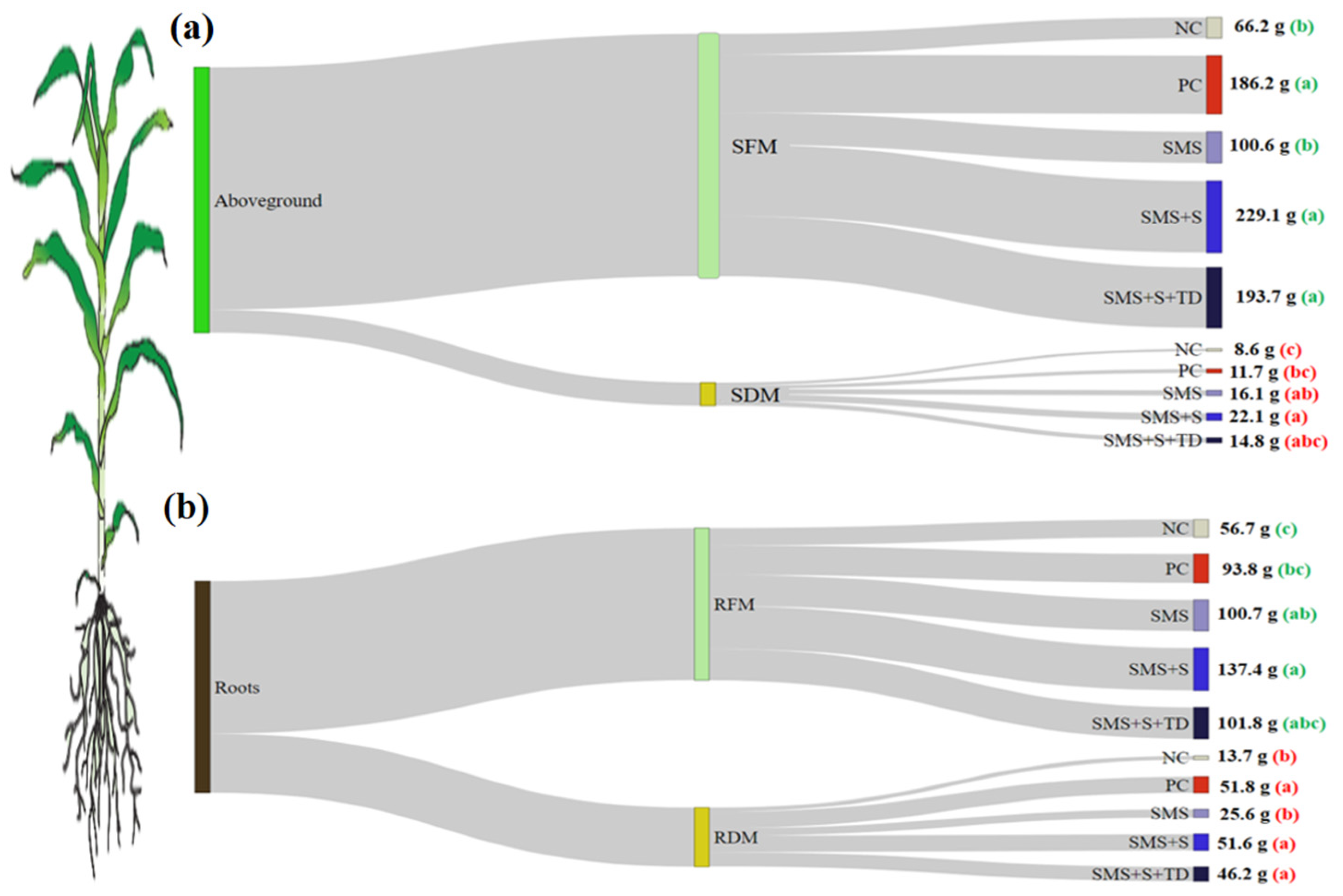
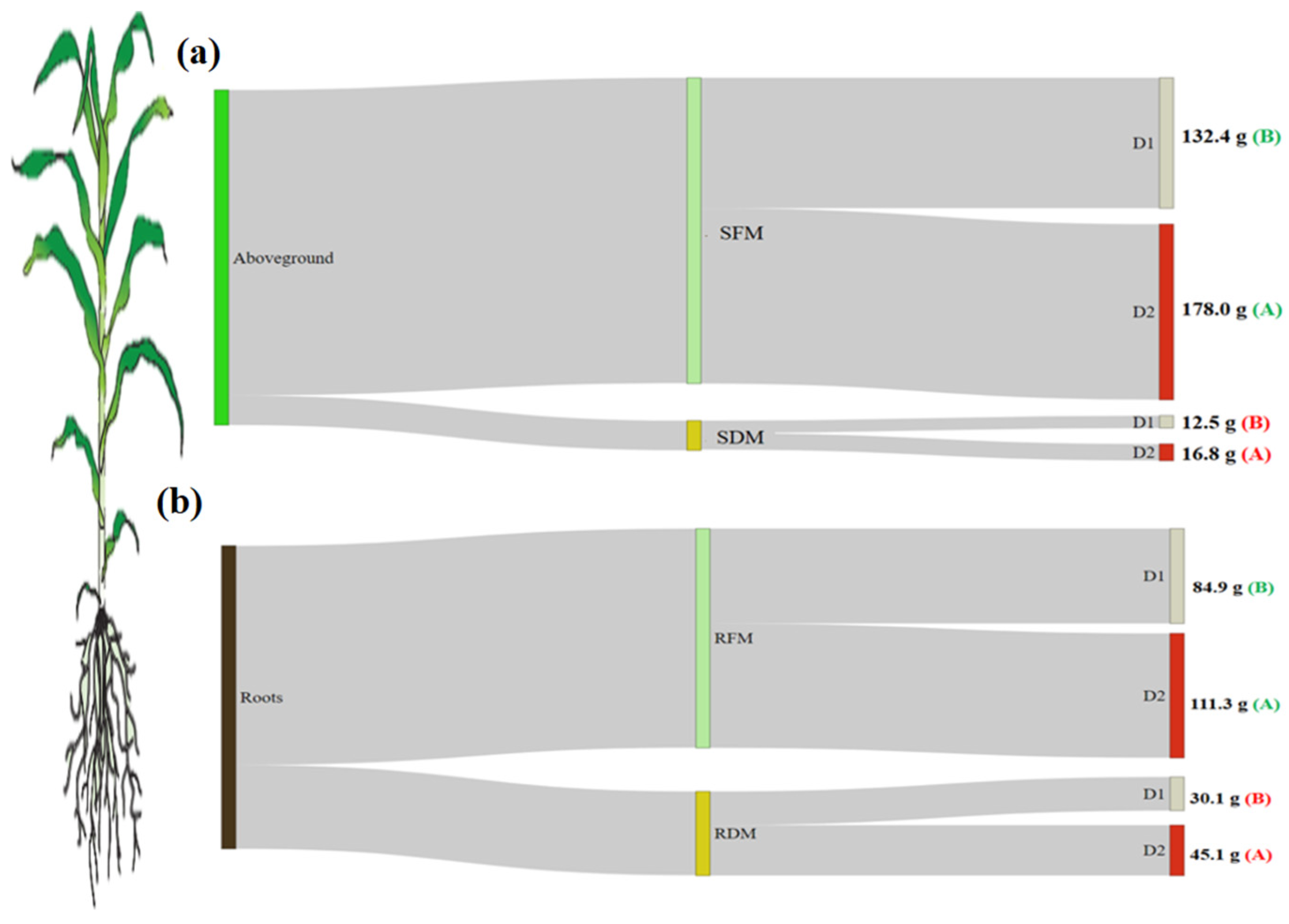
3.2. Biometric Parameters
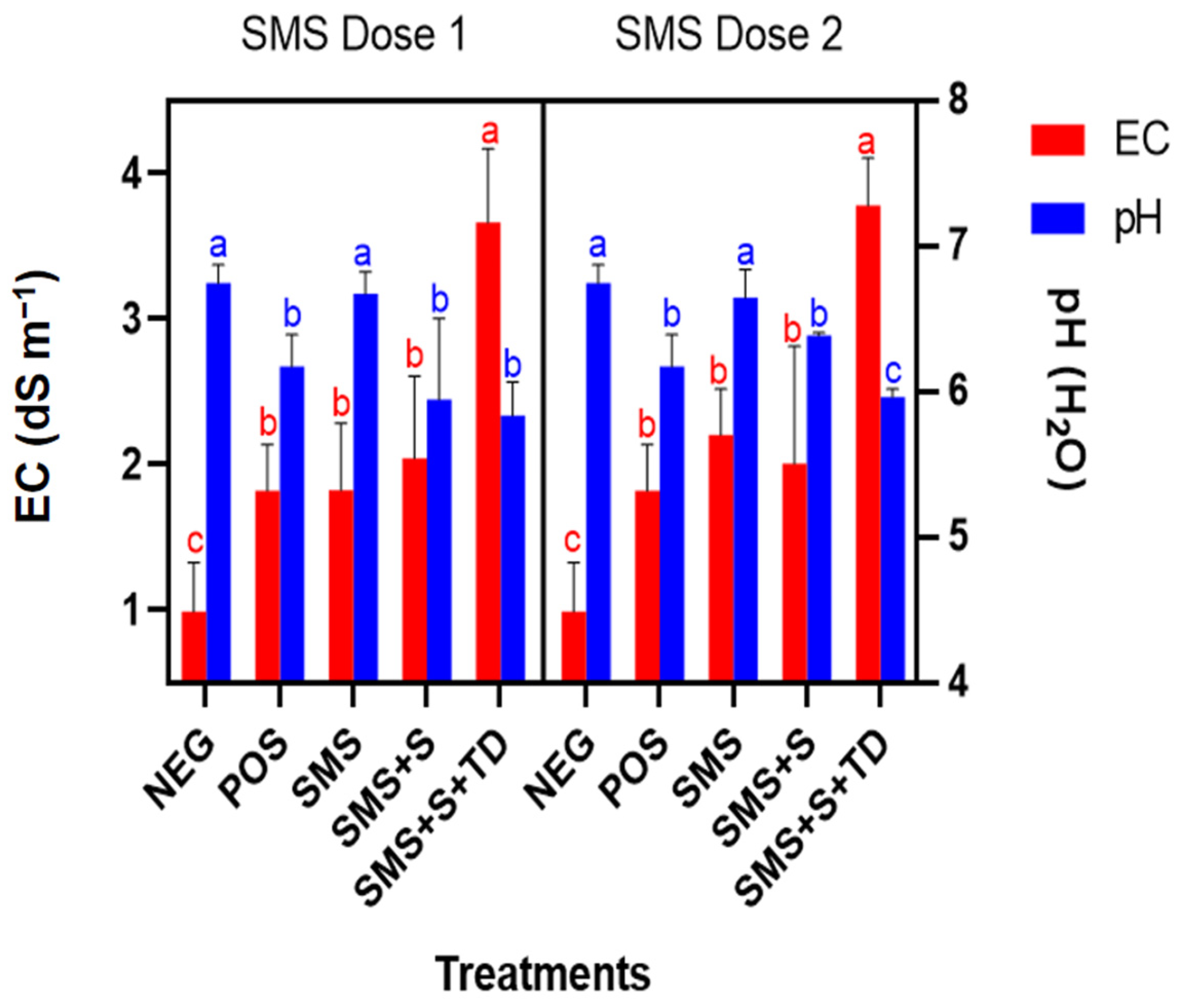
3.3. Soil Electrical Conductivity and pH
3.4. Soil and Leaf Chemical Characteristics
4. Discussion
4.1. Biometric Parameters
4.2. Soil Electrical Conductivity and pH
4.3. Soil and Leaf Chemical Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Wang, S.; Xue, B.; Li, R.; Geng, Y.; Yang, T.; Li, Y.; Dong, H.; Luo, Z.; Tao, W.; et al. Emergy-based indicators of the environmental impacts and driving forces of non-point source pollution from crop production in China. Ecol. Indic. 2021, 121, 107023. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 30 March 2022).
- Tanumihardjo, S.A.; McCulley, L.; Roh, R.; Lopez-Ridaura, S.; Palacios-Rojas, N.; Gunaratna, N.S. Maize agro-food systems to ensure food and nutrition security in reference to the Sustainable Development Goals. Glob. Food Secur. 2020, 25, 100327. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Wang, F.; Xie, Y. Maize (Zea mays) growth and nutrient uptake following integrated improvement of vermicompost and humic acid fertilizer on coastal saline soil. Appl. Soil Ecol. 2019, 142, 147–154. [Google Scholar] [CrossRef]
- Maja, M.M.; Ayano, S.F. The impact of population growth on natural resources and farmers’ capacity to adapt to climate change in low-income countries. Earth Syst. Environ. 2021, 5, 271–283. [Google Scholar] [CrossRef]
- Tadesse, M.; Simane, B.; Abera, W.; Tamene, L.; Ambaw, G.; Recha, J.W.; Mekonnen, K.; Demeke, G.; Nigussie, A.; Solomon, D. The Effect of Climate-Smart Agriculture on Soil Fertility, Crop Yield, and Soil Carbon in Southern Ethiopia. Sustainability 2021, 13, 4515. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Walia, M.K.; Gupta, R.K.; Singh, R.; Dhaliwal, M.K. Effect of manures and fertilizers on soil physical properties, build-up of macro and micronutrients and uptake in soil under different cropping systems: A review. J. Plant Nutr. 2019, 42, 2873–2900. [Google Scholar] [CrossRef]
- Levi, P.G.; Cullen, J.M. Mapping global flows of chemicals: From fossil fuel feedstocks to chemical products. Environ. Sci. Technol. 2018, 52, 1725–1734. [Google Scholar] [CrossRef]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Heinemann: Butterworth, Malaysia, 2020; pp. 25–54. [Google Scholar] [CrossRef]
- Lv, F.; Song, J.; Giltrap, D.; Feng, Y.; Yang, X.; Zhang, S. Crop yield and N2O emission affected by long-term organic manure substitution fertilizer under winter wheat-summer maize cropping system. Sci. Total Environ. 2020, 732, 139321. [Google Scholar] [CrossRef]
- Wan, L.J.; Tian, Y.; He, M.; Zheng, Y.Q.; Lyu, Q.; Xie, R.J.; Ma, Y.Y.; Deng, L.; Yi, S.L. Effects of Chemical Fertilizer Combined with Organic Fertilizer Application on Soil Properties, Citrus Growth Physiology, and Yield. Agriculture 2021, 11, 1207. [Google Scholar] [CrossRef]
- Mostashari-Rad, F.; Nabavi-Pelesaraei, A.; Soheilifard, F.; Hosseini-Fashami, F.; Chau, K.W. Energy optimization and greenhouse gas emissions mitigation for agricultural and horticultural systems in Northern Iran. Energy 2019, 186, 115845. [Google Scholar] [CrossRef]
- Walling, E.; Vaneeckhaute, C. Greenhouse gas emissions from inorganic and organic fertilizer production and use: A review of emission factors and their variability. J. Environ. Manag. 2020, 276, 111211. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fernie, A.R.; Yan, J. The past, present, and future of maize improvement: Domestication, genomics, and functional genomic routes toward crop enhancement. Plant Commun. 2020, 1, 100010. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.; Zhang, D. Effects of fertilizer broadcasting on the excessive use of inorganic fertilizers and environmental sustainability. Sustainability 2018, 10, 759. [Google Scholar] [CrossRef]
- Jang, W.S.; Neff, J.C.; Im, Y.; Doro, L.; Herrick, J.E. The hidden costs of land degradation in US maize agriculture. Earths Future 2021, 9, e2020EF001641. [Google Scholar] [CrossRef]
- Clapp, J.; Isakson, S.R. Risky returns: The implications of financialization in the food system. Dev. Chang. 2018, 49, 437–460. [Google Scholar] [CrossRef]
- Morgan, S.N.; Mason, N.M.; Levine, N.K.; Zulu-Mbata, O. Dis-incentivizing sustainable intensification? The case of Zambia’s maize-fertilizer subsidy program. World Dev. 2019, 122, 54–69. [Google Scholar] [CrossRef]
- Meybeck, A.; Gitz, V. Sustainable diets within sustainable food systems. Proc. Nutr. Soc. 2017, 76, e2020EF001641. [Google Scholar] [CrossRef]
- Hetland, G.; Tangen, J.M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Johnson, E. Antitumor, anti-inflammatory and antiallergic effects of Agaricus blazei mushroom extract and the related medicinal Basidiomycetes mushrooms, Hericium erinaceus and Grifola frondosa: A review of preclinical and clinical studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef]
- Cavalcante, J.L.R.; Gomes, V.F.F.; Kopytowski Filho, J.; Minhoni, M.T.D.A.; Andrade, M.C.N.D. Cultivation of Agaricus blazei in the environmental protection area of the Baturité region under three types of casing soils. Acta Sci. Agron. 2008, 30, 513–517. [Google Scholar] [CrossRef]
- Vieira Junior, W.G.; Centeio Cardoso, R.V.; Fernandes, Â.; Ferreira, I.C.F.R.; Barros, L.; Pardo-Giménez, A.; Soares, D.M.M.S.; Zied, D.C. Influence of strains and environmental cultivation conditions on the bioconversion of ergosterol and vitamin D2 in the sun mushroom. J. Sci. Food Agric. 2021, 102, 1699–1706. [Google Scholar] [CrossRef]
- Roy, S.; Barman, S.; Chakraborty, U.; Chakraborty, B. Evaluation of Spent Mushroom Substrate as biofertilizer for growth improvement of Capsicum annuum L. J. Appl. Biol. Biotechnol. 2015, 3, 022–027. [Google Scholar] [CrossRef]
- Rinker, D. Spent mushroom substrate uses. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Giménez, A., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 427–454. [Google Scholar]
- Zied, D.C.; Abreu, C.G.; Alves, L.S.; Prado, E.P.; Pardo-Gimenez, A.; Melo, P.C.; Dias, E.S. Influence of the production environment on the cultivation of lettuce and arugula with spent mushroom substrate. J. Environ. Manag. 2021, 281, 111799. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Shi, Y.; Zhang, L.; Fang, H.; Gao, Y.; Luo, L.; Feng, W.; Hu, X.; Wan, S.; Huang, W.; et al. Effects of spent mushroom substrate-derived biochar on soil CO2 and N2O emissions depend on pyrolysis temperature. Chemosphere 2020, 246, 125608. [Google Scholar] [CrossRef] [PubMed]
- Becher, M.; Banach-Szott, M.; Godlewska, A. Organic Matter Properties of Spent Button Mushroom Substrate in the Context of Soil Organic Matter Reproduction. Agronomy 2021, 11, 204. [Google Scholar] [CrossRef]
- Tuhy, Ł.; Samoraj, M.; Witkowska, Z.; Wilk, R.; Chojnacka, K. Using spent mushroom substrate as the base for organic-mineral micronutrient fertilizer–field tests on maize. BioResources 2015, 10, 5709–5719. [Google Scholar] [CrossRef]
- Li, F.; Kong, Q.; Zhang, Q.; Wang, H.; Wang, L.; Luo, T. Spent mushroom substrates affect soil humus composition, microbial biomass and functional diversity in paddy fields. Appl. Soil Ecol. 2020, 149, 103489. [Google Scholar] [CrossRef]
- Othman, N.Z.; Sarjuni, M.N.H.; Rosli, M.A.; Nadri, M.H.; Yeng, L.H.; Ying, O.P.; Sarmidi, M.R. Spent mushroom substrate as biofertilizer for agriculture application. In Valorisation of Agro-industrial Residues—Volume I: Biological Approaches; Zakaria, Z.A., Boopathy, R., Dib, J.R., Eds.; Springer: Cham, Switzerland, 2020; pp. 37–57. [Google Scholar] [CrossRef]
- Paredes, C.; Medina, E.; Bustamante, M.A.; Moral, R. Effects of spent mushroom substrates and inorganic fertilizer on the characteristics of a calcareous clayey-loam soil and lettuce production. Soil Use Manag. 2016, 32, 487–494. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E. The Effect of Fertilization with Spent Mushroom Substrate and Traditional Methods of Fertilization of Common Thyme (Thymus vulgaris L.) on Yield Quality and Antioxidant Properties of Herbal Material. Agronomy 2021, 11, 329. [Google Scholar] [CrossRef]
- Setiyono, T.D.; Walters, D.T.; Cassman, K.G.; Witt, C.; Dobermann, A. Estimating maize nutrient uptake requirements. Field Crops Res. 2010, 118, 158–168. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Malavolta, E. Manual de Química Agrícola: Adubos e Adubação; Agronômica Ceres: São Paulo, Brazil, 1981. [Google Scholar]
- Van Raij, B.; Cantarela, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem para o Estado de São Paulo; Instituto Agronômico/Fundação IAC: Campinas, Brazil, 1997.
- Hafez, M.; Popov, A.I.; Rashad, M. Integrated use of bio-organic fertilizers for enhancing soil fertility–plant nutrition, germination status and initial growth of corn (Zea mays L.). Environ. Sci. Technol. 2021, 21, 101329. [Google Scholar] [CrossRef]
- Carmo, D.L.; Silva, C.A. Electrical conductivity and corn growth in contrasting soils affected by liming application at various levels. Pesqu. Agropecu. Bras. 2016, 51, 1762–1772. [Google Scholar] [CrossRef]
- Borges, W.L.B.; Freitas, R.S.D.; Mateus, G.P.; Sá, M.E.D.; Alves, M.C. Absorção de nutrientes e alterações químicas em Latossolos cultivados com plantas de cobertura em rotação com soja e milho. Rev. Bras. Cienc. Solo 2014, 38, 252–261. [Google Scholar] [CrossRef][Green Version]
- Michalovicz, L.; Müller, M.M.L.; Foloni, J.S.S.; Kawakami, J.; Nascimento, R.D.; Kramer, L.F.M. Soil fertility, nutrition and yield of maize and barley with gypsum application on soil surface in no-till. Rev. Bras. Cienc. Solo 2014, 38, 1496–1505. [Google Scholar] [CrossRef][Green Version]
- Friendly, M.; Fox, J.; Friendly, M.M. Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis. R Package Candisc Version: 0.6-5. Available online: https://cran.r-project.org/web/packages/candisc/candisc.pdf (accessed on 20 March 2022).
- Zhang, R.H.; Zeng-Qiang, D.; Zhi-Guo, L.I. Use of spent mushroom substrate as growing media for tomato and cucumber seedlings. Pedosphere 2012, 22, 333–342. [Google Scholar] [CrossRef]
- Tuhy, Ł.; Samoraj, M.; Michalak, I.; Chojnacka, K. The application of biosorption for production of micronutrient fertilizers based on waste biomass. Appl. Biochem. Biotechnol. 2014, 174, 1376–1392. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Wang, Y. Optimizing the amount of pig manure in the vermicomposting of spent mushroom (Lentinula) substrate. PeerJ 2020, 8, e10584. [Google Scholar] [CrossRef]
- Elaamer, H. The Effect of Spent Mushroom (Agaricus bisporus) Compost on the Indigenous Rhizosphere Microbiota in Strawberry Cultivation. Master’s Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2020. [Google Scholar]
- Llarena-Hernández, C.R.; Largeteau, M.L.; Ferrer, N.; Regnault-Roger, C.; Savoie, J.M. Optimization of the cultivation conditions for mushroom production with European wild strains of Agaricus subrufescens and Brazilian cultivars. J. Sci. Food Agric. 2014, 94, 77–84. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, Z.; Wang, H. Effects of Gleditsia sinensis pod powder, coconut shell biochar and rice husk biochar as additives on bacterial communities and compost quality during vermicomposting of pig manure and wheat straw. J. Environ. Manag. 2021, 295, 113136. [Google Scholar] [CrossRef]
- Buvaneshwari, S.; Riotte, J.; Sekhar, M.; Sharma, A.K.; Helliwell, R.; Kumar, M.M.; Braun, J.J.; Ruiz, L. Potash fertilizer promotes incipient salinization in groundwater irrigated semi-arid agriculture. Sci. Rep. 2020, 10, 3691. [Google Scholar] [CrossRef]
- George, E.; Horst, W.J.; Neumann, E. Adaptation of plants to adverse chemical soil conditions. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 409–472. [Google Scholar]
- Frąc, M.; Pertile, G.; Panek, J.; Gryta, A.; Oszust, K.; Lipiec, J.; Usowicz, B. Mycobiome composition and diversity under the long-term application of spent mushroom substrate and chicken manure. Agronomy 2021, 11, 410. [Google Scholar] [CrossRef]
- Adamtey, N.; Musyoka, M.W.; Zundel, C.; Cobo, J.G.; Karanja, E.; Fiaboe, K.K.; Muriuki, A.; Mucheru-Muna, M.; Vanlauwe, B.; Berset, E.; et al. Productivity, profitability and partial nutrient balance in maize-based conventional and organic farming systems in Kenya. Agric. Ecosyst. Environ. 2016, 235, 61–79. [Google Scholar] [CrossRef]
- Coles, P.S.; Nogin, G.; Fidanza, M.; Roth, G. Evaluation of Fresh Mushroom Compost in a Field Corn Production System. Compost Sci. Util. 2020, 28, 76–86. [Google Scholar] [CrossRef]
- Oladele, S.O. Changes in physicochemical properties and quality index of an Alfisol after three years of rice husk biochar amendment in rainfed rice–Maize cropping sequence. Geoderma 2019, 353, 359–371. [Google Scholar] [CrossRef]
- Corwin, D.L.; Yemoto, K. Salinity: Electrical conductivity and total dissolved solids. Soil Sci. Soc. Am. J. 2020, 84, 1442–1461. [Google Scholar] [CrossRef]
- Meng, X.; Liu, B.; Zhang, H.; Wu, J.; Yuan, X.; Cui, Z. Co-composting of the biogas residues and spent mushroom substrate: Physicochemical properties and maturity assessment. Bioresour. Technol. 2019, 276, 281–287. [Google Scholar] [CrossRef]
- Hřebečková, T.; Wiesnerová, L.; Hanč, A. Change in agrochemical and biochemical parameters during the laboratory vermicomposting of spent mushroom substrate after cultivation of Pleurotus ostreatus. Sci. Total Environ. 2020, 739, 140085. [Google Scholar] [CrossRef]
- Scheberl, L.; Scharenbroch, B.C.; Werner, L.P.; Prater, J.R.; Fite, K.L. Evaluation of soil pH and soil moisture with different field sensors: Case study urban soil. Urban For. Urban Green. 2019, 38, 267–279. [Google Scholar] [CrossRef]
- Ngan, N.M.; Riddech, N. Use of Spent Mushroom Substrate as an Inoculant Carrier and an Organic Fertilizer and Their Impacts on Roselle Growth (Hibiscus sabdariffa L.) and Soil Quality. Waste Biomass Valoriz. 2021, 12, 3801–3811. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Z.; Zhang, M.; Shi, Y.; Zhu, Q.; Sun, Y.; Zhou, H.; Li, C.; Yang, Y.; Geng, J. Improving crop yields, nitrogen use efficiencies, and profits by using mixtures of coated controlled-released and uncoated urea in a wheat-maize system. Field Crops Res. 2017, 205, 106–115. [Google Scholar] [CrossRef]
- Garcia, P.L.; Sermarini, R.A.; Filho, C.R.D.S.A.; Bendassolli, J.A.; Boschiero, B.N.; Trivelin, P.C.O. 15N-Fertilizer recovery in maize as an additional strategy for understanding nitrogen fertilization management with blends of controlled-release and conventional urea. Agronomy 2020, 10, 1932. [Google Scholar] [CrossRef]
- Garcia, P.L.; Sermarini, R.A.; Trivelin, P.C.O. Effect of nitrogen rates applying controlled-release and conventional urea blend in maize. J. Plant Nutr. 2019, 42, 2199–2208. [Google Scholar] [CrossRef]
- Ozlu, E.; Kumar, S. Response of soil organic carbon, pH, electrical conductivity, and water stable aggregates to long-term annual manure and inorganic fertilizer. Soil Sci. Soc. Am. J. 2018, 82, 1243–1251. [Google Scholar] [CrossRef]
- Lipiec, J.; Usowicz, B.; Kłopotek, J.; Turski, M.; Frąc, M. Effects of Application of Recycled Chicken Manure and Spent Mushroom Substrate on Organic Matter Acidity and Hydraulic Properties of Sandy Soils. Materials 2021, 14, 4036. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.M.G.; Lobato, E. Cerrado: Correção do Solo e Adubação; Embrapa Cerrados: Planaltina, Brazil, 2004. [Google Scholar]
- Kuśmirek, E. The rate of release of macronutrients from new organic-mineral fertilizers. Chall. Mod. Technol. 2018, 9, 3–17. [Google Scholar] [CrossRef]
- García-Hernández, J.L.; Valdez-Cepeda, R.D.; Murillo-Amador, B.; Beltrán-Morales, F.A.; Ruiz-Espinoza, F.H.; Orona-Castillo, I.; Flores-Hernández, A.; Troyo-Diéguez, E. Preliminary compositional nutrient diagnosis norms in Aloe vera L. grown on calcareous soil in an arid environment. Environ. Exp. Bot. 2006, 58, 244–252. [Google Scholar] [CrossRef]
- Uribe, R.A.M.; Silvério, P.C.; Costa, G.H.G.; Nogueira, L.C.; Leite, L.A.R. Chloride levels in biomass sorghum due to fertilization sources. Biomass Bioenergy 2020, 143, 105845. [Google Scholar] [CrossRef]
- Geng, J.; Yang, X.; Huo, X.; Chen, J.; Lei, S.; Li, H.; Lang, Y.; Liu, Q. Determination of the best controlled-release potassium chloride and fulvic acid rates for an optimum cotton yield and soil available potassium. Front. Plant Sci. 2020, 11, 562335. [Google Scholar] [CrossRef] [PubMed]
- Braga, B.B.; Carvalho, T.R.A.; Brosinsky, A.; Foerster, S.; Medeiros, P.H.A. From waste to resource: Cost-benefit analysis of reservoir sediment reuse for soil fertilization in a semiarid catchment. Sci. Total Environ. 2019, 670, 158–169. [Google Scholar] [CrossRef]
- Zhang, M.; Geng, Y.; Cao, G.; Wang, L.; Wang, M.; Stephano, M.F. Magnesium accumulation, partitioning and remobilization in spring maize (Zea mays L.) under magnesium supply with straw return in northeast China. J. Sci. Food Agric. 2020, 100, 2568–2578. [Google Scholar] [CrossRef]
- Stewart, D.P.C.; Cameron, K.C.; Cornforth, I.S.; Main, B.E. Release of sulphate-sulphur, potassium, calcium and magnesium from spent mushroom compost under field conditions. Biol. Fertil. Soils 2000, 31, 128–133. [Google Scholar] [CrossRef]
- Zenda, T.; Liu, S.; Dong, A.; Duan, H. Revisiting sulphur—The once neglected nutrient: It’s roles in plant growth, metabolism, stress tolerance and crop production. Agriculture 2021, 11, 626. [Google Scholar] [CrossRef]
- Häring, V.; Manka’abusi, D.; Akoto-Danso, E.K.; Werner, S.; Atiah, K.; Steiner, C.; Lompo, D.J.P.; Adiku, S.; Buerkert, A.; Marschner, B. Effects of biochar, waste water irrigation and fertilization on soil properties in West African urban agriculture. Sci. Rep. 2017, 7, 10738. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Zhu, J.; Wang, Z.; Baig, S.A.; Fang, L.; Hu, B.; Xu, X. Release characteristics and control of nitrogen, phosphate, organic matter from spent mushroom compost amended soil in a column experiment. Process Saf. Environ. Prot. 2015, 98, 417–423. [Google Scholar] [CrossRef]

| Treatment | N 1 (mg dm3) | P 1 (mg dm3) | K 1 (mg dm3) | SMS (g dm3) |
|---|---|---|---|---|
| SMS Dose 1 | ||||
| SMS | N.A. | N.A. | N.A. | 22.75 |
| SMS + S | 300 | 200 | 300 | 22.75 |
| SMS + S + TD 2 | 495 (195) | 200 | 550 (250) | 22.75 |
| SMS Dose 2 | ||||
| SMS | N.A. | N.A. | N.A. | 45.5 |
| SMS + S | 300 | 200 | 300 | 45.5 |
| SMS + S + TD 2 | 495 (195) | 200 | 550 (250) | 45.5 |
| Control | ||||
| NC | N.A. | N.A. | N.A. | N.A. |
| PC 2 | 495 (195) | 200 | 550 (250) | N.A. |
| Analysis of Variance | Source of Variation | Analysis of Variance | Source of Variation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SMS Dose | SMS Management | CV (%) | SMS Dose | SMS Management | CV (%) | ||||
| F-calculated | G | 0.28 ns | 9.93 ** | 8.9 | F-calculated | N leaf | 1.28 ns | 96.27 ** | 11.7 |
| ESI | 0.66 ns | 28.11 ** | 15.8 | P leaf | 0.05 ns | 28.62 ** | 13.2 | ||
| NL | 5.60 * | 29.30 ** | 13.7 | K leaf | 0.09 ns | 6.37 ** | 22.4 | ||
| H | 13.17 ** | 17.00 ** | 10.0 | Ca leaf | 2.45 ns | 1.66 ns | 34.8 | ||
| D | 7.87 ** | 20.65 ** | 17.1 | Mg leaf | 0.26 ns | 3.56 * | 48.1 | ||
| SFM | 13.18 ** | 23.85 ** | 26.1 | S leaf | 2.08 ns | 10.85 ** | 9.9 | ||
| RFM | 7.76 ** | 7.36 ** | 33.1 | P soil | 0.09 ns | 3.58 * | 12.3 | ||
| RL | 0.04 ns | 4.01 ** | 20.5 | K soil | 13.18 ** | 34.25 ** | 19.1 | ||
| SDM | 6.52 * | 7.25 ** | 21.2 | Ca soil | 0.28 ns | 3.37 * | 38.2 | ||
| RDM | 16.07 ** | 16.58 ** | 34.4 | Mg soil | 1.04 ns | 10.45 ** | 13.3 | ||
| LA | 13.13 ** | 25.20 ** | 28.1 | S soil | 0.52 ns | 50.07 ** | 29.5 | ||
| pH | 3.14 ns | 28.14 ** | 3.7 | OM | 1.65 ns | 3.69 * | 17.9 | ||
| EC | 0.61 ns | 57.27 ** | 21.5 | ||||||
| SMS Management | G (%) | ESI | |||
|---|---|---|---|---|---|
| 10 DAS | |||||
| NC | 95.8 ab | 11.5 a | |||
| PC | 79.1 bc | 6.9 b | |||
| SMS | 100.0 a | 11.3 a | |||
| SMS + S | 75.02 c | 4.2 b | |||
| NL (un) | D (mm per plant) | RL (cm per plant) | |||
| 70 DAS | |||||
| NC | 6.2 c | 10.7 c | 31.2 b | ||
| PC | 9.3 a | 16.1 a | 40.6 a | ||
| SMS | 7.6 b | 13.5 b | 36.4 ab | ||
| SMS + S | 9.6 a | 18.0 a | 38.1 ab | ||
| SMS + S + TD | 10.0 a | 16.8 a | 40.3 a | ||
| SMS Dose | G (%) | ESI | NL (un) | D (mm per plant) | RL (cm per plant) |
| 10 DAS | 70 DAS | ||||
| D1 | 88.5 | 8.7 | 8.2 B | 14.2 B | 37.2 |
| D2 | 86.4 | 8.2 | 8.9 A | 15.8 A | 37.5 |
| SMS Management | OM (g dm−3) | P Soil 1 (mg dm−3) | K Soil 1 (mmolc dm−3) | Ca Soil 1 (mmolc dm−3) | Mg Soil 1 (mmolc dm−3) | S Soil (mg dm−3) |
|---|---|---|---|---|---|---|
| NC | 6.8 b | 5.8 ab | 1.8 b | 26.2 ab | 8.9 a | 4.4 c |
| PC | 7.6 ab | 5.4 ab | 2.3 b | 23.1 b | 6.8 c | 3.2 c |
| SMS | 8.1 a | 5.1 b | 2.3 b | 31.1 ab | 9.1 a | 13.2 a |
| SMS + S | 7.9 ab | 5.6 ab | 2.2 b | 32.8 ab | 7.3 bc | 8.5 b |
| SMS + S + TD | 8.0 a | 6.1 a | 4.0 a | 39.2 a | 8.2 ab | 15.9 a |
| SMS Dose | O.M. (g dm−3) | P soil 1 (mg dm−3) | K soil 1 (mmolc dm−3) | Ca soil 1 (mmolc dm−3) | Mg soil 1 (mmolc dm−3) | S soil (mg dm−3) |
| D1 | 7.6 | 5.6 | 2.3 B | 29.7 | 7.9 | 8.8 |
| D2 | 7.9 | 5.7 | 2.8 A | 31.3 | 8.2 | 9.3 |
| SMS Management | N Leaf (g kg−1) | P Leaf (g kg−1) | K Leaf (g kg−1) | Ca Leaf (g kg−1) | Mg Leaf (g kg−1) | S Leaf (g kg−1) |
|---|---|---|---|---|---|---|
| NC | 13.3 c | 1.1 c | 3.5 c | 2.6 | 2.1 ab | 1.2 c |
| PC | 22.4 b | 1.9 a | 4.3 bc | 3.5 | 3.9 a | 1.3 bc |
| SMS | 12.9 c | 1.4 b | 5.0 ab | 3.1 | 2.3 ab | 1.5 a |
| SMS + S | 22.9 b | 1.7 a | 5.5 a | 2.7 | 3.4 ab | 1.4 ab |
| SMS + S + TD | 28.1 a | 1.9 a | 4.5 abc | 3.2 | 1.9 b | 1.5 a |
| SMS Dose | N leaf (g kg−1) | P leaf (g kg−1) | K leaf (g kg−1) | Ca leaf (g kg−1) | Mg leaf (g kg−1) | S leaf (g kg−1) |
| D1 | 19.6 | 1.6 | 4.5 | 2.8 | 2.6 | 1.3 |
| D2 | 20.2 | 1.6 | 4.7 | 3.2 | 2.8 | 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, L.d.S.; Caitano, C.E.C.; Ferrari, S.; Vieira Júnior, W.G.; Heinrichs, R.; de Almeida Moreira, B.R.; Pardo-Giménez, A.; Zied, D.C. Application of Spent Sun Mushroom Substrate in Substitution of Synthetic Fertilizers at Maize Topdressing. Agronomy 2022, 12, 2884. https://doi.org/10.3390/agronomy12112884
Alves LdS, Caitano CEC, Ferrari S, Vieira Júnior WG, Heinrichs R, de Almeida Moreira BR, Pardo-Giménez A, Zied DC. Application of Spent Sun Mushroom Substrate in Substitution of Synthetic Fertilizers at Maize Topdressing. Agronomy. 2022; 12(11):2884. https://doi.org/10.3390/agronomy12112884
Chicago/Turabian StyleAlves, Lucas da Silva, Cinthia Elen Cardoso Caitano, Samuel Ferrari, Wagner Gonçalves Vieira Júnior, Reges Heinrichs, Bruno Rafael de Almeida Moreira, Arturo Pardo-Giménez, and Diego Cunha Zied. 2022. "Application of Spent Sun Mushroom Substrate in Substitution of Synthetic Fertilizers at Maize Topdressing" Agronomy 12, no. 11: 2884. https://doi.org/10.3390/agronomy12112884
APA StyleAlves, L. d. S., Caitano, C. E. C., Ferrari, S., Vieira Júnior, W. G., Heinrichs, R., de Almeida Moreira, B. R., Pardo-Giménez, A., & Zied, D. C. (2022). Application of Spent Sun Mushroom Substrate in Substitution of Synthetic Fertilizers at Maize Topdressing. Agronomy, 12(11), 2884. https://doi.org/10.3390/agronomy12112884







