Effects on Lettuce Yield Parameters and Toxicological Safety Assessment of a Plant-Derived Formulation Based on Rosemary and Eucalyptus Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Compound
2.2. Lettuce Cultivation
2.3. Plant Physiology and Growth Parameters
2.4. Total Phenolics Content and Antioxidant Activity
2.5. Hydrogen Peroxide Content, Lipid Peroxidation, and Antioxidant Enzymes Activity
2.6. Plant Nutrient Content
2.7. Fresh Produce Quality
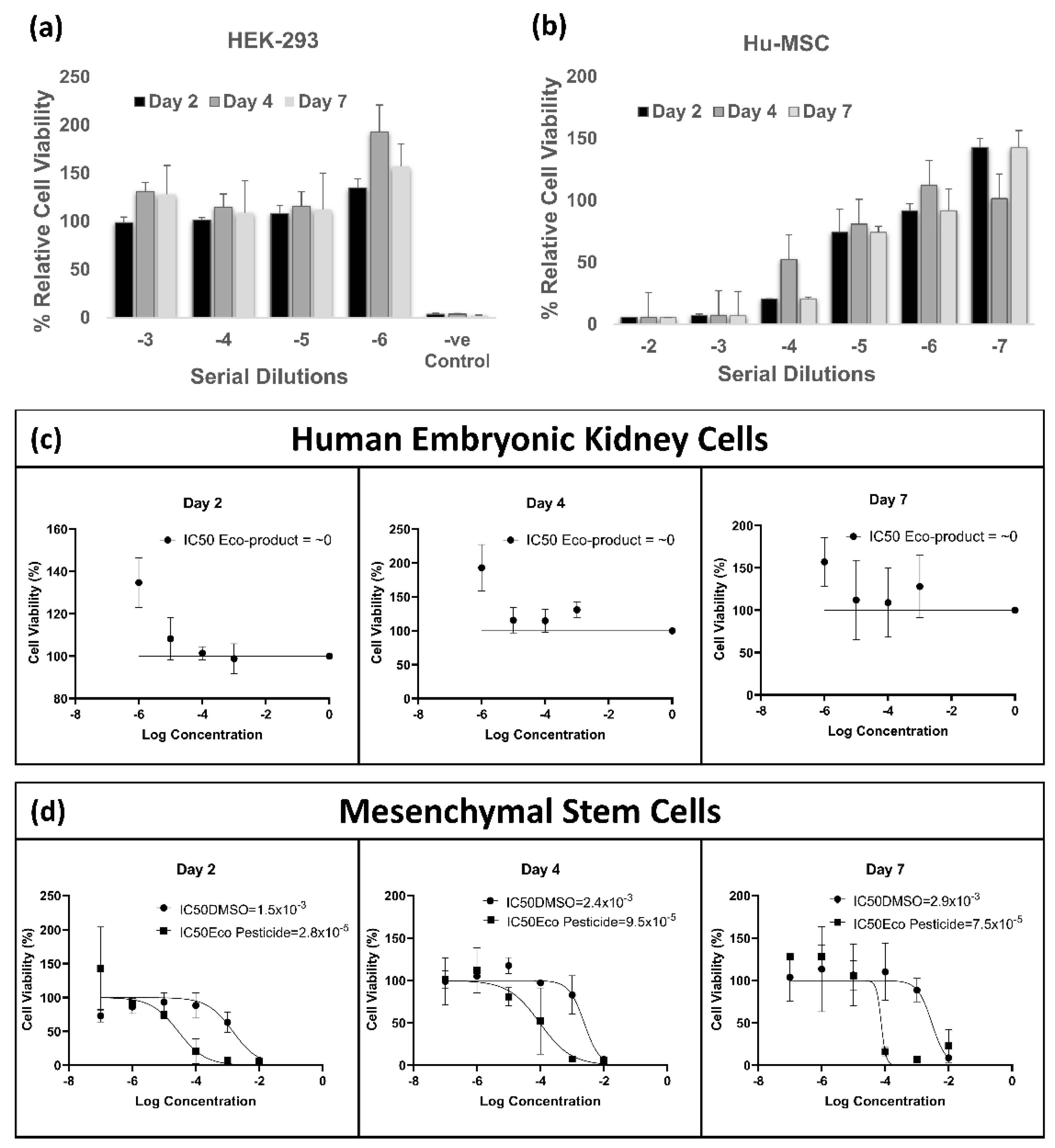
2.8. Cytotoxicity Assay
2.9. Animals
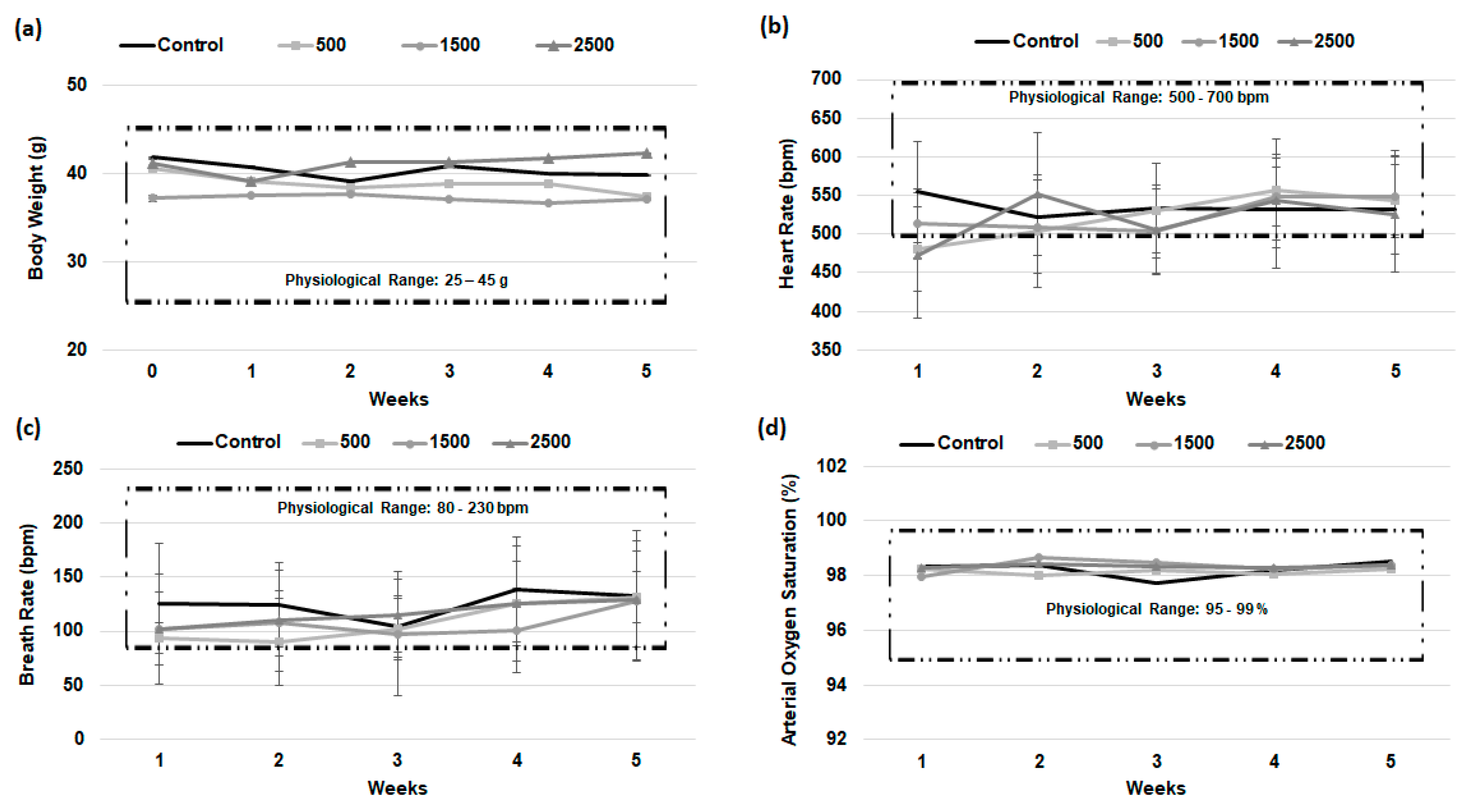
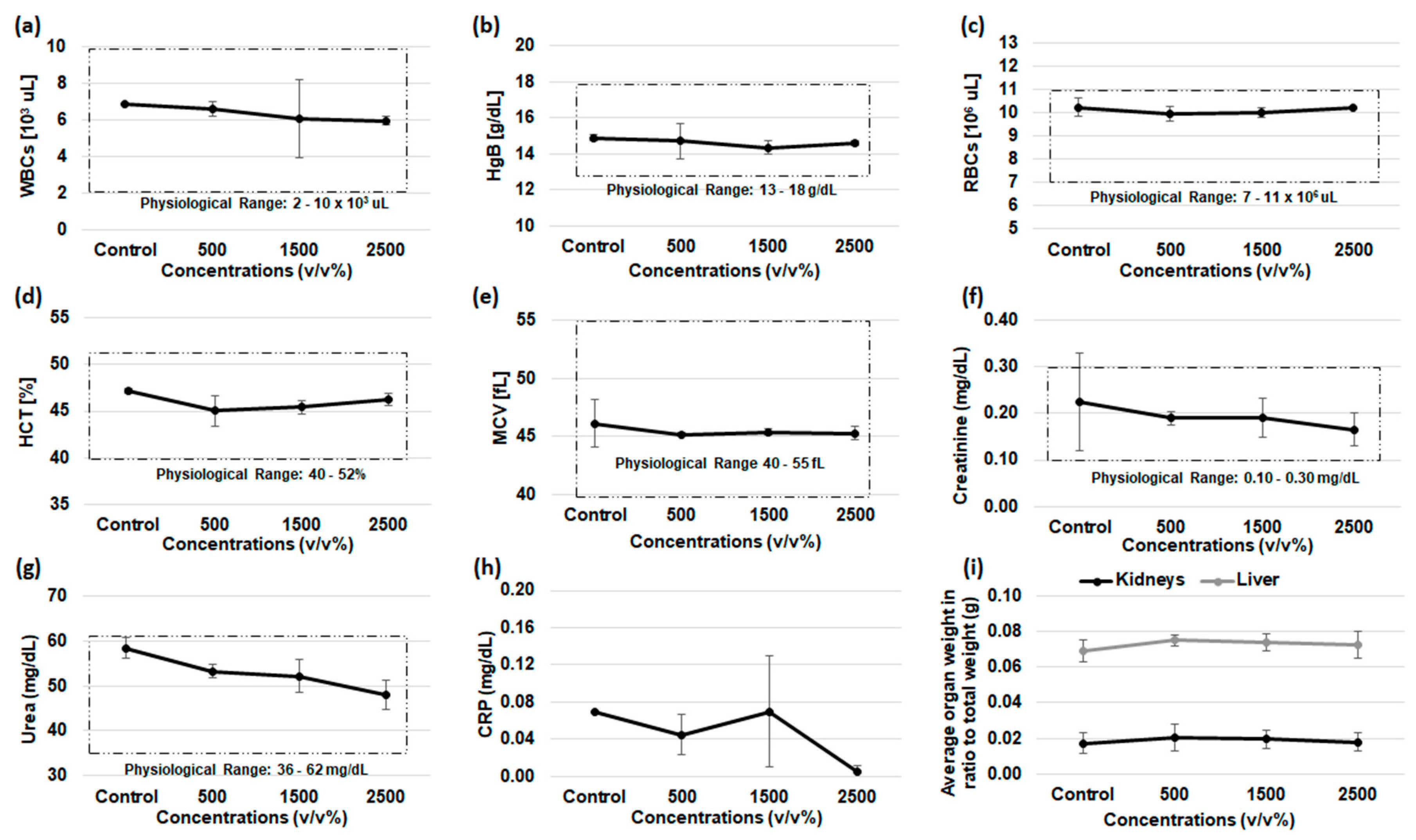
2.10. Acute Oral Toxicity
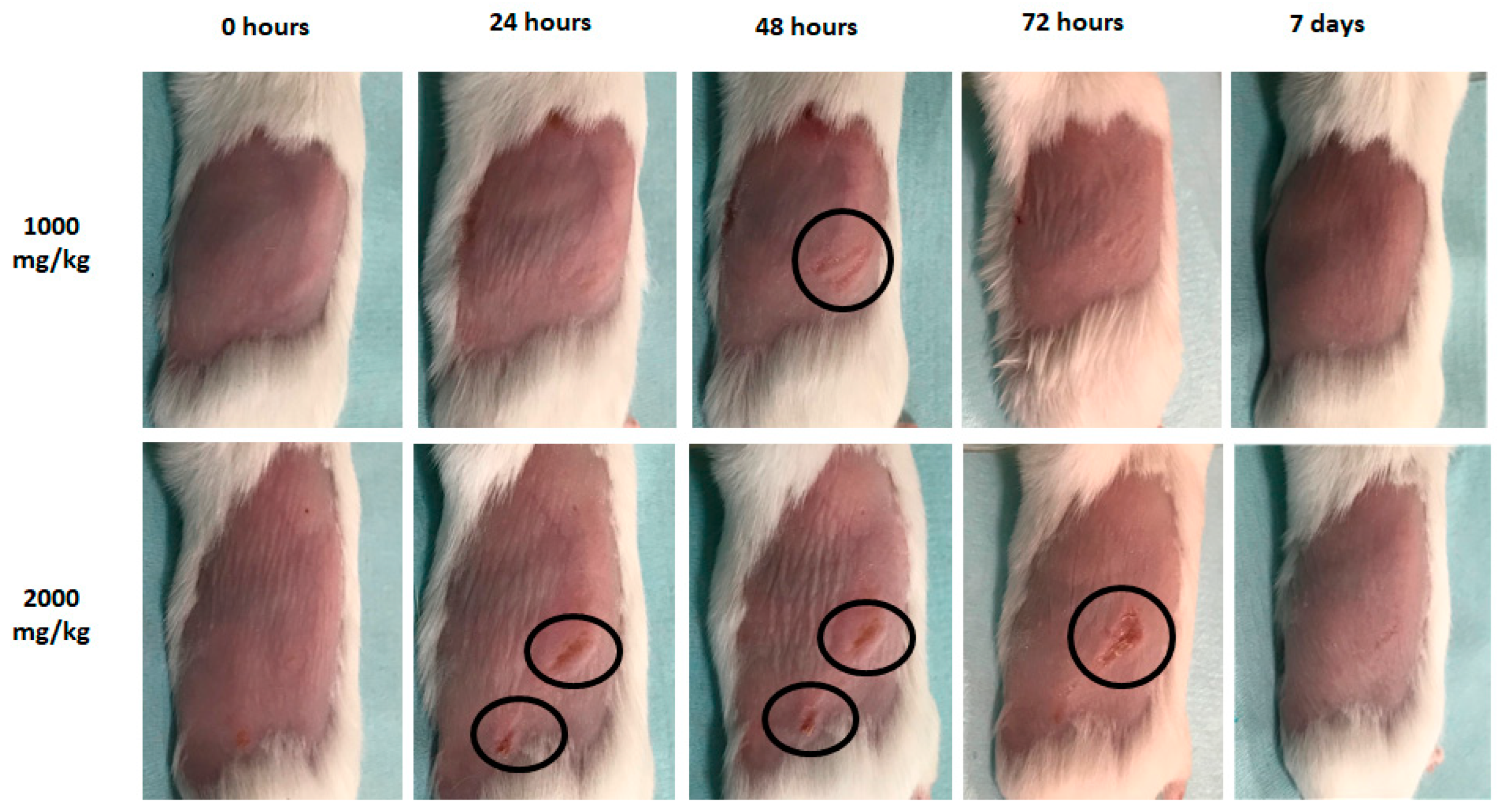
2.11. Acute Dermal Toxicity
2.12. Statistical Analysis
3. Results
3.1. Plant Growth and Physiological Parameters
3.2. Total Phenolic Content and Antioxidant Activity
3.3. Damage Index and Antioxidant Enzyme Activity
3.4. Nutrient Content
3.5. Fresh Produce Quality
3.6. In Vitro Cytotoxicity Assay
3.7. Acute Oral Toxicity
3.8. Acute Dermal toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucini, L.; Colla, G.; Moreno, M.B.M.; Bernardo, L.; Cardarelli, M.; Terzi, V.; Bonini, P.; Rouphael, Y. Inoculation of Rhizoglomus Irregulare or Trichoderma Atroviride Differentially Modulates Metabolite Profiling of Wheat Root Exudates. Phytochemistry 2019, 157, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.; Chrysargyris, A.; Sivakumar, D.; Loulakakis, K. Vapour or Dipping Applications of Methyl Jasmonate, Vinegar and Sage Oil for Pepper Fruit Sanitation towards Grey Mould. Postharvest Biol. Technol. 2016, 118, 120–127. [Google Scholar] [CrossRef]
- Tzortzakis, N. Ozone: A Powerful Tool for the Fresh Produce Preservation. In Postharvest Management Approaches for Maintaining Quality of Fresh Produce; Springer International Publishing: Cham, Switzerland, 2016; pp. 175–207. [Google Scholar]
- Tzortzakis, N.; Singleton, I.; Barnes, J. Deployment of Low-Level Ozone-Enrichment for the Preservation of Chilled Fresh Produce. Postharvest Biol. Technol. 2007, 43, 261–270. [Google Scholar] [CrossRef]
- Meher, S.M.; Bodhankar, S.L.; Kumar, A.; Dhuley, J.N.; Khodape, D.J.; Naik, S.R. Toxicity Studies of Microbial Insecticide Bacillus Thuringiensis Var. Kenyae in Rats, Rabbits, and Fish. Int. J. Toxicol. 2002, 21, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Toward a Sustainable Agriculture Through Plant Biostimulants from Experimental Data to Practical Applications; MDPI AG: Basel, Switzerland, 2020. [Google Scholar]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- EUR-Lex-32019R1009-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583934899523&uri=CELEX:32019R1009 (accessed on 20 September 2022).
- Rouphael, Y.; Colla, G. Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Tortella, G.; Rubilar, O.; Fincheira, P.; Benavides-Mendoza, A. Biostimulation and Toxicity: The Magnitude of the Impact of Nanomaterials in Microorganisms and Plants. J. Adv. Res. 2021, 31, 113–126. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Tsige, A.A.; Opara, U.L.; Fawole, O.A. Application of Gum Arabic and Methyl Cellulose Coatings Enriched with Thyme Oil to Maintain Quality and Extend Shelf Life of “Acco” Pomegranate Arils. Plants 2020, 9, 1690. [Google Scholar] [CrossRef]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and Safety Attributes on Shredded Carrots by Using Origanum Majorana and Ascorbic Acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Stavropoulou, A.; Loulakakis, K.; Magan, N.; Tzortzakis, N. Origanum Dictamnus Oil Vapour Suppresses the Development of Grey Mould in Eggplant Fruit In Vitro. BioMed Res. Int. 2014, 2014, 562679. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.; Xylia, P.; Chrysargyris, A. Sage Essential Oil Improves the Effectiveness of Aloe Vera Gel on Postharvest Quality of Tomato Fruit. Agronomy 2019, 9, 635. [Google Scholar] [CrossRef]
- Mossa, A.-T.H.; Mohafrash, S.M.M.; Chandrasekaran, N. Safety of Natural Insecticides: Toxic Effects on Experimental Animals. BioMed Res. Int. 2018, 2018, 4308054. [Google Scholar] [CrossRef]
- Bullangpoti, V.; Mujchariyakul, W.; Laksanavilat, N.; Junhirun, P. Acute Toxicity of Essential Oil Compounds (Thymol and 1,8-Cineole) to Insectivorous Guppy, Poecilia Reticulata Peters, 1859. Agric. Nat. Resour. 2018, 52, 190–194. [Google Scholar] [CrossRef]
- Xylia, P.; Ioannou, I.; Chrysargyris, A.; Stavrinides, M.C.; Tzortzakis, N. Quality Attributes and Storage of Tomato Fruits as Affected by an Eco-Friendly, Essential Oil-Based Product. Plants 2021, 10, 1125. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Charalambous, S.; Xylia, P.; Litskas, V.; Stavrinides, M.; Tzortzakis, N. Assessing the Biostimulant Effects of a Novel Plant-Based Formulation on Tomato Crop. Sustainability 2020, 12, 8432. [Google Scholar] [CrossRef]
- Lucarini, M.; D’Evoli, L.; Tufi, S.; Gabrielli, P.; Paoletti, S.; di Ferdinando, S.; Lombardi-Boccia, G. Influence of Growing System on Nitrate Accumulation in Two Varieties of Lettuce and Red Radicchio of Treviso. J. Sci. Food Agric. 2012, 92, 2796–2799. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikolaidou, E.; Stamatakis, A.; Tzortzakis, N. Vegetative, Physiological, Nutritional and Antioxidant Behavior of Spearmint (Mentha spicata L.) in Response to Different Nitrogen Supply in Hydroponics. J. Appl. Res. Med. Aromat. Plants 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Borland, A.; Singleton, I.; Barnes, J. Impact of Atmospheric Ozone-Enrichment on Quality-Related Attributes of Tomato Fruit. Postharvest Biol. Technol. 2007, 45, 317–325. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The Combined and Single Effect of Salinity and Copper Stress on Growth and Quality of Mentha Spicata Plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents; OECD: Paris, France, 2008. [Google Scholar]
- OECD. Test No. 402: Acute Dermal Toxicity; OECD: Paris, France, 2017. [Google Scholar]
- Ewald, A.J.; Werb, Z.; Egeblad, M. Monitoring of Vital Signs for Long-Term Survival of Mice under Anesthesia: FIGURE 1. Cold Spring Harb. Protoc. 2011, 2011, pdb.prot5563. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Zhao, X.; Gao, S.; Hong, C.; Vatner, D.E.; Vatner, S.F. Heart Rate and Electrocardiography Monitoring in Mice. Curr. Protoc. Mouse Biol. 2011, 1, 123–139. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Appraisal of the Safety of Chemicals in Foods, Drugs, and Cosmetics; Association of Food & Drug Officials of the United States: Montgomery, MA, USA, 1959. [Google Scholar]
- ECETOC. TR 066—Skin Irritation and Corrosion: Reference Chemicals Data Bank; Technical Report No.66; ECTOC: Brussels, Belgium, 1995. [Google Scholar]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of Seaweed Extracts From Laminaria and Ascophyllum nodosum Spp. as Biostimulants in Zea mays L. Using a Combination of Chemical, Biochemical and Morphological Approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef]
- Markets and Markets. Biostimulants Market by Active Ingredient (Humic Substances, Amino Acids, Seaweed Extracts, Microbial Amendments), Crop Type (Fruits & Vegetables, Cereals, Turf & Ornamentals), Application Method, Form and Region—Global Forecast to 2026. Available online: https://www.marketsandmarkets.com/Market-Reports/biostimulant-market-1081.html?gclid=CjwKCAiA68ebBhB-EiwALVC-NgJ5E9kFF6s8qezM-o_iNap1s0zck8Q9GCjrCQavF43bxQ382khVHhoCqdkQAvD_BwE (accessed on 30 June 2022).
- de Sousa Silva, T.; Silva, A.P.S.E.; de Almeida Santos, A.; Ribeiro, K.G.; de Souza, D.C.; Bueno, P.A.A.; Marques, M.M.M.; de Almeida, P.M.; Peron, A.P. Cytotoxicity, Genotoxicity, and Toxicity of Plant Biostimulants Produced in Brazil: Subsidies for Determining Environmental Risk to Non-Target Species. Water Air Soil Pollut. 2020, 231, 233. [Google Scholar] [CrossRef]
- Rawi, S.M.; Seif Al Nassr, F.M. Zinc Sulphate and Vitamin E Alleviate Reproductive Toxicity Caused by Aluminium Sulphate in Male Albino Rats. Toxicol. Ind. Health 2015, 31, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, A.P.; Everds, N. Haematology of the Mouse. In The Laboratory Mouse, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 331–347. [Google Scholar]
- Wquimby, F.; Hluong, R. Clinical Chemistry of the Laboratory Mouse. In The Mouse in Biomedical Research; Elsevier: Amsterdam, The Netherlands, 2007; pp. 171–216. [Google Scholar]
- Rodenak-Kladniew, B.; Castro, A.; Stärkel, P.; Galle, M.; Crespo, R. 1,8-Cineole Promotes G0/G1 Cell Cycle Arrest and Oxidative Stress-Induced Senescence in HepG2 Cells and Sensitizes Cells to Anti-Senescence Drugs. Life Sci. 2020, 243, 117271. [Google Scholar] [CrossRef]
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Yoshimura, Y.; Suzuki, M.; Shuto, K.; Ohkohchi, N.; et al. Antitumor Effect of 1, 8-Cineole against Colon Cancer. Oncol. Rep. 2013, 30, 2647–2652. [Google Scholar] [CrossRef]
- Soltanian, S.; Mohamadi, N.; Rajaei, P.; Khodami, M.; Mohammadi, M. Phytochemical Composition, and Cytotoxic, Antioxidant, and Antibacterial Activity of the Essential Oil and Methanol Extract of Semenovia Suffruticosa. Avicenna J. Phytomed. 2019, 9, 143–152. [Google Scholar]
- Juergens, U. Anti-Inflammatory Properties of the Monoterpene 1.8-Cineole: Current Evidence for Co-Medication in Inflammatory Airway Diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Simsek, M.; Duman, R. Investigation of Effect of 1,8-Cineole on Antimicrobial Activity of Chlorhexidine Gluconate. Pharmacogn. Res. 2017, 9, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Xylia, P.; Chrysargyris, A.; Ahmed, Z.F.R.; Tzortzakis, N. Application of Rosemary and Eucalyptus Essential Oils and Their Main Component on the Preservation of Apple and Pear Fruits. Horticulturae 2021, 7, 479. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.A.; Rao, V.S.N. Mast Cell Involvement in the Rat Paw Oedema Response to 1,8-Cineole, the Main Constituent of Eucalyptus and Rosemary Oils. Eur. J. Pharmacol. 1997, 331, 253–258. [Google Scholar] [CrossRef]
- Santos, F.A.; Rao, V.S.N. Inflammatory Edema Induced by 1,8-Cineole in the Hindpaw of Rats: A Model for Screening Antiallergic and Anti-Inflammatory Compounds. Phytomedicine 1998, 5, 115–119. [Google Scholar] [CrossRef]
- Santos, F.A.; Silva, R.M.; Tomé, A.R.; Rao, V.S.N.; Pompeu, M.M.L.; Teixeira, M.J.; de Freitas, L.A.R.; de Souza, V.L. 1,8-Cineole Protects against Liver Failure in an in-Vivo Murine Model of Endotoxemic Shock. J. Pharm. Pharmacol. 2010, 53, 505–511. [Google Scholar] [CrossRef]
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Eighth Revised Edition. New York and Geneva, 2019. Available online: https://unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev08/ST-SG-AC10-30-Rev8e.pdf (accessed on 30 June 2022).
| Plant Growth | Control | CP | EP-1x | EP-2x |
|---|---|---|---|---|
| Leaf length (cm) | 27.75 ± 0.64a | 27.83 ± 0.38a | 26.92 ± 0.47ab | 25.83 ± 0.46b |
| Leaf number | 21.00 ± 1.18a | 20.83 ± 1.01a | 22.67 ± 0.61a | 21.00 ± 0.37a |
| Plant biomass (g) | 116.44 ± 9.17b | 111.29 ± 7.41b | 140.42 ± 6.91a | 112.55 ± 6.83b |
| Plant dry weight (g) | 7.16 ± 0.55a | 7.38 ± 0.71a | 7.76 ± 0.37a | 7.32 ± 0.35a |
| Stomatal conductance (s cm−1) | 1.22 ± 0.04c | 1.34 ± 0.05bc | 1.41 ± 0.04ab | 1.50 ± 0.07a |
| Chlorophyll fluorescence (Fv/Fm) | 0.812 ± 0.004a | 0.813 ± 0.002a | 0.803 ± 0.007a | 0.812 ± 0.004a |
| Chlorophyll a (mg g−1 Fw) | 0.92 ± 0.03ab | 0.97 ± 0.02a | 0.86 ± 0.05b | 0.91 ± 0.02ab |
| Chlorophyll b (mg g−1 Fw) | 0.26 ± 0.01ab | 0.27 ± 0.01a | 0.24 ± 0.01b | 0.25 ± 0.01ab |
| Total Chlorophylls (mg g−1 Fw) | 1.17 ± 0.04ab | 1.24 ± 0.03a | 1.10 ± 0.06b | 1.16 ± 0.02ab |
| Control | CP | EP-1x | EP-2x | |
|---|---|---|---|---|
| Total phenols (μmol GAE g−1 Fw) | 1.69 ± 0.06a | 1.14 ± 0.11b | 1.05 ± 0.04b | 1.49 ± 0.12a |
| DPPH (mg Trolox g−1 Fw) | 2.55 ± 0.12a | 1.78 ± 0.12b | 1.86 ± 0.10b | 2.45 ± 0.21a |
| FRAP (mg Trolox g−1 Fw) | 1.86 ± 0.11a | 1.04 ± 0.12b | 1.08 ± 0.05b | 1.68 ± 0.12a |
| MDA (nmol g−1) | 10.91 ± 0.37b | 12.30 ± 0.37a | 12.43 ± 0.63a | 12.38 ± 0.45a |
| H2O2 content (μmol g−1) | 0.12 ± 0.00c | 0.11 ± 0.01c | 0.29 ± 0.01a | 0.25 ± 0.00b |
| CAT (unit mg protein−1) | 30.85 ± 3.71b | 37.85 ± 0.78a | 29.43 ± 1.37b | 29.44 ± 2.89b |
| SOD (unit mg protein−1) | 3.19 ± 0.2c | 4.52 ± 0.19a | 3.77 ± 0.05bc | 3.89 ± 0.48ab |
| POD (unit mg protein−1) | 0.67 ± 0.01c | 1.03 ± 0.01a | 0.60 ± 0.02d | 0.87 ± 0.02b |
| N (g kg−1 Dw) | 29.58 ± 1.41a | 28.18 ± 2.44a | 32.23 ± 0.88a | 27.42 ± 1.46a |
| K (g kg−1 Dw) | 42.04 ± 3.54a | 42.99 ± 2.38a | 40.55 ± 2.84a | 42.35 ± 1.08a |
| P (g kg−1 Dw) | 3.75 ± 0.06ab | 4.08 ± 0.14a | 3.60 ± 0.11b | 3.70 ± 0.16ab |
| Ca (g kg−1 Dw) | 8.66 ± 0.48a | 8.23 ± 0.61a | 10.75 ± 0.30a | 8.44 ± 0.56a |
| Mg (g kg−1 Dw) | 6.88 ± 0.14a | 6.71 ± 0.31a | 6.65 ± 0.11a | 6.88 ± 0.14a |
| Na (g kg−1 Dw) | 8.66 ± 0.48b | 8.23 ± 0.61b | 10.75 ± 0.30a | 8.44 ± 0.56b |
| Quality Attributes | Control | CP | EP-1x | EP-2x |
|---|---|---|---|---|
| TSS (°Brix) | 5.51 ± 0.10b | 6.16 ± 0.40a | 6.40 ± 0.05a | 6.30 ± 0.06a |
| TA (malic acid g kg−1) | 0.50 ± 0.002ab | 0.39 ± 0.062b | 0.58 ± 0.031a | 0.38 ± 0.033b |
| AA (mg 100 g−1 Fw) | 6.33 ± 0.17a | 5.35 ± 0.46b | 4.50 ± 0.47b | 4.84 ± 0.11b |
| Colour L* | 41.14 ± 0.77b | 45.22 ± 0.59a | 42.72 ± 0.36b | 42.85 ± 0.74b |
| Colour a* | −16.52 ± 0.45a | −18.46 ± 0.30b | −16.94 ± 0.12a | −17.00 ± 0.21a |
| Colour b* | 23.39 ± 1.04b | 27.92 ± 0.95a | 23.88 ± 0.36b | 25.04 ± 0.38b |
| Hue | 125.33 ± 0.55a | 123.54 ± 0.51b | 125.35 ± 0.26a | 124.17 ± 0.17ab |
| Chroma | 28.64 ± 1.10b | 33.48 ± 0.95a | 29.28 ± 0.35b | 30.26 ± 0.43b |
| Colour index | −17.3 ± 0.67c | −14.7 ± 0.45a | −16.62 ± 0.21bc | −15.88 ± 0.34ab |
| Marketability (1–10) | 9.17 ± 0.22a | 7.22 ± 0.14c | 8.83 ± 0.14a | 8.11 ± 0.14b |
| Appearance (1–10) | 8.89 ± 0.21a | 7.00 ± 0.24c | 8.61 ± 0.13ab | 8.28 ± 0.06b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapnisis, K.; Chrysargyris, A.; Prokopi, M.; Varda, E.; Kokkinidou, D.; Samourides, A.; Xylia, P.; Onisiforou, P.; Stavrinides, M.; Tzortzakis, N.; et al. Effects on Lettuce Yield Parameters and Toxicological Safety Assessment of a Plant-Derived Formulation Based on Rosemary and Eucalyptus Essential Oils. Agronomy 2022, 12, 2861. https://doi.org/10.3390/agronomy12112861
Kapnisis K, Chrysargyris A, Prokopi M, Varda E, Kokkinidou D, Samourides A, Xylia P, Onisiforou P, Stavrinides M, Tzortzakis N, et al. Effects on Lettuce Yield Parameters and Toxicological Safety Assessment of a Plant-Derived Formulation Based on Rosemary and Eucalyptus Essential Oils. Agronomy. 2022; 12(11):2861. https://doi.org/10.3390/agronomy12112861
Chicago/Turabian StyleKapnisis, Konstantinos, Antonios Chrysargyris, Marianna Prokopi, Eleni Varda, Despoina Kokkinidou, Andreas Samourides, Panayiota Xylia, Pavlina Onisiforou, Menelaos Stavrinides, Nikolaos Tzortzakis, and et al. 2022. "Effects on Lettuce Yield Parameters and Toxicological Safety Assessment of a Plant-Derived Formulation Based on Rosemary and Eucalyptus Essential Oils" Agronomy 12, no. 11: 2861. https://doi.org/10.3390/agronomy12112861
APA StyleKapnisis, K., Chrysargyris, A., Prokopi, M., Varda, E., Kokkinidou, D., Samourides, A., Xylia, P., Onisiforou, P., Stavrinides, M., Tzortzakis, N., & Anayiotos, A. (2022). Effects on Lettuce Yield Parameters and Toxicological Safety Assessment of a Plant-Derived Formulation Based on Rosemary and Eucalyptus Essential Oils. Agronomy, 12(11), 2861. https://doi.org/10.3390/agronomy12112861








