The Composition of Dissolved Organic Matter in Arable Lands: Does Soil Management Practice Matter?
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Soil Organic Matter Extraction
2.4. Spectroscopic Measurement of DOM Composition
2.5. Soil Water Content, Air Temperature, and Precipitation Monitoring
2.6. Statistical Analysis
3. Results and Discussion
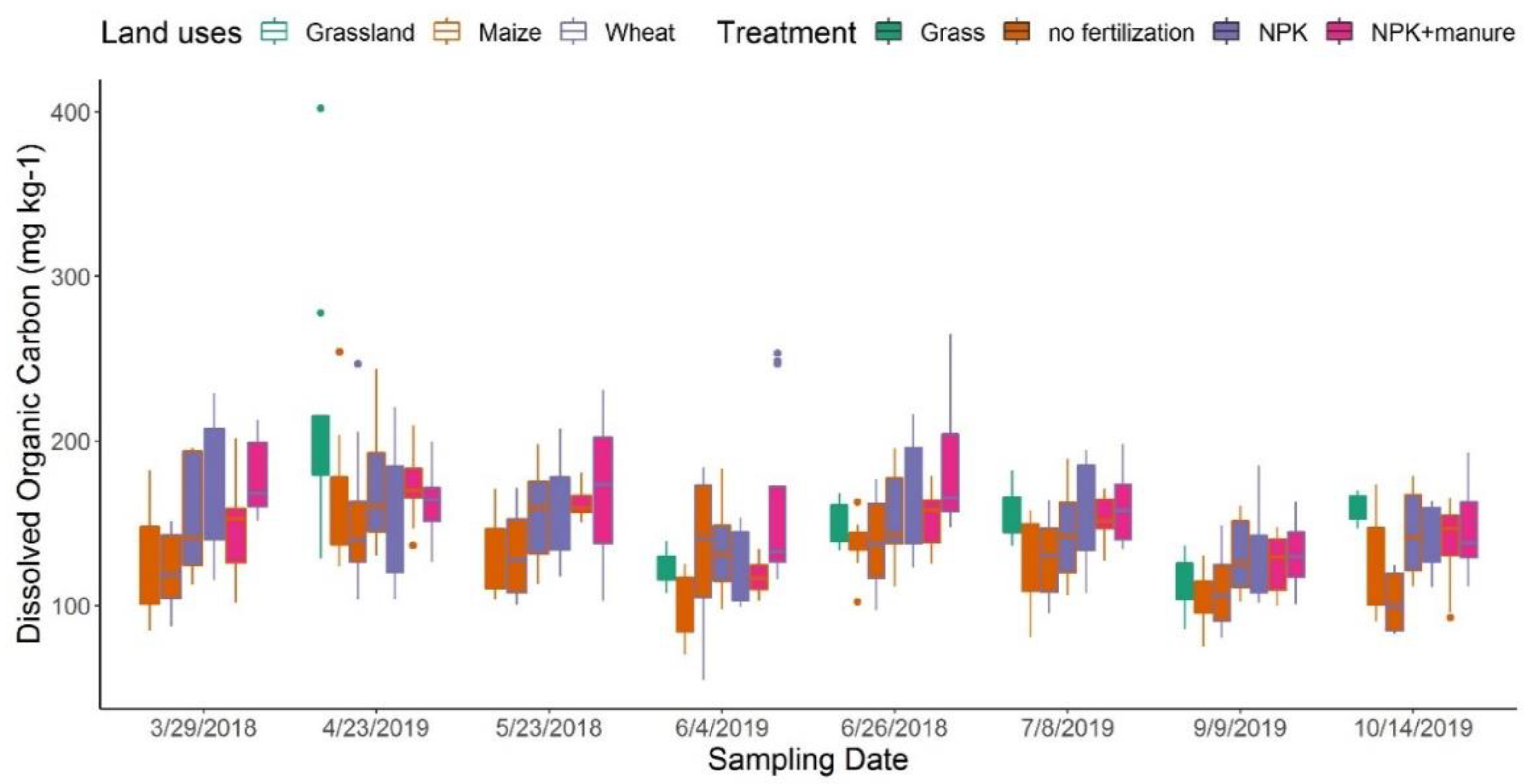
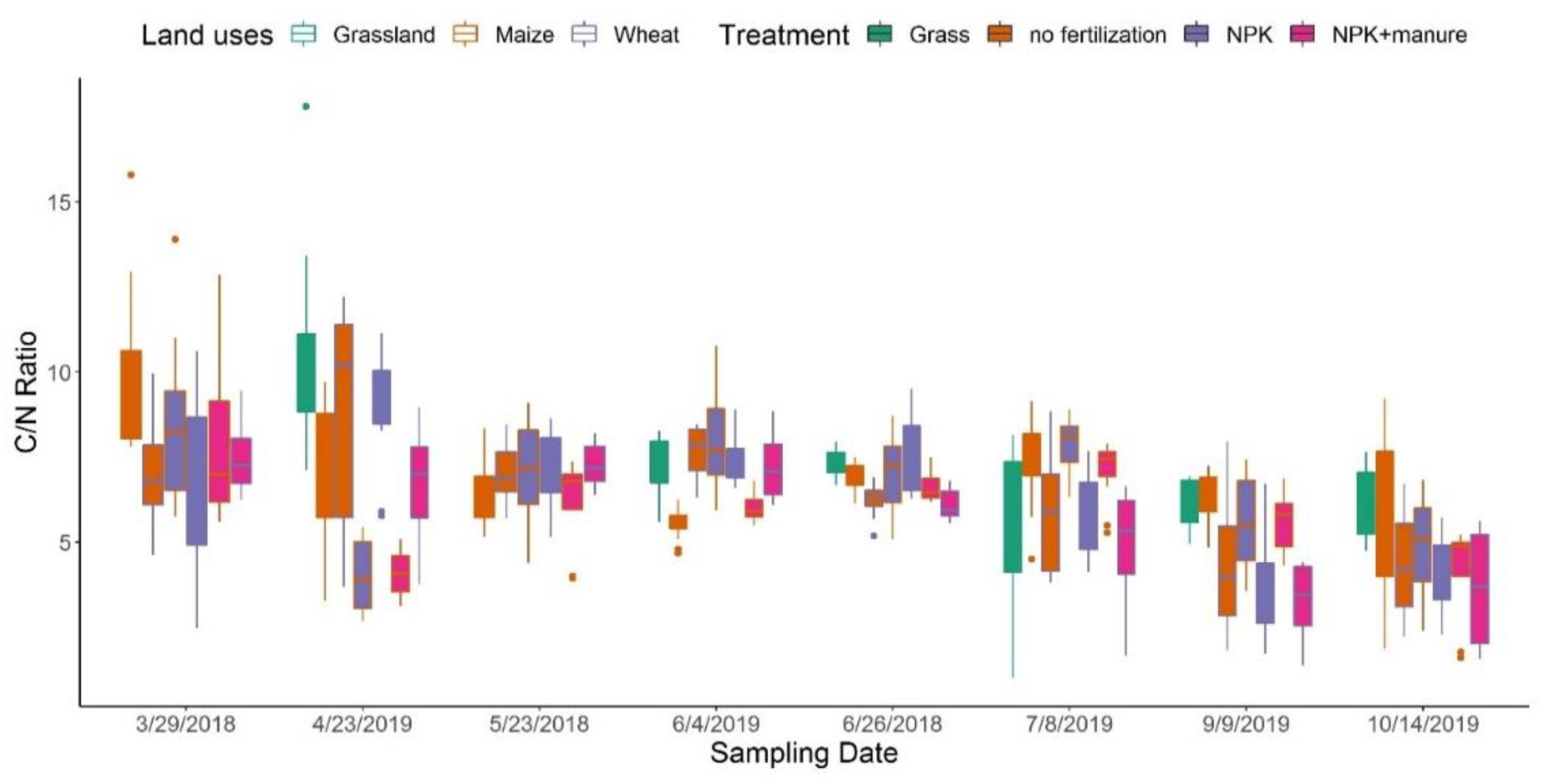
3.1. DOC Contents and DOM Composition across Sampling Dates
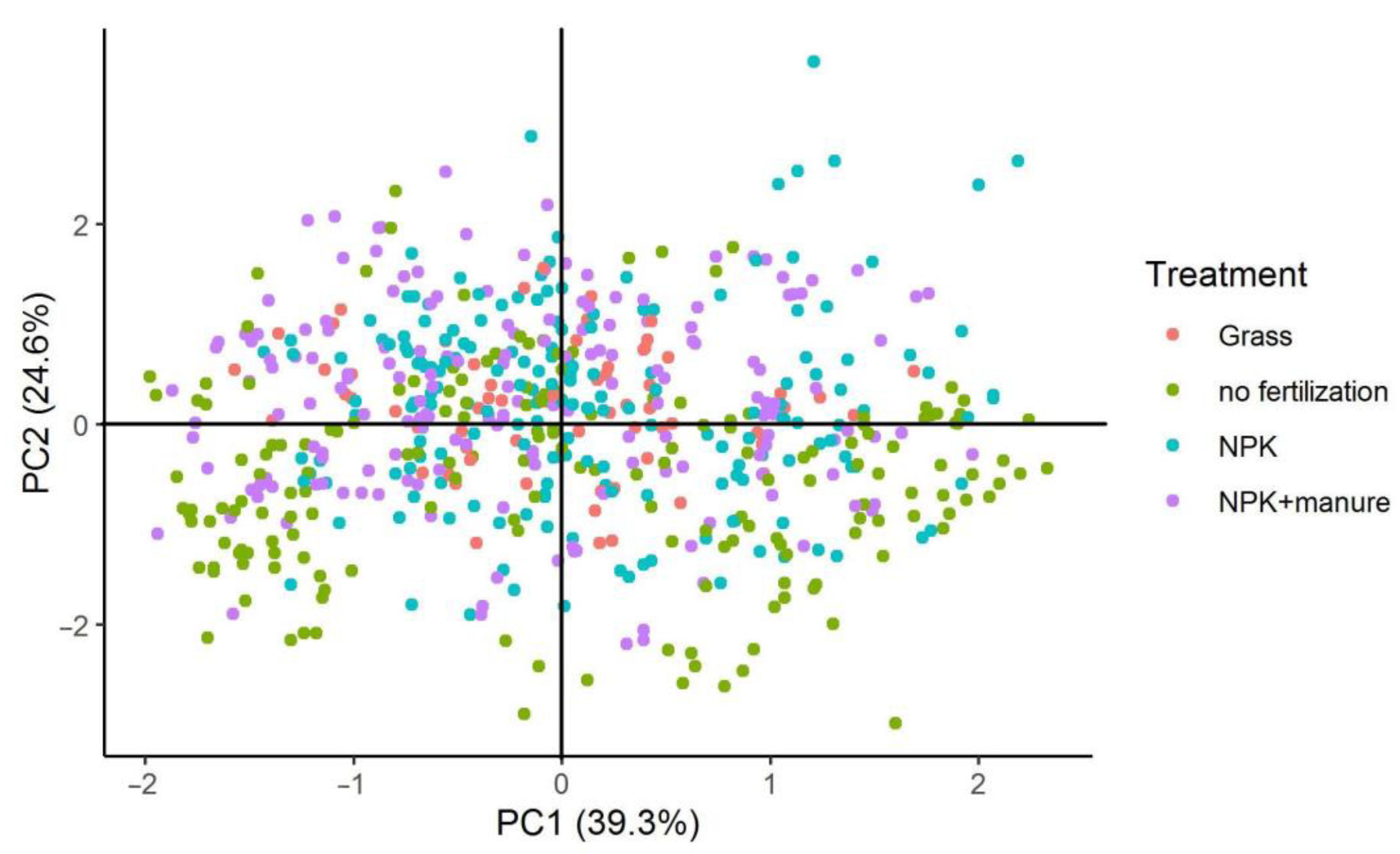
3.2. Effects of Land Use and Fertilization on DOM
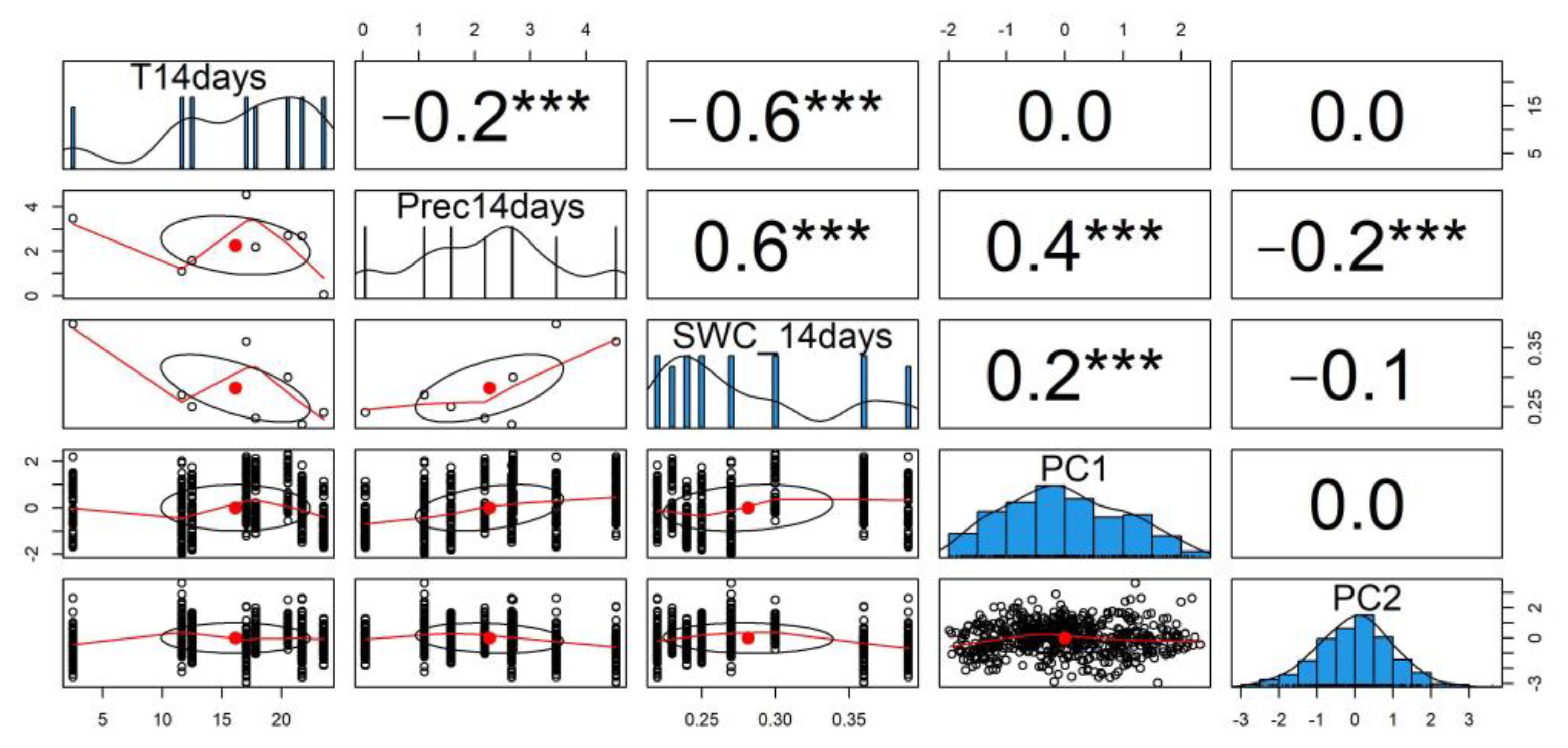
3.3. Effect of Weather Parameters on DOM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, M.; Leifeld, J.; Schmidt, M.W.I.; Smith, P.; Fuhrer, J. Measured soil organic matter fractions can be related to pools in the RothC model. Eur. J. Soil Sci. 2007, 58, 658–667. [Google Scholar] [CrossRef]
- Herbert, B.E.; Bertsch, P.M. Characterization of dissolved and colloidal organic matter in soil solution: A review. Carbon Funct. For. Soils 1995, 63–88. [Google Scholar] [CrossRef]
- von Lützow, M.; Ko, I.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Guggenberger, G.; Zech, W.; Schulten, H.R. Formation and mobilization pathways of dissolved organic matter: Evidence from chemical structural studies of organic matter fractions in acid forest floor solutions. Org. Geochem. 1994, 21, 51–66. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Carter, M.R.; Angers, D.A.; Monreal, C.M.; Ellert, B.H. Towards a minimum data set to assess soil organic matter quality in agricultural soils. Can. J. Soil Sci. 1994, 74, 367–385. [Google Scholar] [CrossRef]
- Silveira, M.L.A. Carbono orgânico dissolvido e biodisponibilidade de N e P como indicadores de qualidade do solo. Sci. Agric. 2005, 62, 502–508. [Google Scholar] [CrossRef]
- Dai, K.H.; David, M.B.; Vance, G.F. Characterization of solid and dissolved carbon in a spruce-fir spodosol. Biogeochemistry 1996, 35, 339–365. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G.; Haumaier, L.; Zech, W. Seasonal variations in the chemical composition of dissolved organic matter in organic forest floor layer leachates of old-growth Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) stands in northeastern Bavaria, Germany. Biogeochemistry 2001, 55, 103–143. [Google Scholar] [CrossRef]
- Tipping, E.; Woof, C.; Rigg, E.; Harrison, A.F.; Ineson, P.; Taylor, K.; Benham, D.; Poskitt, J.; Rowland, A.P.; Bol, R.; et al. Climatic influences on the leaching of dissolved organic matter from upland UK moorland soils, investigated by a field manipulation experiment. Environ. Int. 1999, 25, 83–95. [Google Scholar] [CrossRef]
- Angers, D.A.; Chantigny, M.H.; Rochette, P.; Gagnon, B. Dynamics of soil water-extractable organic C following application of dairy cattle manures. Can. J. Soil Sci. 2006, 86, 851–858. [Google Scholar] [CrossRef]
- Zsolnay, A.; Görlitz, H. Water extractable organic matter in arable soils: Effects of drought and long-term fertilization. Soil Biol. Biochem. 1994, 26, 1257–1261. [Google Scholar] [CrossRef]
- Embacher, A.; Zsolnay, A.; Gattinger, A.; Munch, J.C. The dynamics of water extractable organic matter (WEOM) in common arable topsoils: I. Quantity, quality and function over a three year period. Geoderma 2007, 139, 11–22. [Google Scholar] [CrossRef]
- Gmach, M.R.; Cherubin, M.R.; Kaiser, K.; Cerri, C.E.P. Processes that influence dissolved organic matter in the soil: A review. Sci. Agric. 2020, 77, 1–10. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile organic matter as an indicator of organic matter quality. Soil Biol. Biochem. 2000, 32, 211–219. [Google Scholar] [CrossRef]
- Kahlon, M.S.; Lal, R.; Ann-Varughese, M. Twenty two years of tillage and mulching impacts on soil physical characteristics and carbon sequestration in Central Ohio. Soil Tillage Res. 2013, 126, 151–158. [Google Scholar] [CrossRef]
- Weidhuner, A.; Hanauer, A.; Krausz, R.; Crittenden, S.J.; Gage, K.; Sadeghpour, A. Tillage impacts on soil aggregation and aggregate-associated carbon and nitrogen after 49 years. Soil Tillage Res. 2021, 208, 104878. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Aiken, G.R.; Butler, K.D.; Guillemette, F.; Podgorski, D.C.; Spencer, R.G.M. DOM composition and transformation in boreal forest soils: The effects of temperature and organic-horizon decomposition state. J. Geophys. Res. Biogeosci. 2016, 121, 2727–2744. [Google Scholar] [CrossRef]
- Suleymanov, A.; Gabbasova, I.; Suleymanov, R.; Abakumov, E.; Polyakov, V.; Liebelt, P. Mapping soil organic carbon under erosion processes using remote sensing. Hung. Geogr. Bull. 2021, 70, 49–64. [Google Scholar] [CrossRef]
- Wilson, H.F.; Xenopoulos, M.A. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2009, 2, 37–41. [Google Scholar] [CrossRef]
- Mayer, Z.; Sasvári, Z.; Szentpéteri, V.; Peth Oné Rétháti, B.; Vajna, B.; Posta, K. Effect of Long-Term Cropping Systems on the Diversity of the Soil Bacterial Communities. Agronomy 2019, 9, 878. [Google Scholar] [CrossRef]
- Berzsenyi, Z.; Győrffy, B.; Lap, D. Effect of crop rotation and fertilisation on maize and wheat yields and yield stability in a long-term experiment. Eur. J. Agron. 2000, 13, 225–244. [Google Scholar] [CrossRef]
- Ujvári, G.; Borsodi, A.K.; Megyes, M.; Mucsi, M.; Szili-Kovács, T.; Szabó, A.; Szalai, Z.; Jakab, G.; Márialigeti, K. Comparison of soil bacterial communities from juvenile maize plants of a long-term monoculture and a natural grassland. Agronomy 2020, 10, 341. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and water-extractable organic matter in soils: A review on the influence of land use and management practices. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Shepherd, M.; Bhogal, A.; Barrett, G.; Dyer, C. Dissolved organic nitrogen in agricultural soils: Effects of sample preparation on measured values. Commun. Soil Sci. Plant Anal. 2001, 32, 1523–1542. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Birdwell, J.E.; Engel, A.S. Characterization of dissolved organic matter in cave and spring waters using UV–Vis absorbance and fluorescence spectroscopy. Org. Geochem. 2010, 41, 270–280. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Anderson, D.T. Spectroflourometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef]
- Mann, H.; Whitney, D. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Mathemetical Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Jakab, G.; Vancsik, A.; Filep, T.; Madarász, B.; Zacháry, D.; Ringer, M.; Ujházy, N.; Szalai, Z. Soil organic matter characterisation using alkali and water extraction, and its relation to soil properties. Geoderma Reg. 2022, 28, e00469. [Google Scholar] [CrossRef]
- Filep, T.; Rékási, M. Factors controlling dissolved organic carbon (DOC), dissolved organic nitrogen (DON) and DOC/DON ratio in arable soils based on a dataset from Hungary. Geoderma 2011, 162, 312–318. [Google Scholar] [CrossRef]
- Cattell, R.B. The Scree Test For The Number Of Factors. Multivar. Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef]
- Hatvani, I.G.; Kirschner, A.K.T.; Farnleitner, A.H.; Tanos, P.; Herzig, A. Hotspots and main drivers of fecal pollution in Neusiedler See, a large shallow lake in Central Europe. Environ. Sci. Pollut. Res. 2018, 25, 28884–28898. [Google Scholar] [CrossRef]
- Kaiser An index of factorial simplicity. Psychometrika 1974, 39, 31–36. [CrossRef]
- van den Berg, L.J.L.; Shotbolt, L.; Ashmore, M.R. Dissolved organic carbon (DOC) concentrations in UK soils and the influence of soil, vegetation type and seasonality. Sci. Total Environ. 2012, 427–428, 269–276. [Google Scholar] [CrossRef]
- Wickland, K.P.; Neff, J.C.; Aiken, G.R. Dissolved organic carbon in Alaskan boreal forest: Sources, chemical characteristics, and biodegradability. Ecosystems 2007, 10, 1323–1340. [Google Scholar] [CrossRef]
- Neff, J.C.; Hooper, D.U. Vegetation and climate controls on potential CO2, DOC and DON production in northern latitude soils. Glob. Chang. Biol. 2002, 8, 872–884. [Google Scholar] [CrossRef]
- Akagi, J.; Zsolnay, Á.; Bastida, F. Quantity and spectroscopic properties of soil dissolved organic matter (DOM) as a function of soil sample treatments: Air-drying and pre-incubation. Chemosphere 2007, 69, 1040–1046. [Google Scholar] [CrossRef]
- Alletto, L.; Coquet, Y. Temporal and spatial variability of soil bulk density and near-saturated hydraulic conductivity under two contrasted tillage management systems. Geoderma 2009, 152, 85–94. [Google Scholar] [CrossRef]
- Ghani, A.; Müller, K.; Dodd, M.; Mackay, A. Dissolved organic matter leaching in some contrasting New Zealand pasture soils. Eur. J. Soil Sci. 2010, 61, 525–538. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. Chapter 3. Natural factors influencing the amount of organic matter. In The Importance of Soil Organic Matter Key to Drought-Resistant Soil and Sustained Food Production; Food and Agriculture Organization of the United Nations: Quebec City, QC, Canada, 2005; p. 5. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Aitkenhead, J.A.; McDowell, W.H. Soil C:N ratio as a predictor of annual riverine DOC flux at local and global scales. Glob. Biogeochem. Cycles 2000, 14, 127–138. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Sparks, D. Environmental Soil Chemistry; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 9780080494807. [Google Scholar]
- Manzoni, S.; Porporato, A. Soil carbon and nitrogen mineralization: Theory and models across scales. Soil Biol. Biochem. 2009, 41, 1355–1379. [Google Scholar] [CrossRef]
- Toosi, E.R.; Castellano, M.J.; Singer, J.W.; Mitchell, D.C. Differences in Soluble Organic Matter After 23 Years of Contrasting Soil Management. Soil Sci. Soc. Am. J. 2012, 76, 628–637. [Google Scholar] [CrossRef]
- Kindler, R.; Siemens, J.; Kaiser, K.; Walmsley, D.C.; Bernhofer, C.; Buchmann, N.; Cellier, P.; Eugster, W.; Gleixner, G.; Grunwald, T.; et al. Dissolved carbon leaching from soil is a crucial component of the net ecosystem carbon balance. Glob. Chang. Biol. 2011, 17, 1167–1185. [Google Scholar] [CrossRef]
- USDA-NRCS Soil Survey Staff. Soil Survey Laboratory Information Manual; Burt, R., Ed.; Soil Survey Investigations Report No. 45, Version 2.0; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2011; p. 305. [Google Scholar]
- Filep, T.; Draskovits, E.; Szabó, J.; Koós, S.; László, P.; Szalai, Z. The dissolved organic matter as a potential soil quality indicator in arable soils of Hungary. Environ. Monit. Assess. 2015, 187, 1–12. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence Inner-Filtering Correction for Determining the Humification Index of Dissolved Organic Matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Lugato, E.; Bampa, F.; Panagos, P.; Montanarella, L.; Jones, A. Potential carbon sequestration of European arable soils estimated by modelling a comprehensive set of management practices. Glob. Chang. Biol. 2014, 20, 3557–3567. [Google Scholar] [CrossRef]
- Lajtha, K.; Bowden, R.D.; Crow, S.; Fekete, I.; Kotroczó, Z.; Plante, A.; Simpson, M.J.; Nadelhoffer, K.J. The detrital input and removal treatment (DIRT) network: Insights into soil carbon stabilization. Sci. Total Environ. 2018, 640–641, 1112–1120. [Google Scholar] [CrossRef]
- Cookson, W.R.; Murphy, D.V.; Roper, M.M. Characterizing the relationships between soil organic matter components and microbial function and composition along a tillage disturbance gradient. Soil Biol. Biochem. 2008, 40, 763–777. [Google Scholar] [CrossRef]
- Roper, M.M.; Gupta, V.V.S.R.; Murphy, D.V. Tillage practices altered labile soil organic carbon and microbial function without affecting crop yields. Aust. J. Soil Res. 2010, 48, 274–285. [Google Scholar] [CrossRef]
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.H.; Mäder, P.; Bünemann, E.K.; de Goede, R.; Brussaard, L.; Xu, M.; Ferreira, C.S.S.; et al. Effects of agricultural management practices on soil quality: A review of long-term experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Ohno, T.; Fernandez, I.J.; Hiradate, S.; Sherman, J.F. Effects of soil acidification and forest type on water soluble soil organic matter properties. Geoderma 2007, 140, 176–187. [Google Scholar] [CrossRef]


| Maize and Wheat Monocultures | Grassland | |||
|---|---|---|---|---|
| Horizon | A | C | A | C |
| Depth (cm) | 0–40 | 60– | 0–30 | 60– |
| SOC (g kg−1) | 10.9 ± 0.2 * | 0.5 ± 0.1 | 26.1 ± 0.6 * | 0.5 ± 0.1 |
| C:N | 18.8 ± 4.0 * | ND | 20.7 ± 1.6 * | ND |
| pH(KCl) | 7.7 ± 0.2 * | 7.9 ± 0.0 | 7.1 ± 0.2 * | 7.6 ± 0.0 |
| Clay (%, v/v) | 24.9 ± 3.3 * | 24.5 ± 0.7 | 20.7 ± 0.6 * | 18.8 ± 0.3 |
| Silt (%, v/v) | 45.6 ± 1.9 * | 44.9 ± 0.9 | 56.3 ± 3.6 * | 30.7 ± 3.1 |
| Sand (%, v/v) | 29.4 ± 2.3 * | 30.6 ± 1.5 | 22.9 ± 2.1 * | 50.5 ± 3.3 |
| Application Date | Treatment | Maize | Wheat | Sampling | ||||
|---|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | N | P2O5 | K2O | Date | ||
| 3 April 2018. | A | - | - | - | - | - | - | 29 March 2018. |
| B | - | - | - | 50 | - | - | 23 May 2018. | |
| C | - | - | - | 56 | - | - | 26 June 2018. | |
| 28 March 2019. | A | - | - | - | - | - | - | 23 April 2019. |
| B | 25 | - | - | 25 | - | - | 4 June 2019. | |
| C | 25 | - | - | 25 | - | - | 8 July 2019. | |
| 11 October 2019. | A | - | - | - | - | - | - | 9 September 2019. |
| B | 100 | 45 | 50 | 50 | 45 | 50 | 14 October 2019. | |
| C | 112 | 45 | 50 | 56 | 45 | 50 | ||
| Variables | Principal Components | |
|---|---|---|
| PC1 | PC2 | |
| Dissolved nitrogen (DN) | 0.038 | 0.744 |
| Dissolved organic carbon (DOC) | −0.530 | 0.658 |
| Specific ultraviolet absorbance at 254 nm (SUVA254) | −0.396 | −0.499 |
| Biological index (BIX) | 0.894 | 0.023 |
| Fluorescent index (FI) | 0.848 | 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Graiti, T.; Jakab, G.; Ujházy, N.; Vancsik, A.; Fodor, N.; Árendás, T.; Madarász, B.; Barcza, Z.; Márialigeti, K.; Szalai, Z. The Composition of Dissolved Organic Matter in Arable Lands: Does Soil Management Practice Matter? Agronomy 2022, 12, 2797. https://doi.org/10.3390/agronomy12112797
Al-Graiti T, Jakab G, Ujházy N, Vancsik A, Fodor N, Árendás T, Madarász B, Barcza Z, Márialigeti K, Szalai Z. The Composition of Dissolved Organic Matter in Arable Lands: Does Soil Management Practice Matter? Agronomy. 2022; 12(11):2797. https://doi.org/10.3390/agronomy12112797
Chicago/Turabian StyleAl-Graiti, Thulfiqar, Gergely Jakab, Noémi Ujházy, Anna Vancsik, Nándor Fodor, Tamás Árendás, Balázs Madarász, Zoltán Barcza, Károly Márialigeti, and Zoltán Szalai. 2022. "The Composition of Dissolved Organic Matter in Arable Lands: Does Soil Management Practice Matter?" Agronomy 12, no. 11: 2797. https://doi.org/10.3390/agronomy12112797
APA StyleAl-Graiti, T., Jakab, G., Ujházy, N., Vancsik, A., Fodor, N., Árendás, T., Madarász, B., Barcza, Z., Márialigeti, K., & Szalai, Z. (2022). The Composition of Dissolved Organic Matter in Arable Lands: Does Soil Management Practice Matter? Agronomy, 12(11), 2797. https://doi.org/10.3390/agronomy12112797









