Abstract
An immune system is a protective mechanism that shields plants from environmental stresses. This primary function is to maintain optimal circumstances for the growth and development of plant tissues while avoiding harm from biotic and abiotic stress factors. Plants subjected to various stressors initiate stress signaling cascades that affect multiple gene expressions and induce adaptation. These signaling pathways are coordinated by transcription factors, non-coding RNAs, RNA-binding proteins, and protein–protein interaction networks. Several studies have focused on various immune systems, but no study has collected all of them together to illustrate them efficiently. According to this review, stress-responsive genes encode ion and water transporters, enzymes, and transcription factors, making plants more resistant to biological and abiotic challenges. Plants have also evolved anti-pathogen defense systems such as regulatory hormone pathways, reactive oxygen species generation, gene expression, programmed cell death, and cell survival. Plants produce short RNAs in response to a viral attack, which silences the offensive genome and creates complex epigenetic regulatory mechanisms such as histone changes, chromatin remodeling, and DNA methylation to protect plants from pathogens. This review provides an in-depth description of proteins, effectors, and pathways included in plant resistance against environmental stresses and offers details on future trends, such as metabolic pathways and genetic engineering, to improve the protection of plants against stress-induced responses.
1. Introduction
Undoubtedly, all living organisms’ survival depends on plants. Most food, sanitary, and industrial products are derived directly or indirectly from plants. Given the high conservation of different organisms in biological systems, plant discoveries also have important implications for human health. According to the abovementioned, the importance of plants’ survival and development becomes more apparent. Plant immunity is the innate or induced capacity of plants to eliminate pathogens such as bacteria, fungi, viruses, nematodes, and insects. Plants have also developed immune mechanisms to avoid herbivores [1]. Plant defense against abiotic or biotic perturbations is critical for plant adaptability and survival under suboptimal growth or adverse conditions.
Despite the many similarities between plant and animal immunities, there are also notable differences. For instance, plants lack antibodies, T cells, and circulating immune cells [2]. Plant immune responses can be categorized into functionally different classes: (1) basal responses, including the transcriptional regulation of genes in response to pathogen-associated molecular patterns (PAMPs), (2) hypersensitive responses, which involve the apoptosis of cells at the site of infection, (3) systemic acquired protection, which renders the entire plant resistant to infection, (4) jasmonic acid (JA)/ethylene (ET) pathway responses, which protect the whole plant and neighboring plants from herbivores, and (5) non-host immunity [3,4,5,6].
Environmental changes are usually unfavorable, posing severe threats to plant growth and development [7]. Abiotic stresses under adverse ecological conditions include drought, high or low temperatures, lack of nutrients, excess salt, and heavy metals in the soil. Besides biotic stresses, abiotic stresses significantly affect crop yield and wild plant species distribution. Drought, salinity, and extreme temperatures are the abiotic stresses affecting the geographic distribution and limiting crop yield. The harmful effects of biological and abiotic stresses on plants are worsening because extreme weather is occurring more often [8]. In a natural environment, the ability of plants to cope with abiotic stresses is influenced by microbial communities composed of bacteria, fungi, and oomycetes that inhabit the host plant without causing disease. Upon recognizing environmental stresses via specific sensing mechanisms, plants adapt their growth, metabolism, and development. Hence, like almost all living organisms, plants have developed signaling pathways to sense changes in their environment and adjust their metabolism and biological functions to prevent stress-related damage [7]. Osmotic stress caused by drought has been recognized as the most common abiotic stress, followed by salinity stress [9], causing ion toxicity [7]. The secondary effects of drought and salinity stress are incredibly complex. They include oxidative stress, metabolic dysfunction, and damage to various cell components, such as membrane lipids, proteins, and nucleic acids [10]. Although primary stress signals can induce immune responses in plants, secondary stress signals are the main triggers of plant immune responses [11]. Extensive crosstalk occurs between the signal transduction pathways induced by drought and salt stress. For instance, the hyperosmotic signals induced by drought and salt stress promote the accumulation of the plant hormone abscisic acid (ABA), which is responsible for numerous plant biological responses [12]. This review summarizes the essential principles of immunity in biological defenses such as PAMP and effector-triggered immunity (ETI) stimulators, systematic immunity propagation in plants, host innate immunity, and signal transduction. The importance of epigenetics, such as histone modifications, chromatin remodeling, DNA methylation, and non-coding RNAs, has been discussed. This review discusses recent research on how stress affects gene expression, metabolism, physiology, and plant growth.
2. Biological Defenses Mediate Plant Immunity
The crosstalk among hormone signal transduction pathways in response to invading pathogens can be antagonistic or synergistic. Salicylic acid (SA) and JA are the predominant hormonal pathways in plant defense [13]. Plant defense mechanisms are versatile and tailored according to the pathogens’ characteristics. The SA pathway is induced primarily in response to biotrophic pathogens, whereas the JA/ET pathway is involved in the defense against herbivore or necrotrophic pathogen infections [14]. Typically, only one of these pathways is activated to preserve energy and resources [15]. Although JA, ET, and SA are the predominant hormones in plant defense, additional hormones play a supporting role. These hormones include ABA, auxins, gibberellins, cytokinins, and brassinosteroids. SA has a vital role in the fight against biotrophic pathogens, whereas JA and ET are critical defense hormones in response to herbivores and necrotrophic pathogens. While the JA/ET and SA pathways have been the most antagonistic, studies in mutant plants have suggested a synergistic effect under certain conditions [16]. In pattern-triggered immunity (PTI), PAMPs such as bacterial flagellin and fungal chitin are detected by transmembrane pattern recognition receptors (PRRs), which initiate PTI on the cell surface. Upon activation, PRRs initiate complex defense signaling cascades [17].
Plant immunity relies on innate immune receptors, which are expressed in every cell and recognize invasion signals, inducing PTI or ETI [18]. To prevent pathogen infection, plants use receptor barriers for innate defense. Microbial or altered host molecules are recognized by PRRs in the plasma membrane, activating PTI [19,20]. Damaged endogenous molecules, microbe-associated molecular patterns (MAMPs), and danger-associated molecular patterns are recognized and bound to by PRRs. Activation of PRR by cytosolic Ca2+ and apoplastic reactive oxygen species (ROS) responses promotes phosphorylation and activates the NADPH oxidase respiratory burst oxidase D (RBOHD). RBOHD activation results in the rapid production of ROS in a calcium-dependent or calcium-independent manner. Increases in ROS and cytosolic Ca2+ cause the activation of Ca2+-dependent protein kinases, mitogen-activated protein kinases (MAPKs), and defense hormone networks, as well as a lot of transcriptional, translational, and metabolic reprogramming [21] (Figure 1).
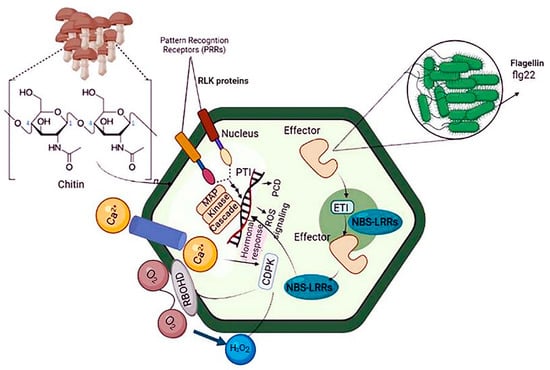
Figure 1.
Schematic diagram of plant immunity against bacterial pathogens. Plants use two strategies to respond to pathogen attacks: PTI and ETI. Because ETI recognizes specific proteins injected by the pathogen rather than PAMPs, ubiquitous molecules expressed by all microorganisms, it is more pathogen strain-specific than PTI. The plant must express a resistance protein that recognizes a specific factor protein for ETI to occur. PTI: pattern-triggered immunity, ETI: effector-triggered immunity, and PAMP: pathogen-associated molecular patterns.
MicroRNAs (miRNAs) are central players in these defense cascades, modulating gene expression, metabolism, and plant development [22]. PTI results in distinct physiological phenomena, including stomata closure to prevent pathogen penetration; ROS and nitric oxide (NO) production; translocation of nutrients from the cytosol to the apoplast; deposition of callose; and production of various antimicrobial metabolites and defense hormones [23,24]. Signaling cascades involved in ETI are more robust than PTI-related cascades. ETI is initiated upon plant recognition of pathogen virulence factors known as effector molecules by resistance proteins (R proteins). R proteins can be extracellular or intracellular and recognize pathogen virulence factors directly or indirectly. Most R proteins contain nucleotide-binding sites and leucine-rich repeat domains. The gene family containing these domains is conserved across plant species and includes highly polymorphic genes often found in clusters [25]. Thus, PTI and ETI result in extensive reprogramming of plant gene expression via various receptor proteins, signal transduction cascades, kinases, ROS, hormones, heat shock proteins, and transcription factors, protecting against invading pathogens [26] (Figure 1).
The overlap between the physiological responses involved in PTI and ETI partially brings together plant immune signaling pathways in response to biological stresses. The transcriptional regulation of downstream signaling cascades involved in ETI and PTI is controlled by molecules such as small RNAs, transcription factors, chromatin remodelers, histone modifiers (like methylase and acetylase), and transcriptional regulatory complexes [27,28] (Figure 1).
2.1. PAMP (PTI) and Effector (ETI)-Stimulated Immunity in Plants
The plant immune system is a complex network of active and passive systems that withstand pathogen invasion of a host [29]. Most plant surface barriers, such as wax coatings, rigid cell walls, cuticular lipids [30], antimicrobial enzymes [31], or secondary metabolites [7], stop pathogens from getting into the plant and causing disease symptoms to appear.
PAMPs/apoplastic effectors, intracellular effectors, or changed effector targets are actively detected by receptors in the plasma membrane or R proteins in the cytoplasm, activating PTI and ETI. Helper proteins and guard proteins/decoys play a role in pathogen-derived component co-perception [32]. Pathogens use host proteins (S-proteins) expressed by plant sensitivity genes (S-genes) to help enter and multiply, which leads to host adaptation [33]. To get past these protective layers, pathogens use microbial (or pathogen)-associated molecular patterns (MAMP/PAMP)-stimulated immunity (MTI/PTI) and enhanced T-cell immunity (ETI) [30] (Figure 2).
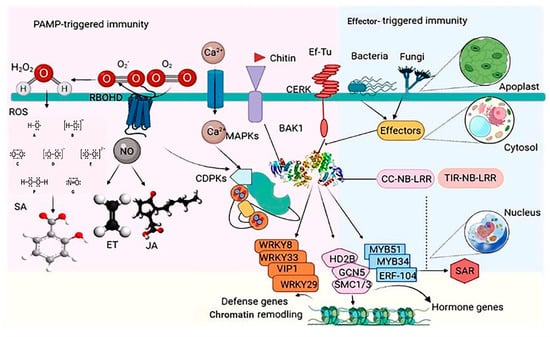
Figure 2.
Schematic view of plant intrinsic immunity PAMP-triggered immunity and ETI in plants. The recognition of microbe-dependent molecular patterns (MAMPs) such as flagellin, elongation factor-Tu (EF-Tu), and chitin by cognate sample assessment receptors (PRRs; FLS2, EFR, and CERK) activates many signaling pathways, resulting in the production of reactive oxygen species (ROS), nitric oxide (NO), Ca2+ flux, and the activation of several protein kinases such as CDPKs and MAP kinases (MAPKs). Pathogens release effector molecules into the plant to repress these primary signaling events. However, some plant varieties may know effectors with the aid of R proteins (CC-NB-LRR and TIR-NB-LRR) to compel a hypersensitive response (HR) and systemic acquired resistance (SAR). Targets that are phosphorylated include the replication agents WRKY33, WRKY8, WRKY29, VIP1, MYB51, MYB34, and ERF104, and the chromatin remodeling factors HD2B, GCN5, and SMC1/3. This activity has a pattern of no single transcriptional reprogramming and infusion of primary advocacy-relevant genes but limits pathogen infection and priming plants versus subsequence attacks. Endogenous phytohormones, such as salicylic acid (SA), jasmonic acid (JA), and ethylene, are also induced and contribute to plant immunity.
2.2. Systemic Propagation of Immunity in Plants
Plants react to their surroundings in a stimulus-specific manner. These reactions frequently converge on multiple phytohormone pathways interacting with one another and presumably fine tune plants’ pathway responses to optimize fitness [34,35]. PTI and ETI are both SA-dependent and elicit a systemic, SA-dependent defense response known as systemic acquired resistance (SAR) [2]. SAR is a type of long-term resistance to a wide range of (hemi-) biotrophic infections [36]. Azelaic acid and glycerol-3-phosphate are also considered as additional signals in SAR [37]. Another common type of induced (pathogen) resistance is induced systemic resistance (ISR), which is triggered by commensal bacteria in the plants rhizosphere [38]. ISR appears to work against a broader spectrum of pathogens than SAR, including both (hemi-) biotrophic and necrotrophic infections. Systemic immunity similar to SAR has been seen in monocots such as maize, barley, wheat, and banana [39,40]. Wheat systemic resistance against Xanthomonas translucens is not related to SA. However, it may be linked to JA/ABAPip (pipecolic acid) and/or WRKY and ETHYLENE RESPONSE FACTORS, which build up in barley petiole exudates after an infection that makes the plant resistant [40] (Figure 2). Systemic immunity against Blumeria graminis f.sp. hordei may depend on SA, whereas Pip induces systemic resistance in barley against both infections [41]. Pip treatment induces NO buildup and primes ROS generation [41], indicating that the Pip pathway of SAR may be conserved between monocotyledonous and dicotyledonous plants (Figure 3). It is important to understand the similarities and differences between induced resistance responses in monocots and dicots. Before using SAR signaling components like those from Arabidopsis to protect cereal crops, it will be important to study SAR in monocots such as barley and wheat.
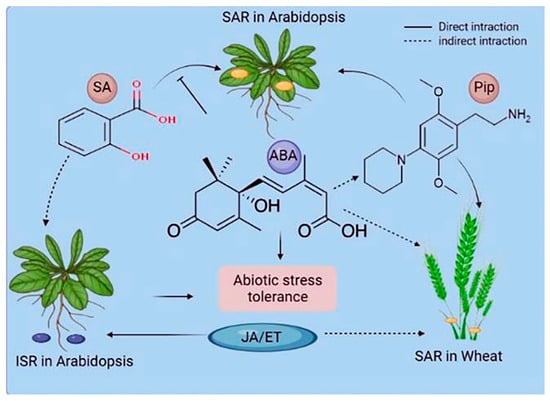
Figure 3.
Phytohormones’ role in systemic acquired resistance (SAR) and induced systemic resistance (ISR). The interactions of stress-associated phytohormones vary based on the systemic resistance mechanism and the plant species. Hormones can directly function in signaling or pathway antagonism or have an indirect or undefined role. ABA denotes abscisic acid, JA denotes jasmonic acid, ET denotes ethylene, and Pip denotes pipecolic acid.
3. Genetic Effects on Plant Immunity
3.1. Signaling Pathways Host Innate Immunity in Plants
Phytopathogens such as biotrophs, hemibiotrophs, and necrotrophs stress the development of their host plants, reducing yields. Plants are constantly in contact with such phytopathogens, making them vulnerable to their attack. Plants must evolve immunity to these attacks to defend themselves. As a result, plants have evolved ways of recognizing and combating disease via PTI and ETI [42].
3.1.1. Pattern-Triggered Immunity
The first step in the immune response is PTI. PAMPs or MAMPs are identified by PRRs and conserved molecular patterns such as lipopolysaccharides, peptidoglycans, chitin, flagellin, EF-Tu, DNA, and ergosterol. The production of ROS, the activation of MAP kinase, and the stimulation of pathogen-responsive gene transcription are some of the well-known MAMP evaluations included in the activation of signaling pathways and hydrolysis [43,44].
3.1.2. Effector-Triggered Immunity
Plants, including R proteins, are able to recognize many effectors released by pathogens to activate their defense systems. ETI is detected by nucleotide-binding oligomerization-like receptors (NOD), which target microorganism-produced effector molecules [45]. The complex network of cell walls and their key protein constituents is one of the most critical aspects of the plant’s immune system. Significant advances in research on how plant viruses affect cell wall remodeling in both susceptible and resistant plants show that cell wall metabolism components can change virus transmission and turn on apoplast- and symplast-based defense mechanisms [46].
3.2. Signal Transduction
3.2.1. ABA Signaling Pathway
ABA is a plant defense-related phytohormone that positively or negatively regulates the immune system, playing a central role in response to abiotic and biotic stresses. The ABA signaling pathway modulates plant growth and development, seed dormancy and maturation, vegetative growth, and stomatal closure in response to various abiotic stimuli. The ABA signaling pathways have been described from hormone receptors to multiple downstream components as a major system that mediates different abiotic stress responses to drought, osmotic, and salt challenges [47,48]. ABA can be synthesized from farnesyl diphosphate (direct C15 pathway) or 9-cis-violaxanthin (indirect C40 pathway). Plants have developed various defense mechanisms to adapt to environmental changes [49]. Under stress, plants accumulate ABA, which serves as a communication molecule among plant cells and initiates various defense mechanisms [50]. Most ABA is synthesized as 9-cis-violaxanthin. In plastids, carotenoids are catabolized into zeaxanthin, which is converted to lutein by zeaxanthin epoxidase. Lutein is converted to all-trans-violaxanthin, which is transformed into 9-cis-violaxanthin or all-trans-neoxanthin; 9-cis-violaxanthin is derived from all-trans-neoxanthin [51]. Under aerobic conditions, 9-cis-epoxycarotenoid dioxygenase converts 9-cis-violaxanthin into 9-cis-neoxanthin and xanthin, which are transferred from the plastid into the cytoplasm [52]. Oxyxanthin is converted into xanthic acid, which serves as a substrate for short-chain alcohol dehydrogenase to generate ABA and abscisic acid. Abscisic aldehyde is further converted into ABA by aldehyde dehydrogenase [53]. Thus, ABA’s binding to its receptor initiates various signaling cascades to modulate different cellular processes, including gene expression. In plants, abiotic stimuli induce activation of MAPKs and SNF1-related protein kinase 2 (SnRK2) family members, which play a central role in osmotic stress responses. In Arabidopsis, osmotic stress and ABA activate all SnRK2 family members except SnRK2.9 [54]. The activities of SnRK2 family members, osmotic stress-activated protein kinase, and SAPK1 in Nicotiana tabacum were inhibited by phosphatase treatment, suggesting the importance of phosphorylation in activating SnRK2 proteins [55]. Numerous phosphorylation sites in SnRK2 have been identified. The serine at position 175 in SnRK2.6 has been extensively characterized and proven essential for SnRK2.6 activation [56,57]. Furthermore, ABA treatment increased in vivo phosphorylation levels [58]. NO-induced SnRK2.6 modification inhibits the activity of SnRK2.6. It has been suggested that NO induction by ABA inactivates SnRK2.6 in guard cells, serving as a negative feedback loop that regulates SnRK2.6 activity in response to ABA pathway activation [59]. Phosphatidic acid is a signaling lipid involved in various stress-induced pathways. McLoughlin et al. [60] found that SnRK2.4 and SnRK2.10 accumulated on the cell membrane by binding to phosphatidic acid, promoting salt resistance and root growth and morphogenesis under salt stress. SnRK2s have been shown to bind to ABA-responsive element-binding factors (ABFs), which, upon phosphorylation, regulate the expression of numerous ABA-induced genes [61]. In wheat, PKABA1 is the phosphorylation substrate of TaABF, a seed-specific ABF [55]. In rice, the serine at position 102 of the ABF TRAB1 is crucial for SAPK8/9/10 activation, regulating downstream targets of the ABA signal transduction pathway in response to osmotic stress [62]. In Arabidopsis, ABF1, AREB1/ABF2, AREB2/ABF4, and ABF3 are the most important transcription factors downstream of SnRK2.2/2.3/2.6 in the ABA pathway during the osmotic stress response [61]. These findings highlight the crucial role of SnRK2s in regulating ABA response gene expression by phosphorylating ABFs. SnRK2s also activate the slow anion channel-associated 1 (SLAC1) protein, which regulates ion balance and controls stomatal closure in response to ABA accumulation [63].
3.2.2. Gibberellic Acid (GA) Signaling Pathway
GA is a tetracyclic diterpenoid belonging to the gibberellin family. It is involved in plant growth, metabolism, and response to abiotic stress [52]. The biosynthetic pathways of GA have been extensively investigated [64]. Ent-copalyl diphosphate synthase, ent-kaurene synthase, ent-kaurene oxidase, ent-kaurenoic acid oxidase, GA13-oxidase, GA20-oxidase (GA20ox), GA3-oxidase (GA3ox), and GA2-oxidase are enzymes essential for GA biosynthesis [65]. Geranylgeranyl diphosphate is converted into the endogen ent-kaurene by ent-copalyl diphosphate synthase and ent-kaurene synthase in plastids. In the endoplasmic reticulum, GA12-aldehyde is formed by ent-kaurene oxidation.
In the cytoplasmic matrix, GA12 and GA53 are oxidized by GA20ox and GA3ox to form different GAs [66] (Figure 4). GA-insensitive and topless-related members of the DELLA (aspartic acid–glutamic acid–leucine–leucine–alanine) protein family and negative regulators of GA signal transduction bind to the promoter of GA20ox after recruitment by the transcription factor GAF1, inhibiting GA biosynthesis. Wu et al. [67] reported the interaction between the zinc finger protein OsLOL1 and the transcription factor OsbZIP58, promoting GA biosynthesis. Serine/threonine phosphorylation has been shown to play an essential role in GA synthesis, as dephosphorylation of the repressor of GA1 induced the transcription of GA20ox and GA3ox [68] (Figure 4).
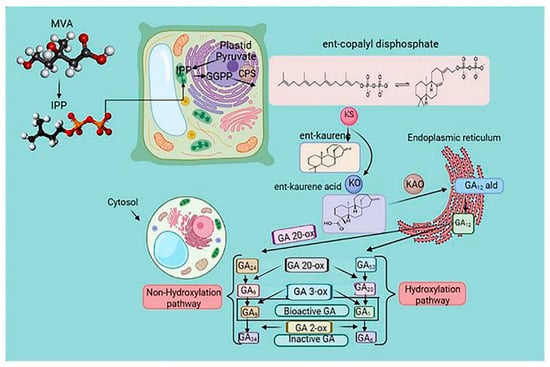
Figure 4.
The gibberellin biosynthesis pathway in higher plants. The cellular location of metabolites in plastids is the endoplasmic reticulum and cytoplasm. Methylerythritol phosphate (MEP) and mevalonic acid (MVA) pathways regulate the biosynthesis of gibberellic acid (GA). Ent-copalyl diphosphate synthase (CPS) catalyzed the conversion of geranylgeranyl diphosphate (GGPP) to the endogen ent-kaurene and ent-kaurene synthase (KS), which is the precursor of GA. The biosynthesis of GA is a process of various enzymatic reactions, and the oxidation reaction of GAox is the critical enzymatic reaction. Arrows indicate a cascade reaction catalyzed by an enzyme. Gibberellin Biosynthetic Pathway in Higher Plants. GGPP, trans-genanylgeranyldiphosphate; CDP, ent-copalyl diphosphate; CPS, CDP synthase; KS, ent-kaurene synthase; KO, ent-kaurene oxidase; KAO, ent-kaurenoic acid oxidase; GA20ox, GA 20-oxidase; and GA3ox, GA 3-oxidase.
Plants sense extracellular GA via the soluble GA receptor GID1. The binding of GA to GID1 is concentration-dependent. At low extracellular GA concentrations, GA does not bind to GID1, and the N-terminal extension domain of GID1 binds to GA-responsive genes, suppressing their transcription. When the concentration of extracellular GA is high, GID1 binds to GA via its C-terminal domain, inducing the transcription of GA-responsive genes. GA binding to GID1 leads to a conformational change in GID1, and the N-terminal extension domain of GID1 forms a hydrophobic surface that binds to DELLA proteins. GA–GID1 complexes bind to DELLA proteins, forming trimers, which bind to the ubiquitin E3 linker complex (SCFSLY1/GID2), leading to DELLA protein degradation by the 26S proteasome [69]. In the absence of GA, GID1 binds to DELLA proteins, activating alternative pathways [70]. Therefore, plants can sense extracellular GA via both GA-dependent and GA-independent pathways. Ca2+, cyclic guanosine monophosphate (cGMP), and NO are critical second messengers in GA signal transduction [71]. These second messengers transform extracellular cues into intracellular signals, triggering various physiological responses. Ca2+ and cGMP are positive regulators of GA signal transduction, whereas NO is a negative regulator of the GA signal pathway. In rice, Ca2+ binds to calmodulin to form Ca2+/calmodulin, which regulates the expression of Ca2+/ATPase-dependent GA-responsive genes [72]. In Arabidopsis, cGMP and the GA response element play essential roles in the GA signaling pathway, modulating the expression of negative regulators of the GA pathway, SPINDLY, and GID1. Lozano-Juste and Leon [71] confirmed that NO reduced the expression levels of SLY (SLEEPY) and promoted the accumulation of DELLA proteins, thereby inhibiting the expression.
3.2.3. ET Signaling Pathway
ET biosynthesis is induced in PTI and ETI, and ET induction is enhanced by SA [39]. ET is involved in response to various abiotic stresses in plants. For instance, low-temperature stress changes the endogenous level of ET. In Arabidopsis seedlings, ET treatment decreased resistance to frost stress, whereas treatment with ET synthesis inhibitors increased resistance to frost stress. This suggests that ET negatively regulates tolerance to low temperatures [73].
In contrast, ET increased the frost resistance in Arabidopsis seedlings grown in soil [74]. The effect of ET on frost resistance may vary depending on the growth conditions [75]. Under salt stress, ET negatively regulated salt tolerance in Arabidopsis. Overexpression of the wheat TaACO1 (aminocyclopropane-1-carboxylate oxidase) gene reduced two crucial transcription factors, DREB1B/CBF1 and DREB1A/CBF3, suggesting the decrease in stress tolerance in Arabidopsis was due to increased salt sensitivity [76]. Although ET promotes stomatal opening by inhibiting ABA-induced stomatal closure, it also promotes stomatal closure by inducing ROS production in guard cells [77]. Methionine-derived 1-aminocyclopropane-carboxylic acid is the primary ET precursor. During the Yang cycle, methionine is converted to S-adenosylmethionine by methionine adenosyltransferase. The ET precursor 1-aminocyclopropane-1-carboxylic acid is formed by 1-aminocyclopropane-1-carboxylic acid synthetase, and 1-aminocyclopropane-carboxylic acid is converted to ET by 1-aminocyclopropane-1-carboxylic acid oxidase. Methionine is replenished by 5-methylthioadenosine, produced simultaneously as 1-aminocyclopropane-carboxylic acid [78] (Figure 5). The ET signaling pathway has been extensively evaluated in Arabidopsis. The ET receptor (ETR) is located on the surface of the endoplasmic reticulum. In the absence of ET, ETR inhibits activation of the ET signaling pathway by binding to CTR. However, ET binds to ETR, releasing the CTR protein in the presence of ET in the cell. Subsequently, CTR activates EIN2 by cleavage; the carboxyl end of EIN2 enters the nucleus, activating the transcription of EIN3, and the resulting EIN3 protein induces ERF expression [79] (Figure 5).
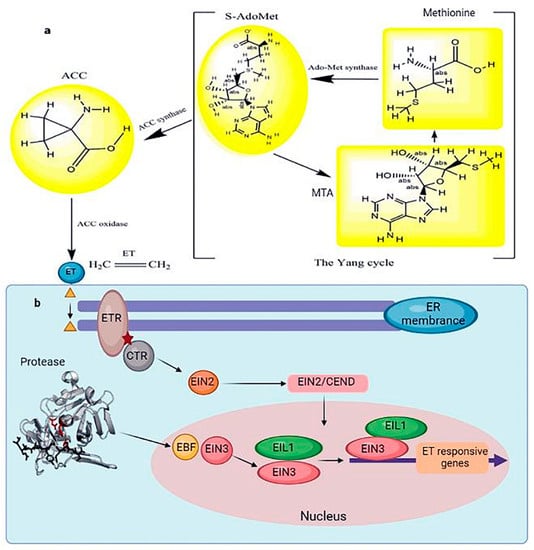
Figure 5.
(a) The schematic Yang cycle to exhibit the biosynthesis of ET in higher plants. In many cells, methionine produces S-adenosylmethionine under the catalysis of the methyl donor methionine adenosyltransferase. Under the catalysis of 1-aminocyclopropane-1-carboxylic acid synthase, the ET precursor 1-aminocyclopropane-1-carboxylic acid is generated. 1-aminocyclopropane-1-carboxylic acid oxidase catalyzes the production of ET from ACC. (b) ET signal transduction in response to abiotic stress. At the same time, 5 methylthioadenosine, also known as MTA, is produced. Methylthioadenosine, in turn, can be utilized to produce new methionine. An enzyme cascade reaction is denoted by an arrow when an enzyme catalyzes it. When ET binds to ETR, it causes the CTR protein to be released. The main controllers of ethylene signaling that support a range of plant responses to ethylene are the transcription factors EIN3/EIL1. The signaling outcome of ethylene, which controls various characteristics of plant development and stress responses, must be modulated correctly according to the spatiotemporal and environmental circumstances. CTR activates EIN2 by degradation, and the carboxyl end of EIN2 translocates into the nucleus and activates EIN3 transcription. Subsequently, EIN3 induces ERF expression, inhibiting ET-responsive gene expression. Bars represent inhibition, and arrows represent activation.
EIN3 is a nuclear protein acting as a plant-specific transcription factor. It plays a critical role in the ET signaling pathway by directly regulating the expression of ET-responsive genes. Arabidopsis has five EIN3 homologous genes (EIL1–5), and EIN3/EIL1 controls the expression of most ET-responsive genes, as reflected by the finding that EIN3/EIL1 defects result in ET insensitivity. Although ET treatment does not alter EIN3 mRNA levels, it causes the accumulation of the EIN3 protein [80]. In the absence of ET, EIN3 binds to the F-box protein EBF, which results in its recruitment to the Cullin protein complex for ubiquitination. Therefore, the level of EIN3 protein in plants is deficient under physiological growth conditions. However, in the presence of ET, a portion of EIN2 translocates into the nucleus, promoting EBF degradation and subsequent EIN3 stabilization [7,81]. Numerous studies have shown that the EIN3/EIL complex promotes salt stress resistance [65] and low-temperature stress [73]. EIN3/EIL1 directly regulates the expression of the ERF transcription factor in plants, and ERF regulates the amount of ET-responsive genes produced in response to abiotic stresses [82,83] (Figure 5).
3.2.4. SA and JA Signaling Pathways
Important roles in plant defense are played by the phytohormones SA and JA. Various studies have shown that SA- and JA-mediated signals interact among themselves (SA-JA crosstalk) to regulate plant innate immunity against pathogens. SA and JA, which are antagonistic defensive hormones involved in defense against biotrophs and necrotrophs, accumulate at high levels during ETI [84]. The plant hormones SA and JA function as critical secondary signaling molecules in plant immunity [39]. In response to plant–pathogen interactions, primary signaling pathways influence the levels and activities of SA and JA [34,85]. Notably, the treatment of cucumber seedlings with SA synthesis inhibitors blocked the accumulation of endogenous SA and rendered plants more susceptible to the damage caused by low temperatures. Inhibition of SA synthesis under low-temperature stress led to the accumulation of hydrogen peroxide, highlighting the importance of SA in response to low-temperature stress by regulating the expression of low-temperature-responsive genes and intracellular hydrogen peroxide levels [86]. JA is involved in tolerance to low temperatures and freezing [75]. Notably, exogenous JA increased frost resistance in Arabidopsis, while mutations in JA biosynthesis genes increased sensitivity to freezing [87]. In addition, JA has also been implicated in salt tolerance. Specifically, the branch of the JA synthesis pathway catalyzed by allene oxide cyclase via MYC2-dependent and ABA-independent pathways is responsible for the JA-mediated increased salt tolerance in plants [88].
3.2.5. Crosstalk in Stress Response Signaling Pathways
Abiotic stresses, such as drought, salinity, and low and high temperatures, initiate systematic responses in plants. Biological crosstalk refers to instances in which one or more components of one signal transduction pathway affect another. This crosstalk can be achieved through various mechanisms, including the interactions among different signaling cascade protein components. In plants, however, stress response pathways are not initiated independently, and apart from local responses, local stress also triggers systemic responses. For example, local salt stress at the root tip can alter calcium and ROS levels in the whole plant, affecting salt tolerance systemically [89]. The zinc finger protein ZAT12 is involved in DNA repair and replication, essential in ensuring cell survival by acclimation to different environmental stress conditions via RBOHD-mediated ROS accumulation [90].
Activating the intracellular Ca2+ signal enhances ROS production and the expression of defense genes, initiating systemic plant defense responses [91]. The guard cell hydrogen-peroxide-resistant 1 (GHR1) protein is a plasma membrane receptor kinase, expressed in guard cells, and regulates the activation of plasma membrane calcium channels [92]. It has been reported that JA responses positively relate to SA [93] and ET [94] responses because of synergistic or feedback regulation. In Arabidopsis, EIN3/EIL1 is regulated by EBF1/2. EIN3/EIL1 regulates the transcriptional repressor jasmonate ZIM domain (JAZ) and causes it to activate ET-responsive gene expression.
Additionally, JAZ inhibits the transcriptional activity of EIN3/EIL1, suppressing the expression of the Erf1 gene. MeJA, a vital component of the JA pathway, increased ET levels in plants [95]. Although JA and ET both positively regulate plant defense responses, they may also have antagonistic effects [96]. Studies have shown that EIN3/EIL1 levels are also regulated by transcription factors activated by other hormones, such as GA. DELLA proteins, transcriptional repressors of the GA signaling pathway, bind to regulatory sequences in Ein3/Eil1 genes, suppressing their transcription [97]. However, in the absence of GA, DELLA and MYC2 bind to JAZs, and the binding of MYC2 to G-box motifs in JA-responsive genes activates their expression. When the GA content increases, DELLA proteins are degraded, and JAZs are released, inhibiting the JA signaling pathway [98]. Yaish et al. [99] demonstrated that OsAP2-39 overexpression induced the upregulation of 9-cis-epoxycarotenoid dioxygenase I, a key enzyme in ABA biosynthesis. OsAP2-39 also plays an essential role in maintaining the balance between ABA and GA in plants by regulating the expression of critical enzymes involved in ABA and GA biosynthesis. In response to abiotic stress in plants, DELLA proteins participate in the dynamic balance between ABA and GA by enhancing the expression of the ubiquitin E3 ligase Xerico, reducing GA levels, and increasing ABA levels [100].
4. Epigenetics Mediate Immunity in Plants
4.1. Histone Modifications
Histone modifications are covalent post-translational modifications in histones, including methylation, phosphorylation, acetylation, ubiquitylation, and sumoylation. These post-translational modifications regulate gene expression by altering chromatin structure or recruiting transcriptional regulators, recognizing specific histone modifications. Histones are crucial for the packaging of DNA into chromosomes. It has become evident that histone modifications regulate diverse biological processes, including transcriptional activation and inactivation, chromosome packaging, and DNA damage repair [101]. The contrasting effects of histone acetyltransferases (HATs) and histone deacetylases (HDACs) regulate the acetylation levels of lysine residues within the N-terminal tail histones. Acetylation is a feature of actively transcribed genes, and some HATs have been shown to function as transcriptional co-activators [78]. In plants, HDACs and HATs control the expression of genes for stress response and development [102]. Thus, HDACs are essential for defense against plant pathogens. According to Chen et al. [103], HDA19 is the predominant HDAC involved in the defense against plant pathogens. HDA19 is a nuclear RPD3/HDA1 family member with HDAC catalytic activity, and its absence has been shown to increase histone acetylation levels by 10-fold [104]. Significantly, increased AtHDAC19 expression levels have enhanced fungal resistance via activation of the ethylene-responsive factor 1, whereas AtHDAC19 silencing has increased susceptibility to fungal infections [105]. It has been suggested that HDA19 and ERF factors may regulate the expression of JA-responsive genes [106]. Additionally, HDA19 was shown to protect against Pseudomonas syringae infection by suppressing SA-responsive gene expression [107], highlighting the extensive crosstalk between the pathways initiated by different plant hormones. Notably, mutations in HDA19 enhanced the expression of SA-responsive genes and provided resistance to biotrophic pathogens [107].
HDAC6 is another HDAC that activates JA-dependent defense mechanisms in plants [108]. It has been suggested that NO suppresses histone deacetylation, ultimately leading to hyperacetylation and subsequent transcription of defense genes [109].
Histone methylation involves transferring one, two, or three methyl groups from S-adenosyl-L-methionine to lysine or arginine residues in histones by histone methyltransferases. Histone methylation regulates gene expression via chromatin-dependent transcriptional repression or activation. In plants, methylation of lysine residues in histone H3 has been well-documented [110]. Notably, H3K27me3 [111], H3K9me2 [112], and H3K4me1/2/3 [113] have been identified as epigenetic modifications in histone H3 that affect DNA packaging and gene expression. For example, in Arabidopsis, H3K27me3 suppressed the expression of over 4000 genes [114]. Interestingly, H3K4me2 and H3K4me3 appear to be mutually exclusive [113].
The WRKY superfamily comprises approximately 100 transcription factors involved in the defense against pathogens and stress [115]. WRKY70 [116], the most extensively investigated WRKY member, regulates plant immunity. H3K4 methylation in the WRKY70 gene has promoted SA-dependent defense responses in plants [117]. In Arabidopsis, WRKY70 links SA- and JA-mediated responses. In wild-type plant cells, P. syringae induced the expression of WRKY70. WRKY70-deficient mutant plants exhibited greater sensitivity to necrotrophic and biotrophic pathogens, including the bacterial pathogens Erwinia carotovora and Erysiphe cichoracearum, as well as the fungi Botrytis cinerea [118]. Similarly, bacterial (Streptomyces) infection in tomato plants increased WRKY70 expression [119]. Protein ubiquitination involves the addition of ubiquitin, a 76-residue peptide, to different proteins. Ubiquitination is mediated by ubiquitin ligases E1, E2, and E3 [120]. Interestingly, among histones, ubiquitination has only been observed in H2B or H2A. Typically, ubiquitination positively regulates gene expression by opening up chromatin. For example, the Arabidopsis genes HUB1 and HUB2 encode monoubiquitination ligase E3 and are essential for plant defense against necrotrophic pathogens [121]. HUB1 and HUB2 were also required for biotrophic pathogen resistance [122] and cuticle alterations [123]. Cotton plants overexpressed with HUB2 were better able to withstand drought, while those whose expression was silenced were more susceptible [124].
4.2. Chromatin Remodeling
Chromatin remodelers are multi-protein complexes that modify histone–DNA interactions via ATP-dependent transfer or modulation of nucleosomes. Pardal et al. [125] reported that chromatin remodeling ATPases control the plant’s immune response mechanisms. Kang et al. [126] described how a novel regulator, CHR19, affects the transcription of genes, influencing plant resistance to various pathogens by acting at the chromatin level. Additionally, it has been shown that VdDpb4 and VdIsw2 play a role in preserving chromatin structure for nucleosome positioning and transcription regulation, which includes genes responsible for DNA repair in response to ROS stress throughout improvement and plant infectious disease [127]. These protein complexes include catalytic subunits of the sucrose non-fermenting 2 (SNF2) family of DNA helicases/ATPases and are recruited to particular promoters via interaction with transcription factors or accessory proteins. SNF2 proteins are evolutionarily conserved and are categorized into subfamilies according to archetypal members [128,129]. Recently, a tomato SNF2 protein was identified, and its potential role in response to diverse stimuli, including ABA, SA, cold, and salt, has been suggested [130].
4.2.1. Photoperiod-Independent Early Flowering 1 (PIE1)
PIE1 is an SWR1-like protein belonging to the SWR1 subfamily of SNF2 proteins. In this way, PIE1 regulates gene expression by replacing the canonical H2A histone with the H2A.Z variant in a replication-independent manner [131,132]. PIE1 is the Arabidopsis SWR1-like complex catalytic component containing various other proteins, including serrated leaves and early flowering (SEF), actin-related protein 6, and H2A.Z [89,133]. PIE1 cooperates with the H2A.Z histone variant encoded by the genes HTA8 (At2g38810), HTA9 (At1g52740), and HTA11 (At3g54560). In Arabidopsis, the SWR1-like complex regulates SA-dependent defense pathways. H2A.Z residues are often targeted for acetylation [134] or methylation [135], modulating the accessibility of chromatin to regulatory proteins [136]. In Arabidopsis, H2A.Z has been implicated in gene silencing regulation in response to DNA methylation [137]. SEF and PIE1 may directly incorporate H2A.Z at the promoters of genes encoding SA pathway inhibitors.
4.2.2. BRAHMA (BRM) and SPLAYED (SYD)
BRM and SYD belong to the SNF2 protein superfamily [129,138] and regulate the development of reproductive and vegetative structures [138]. SYD regulates the expression of the JA-responsive gene PDF1.2a and is essential for initiating JA-mediated defense mechanisms against the necrotrophic pathogen B. cinerea [139]. Specific SYD mutants (syd-4) exhibited enhanced resistance to the bacterial pathogen P. syringae pv. maculicola ES4326 [140]. In plants, the chromatin remodeler BRM was shown to control ABA-responsive gene expression in response to abiotic stress [141]. Importantly, BRM101-mutant plants exhibited dysregulated expression in some defense-related genes, suggesting that BRM plays a vital role in plant defense pathways by modulating the crosstalk between SA and ABA signaling pathways [142].
4.2.3. Decrease in DNA Methylation 1 (DDM1)
DDM1 is a helicase belonging to the LSH subfamily involved in the methylation of genomic DNA. ddm1-mutant Arabidopsis plants exhibited decreased cytosine methylation, TE pathway activation, and histone modification alterations [143]. Furthermore, DDM1 has been shown to regulate the SA signaling pathway, among other plant defense mechanisms [95]. The variations of DDM1 gene expression were discovered in an infection-resistant Arabidopsis strain [144] that controls other chromatin alterations such as histone modifications [145]. Notably, ddm1-mutant plants exhibited remarkable resistance to Hyaloperonospora arabidopsidis [146], indicating the importance of DDM1 in plant defense mechanisms.
4.2.4. CHR5 (Chromatin Remodeling Factor 5)
The chromatin remodeler CHR5 positively regulates gene expression by relaxing the chromatin structure at Snc1, among other genes. Chr5-mutant plants exhibited deficient SNC1 levels and increased susceptibility to avirulent and virulent strains of P. syringae [145] due to enhanced activation of the SA signaling pathway [147].
4.3. DNA Methylation
DNA methylation is an epigenetic mechanism regulating gene expression by adding methyl groups to DNA. In many eukaryotes, methylation of the fifth carbon of cytosine is a critical mechanism for modulating chromatin structure, thereby regulating DNA recombination, gene expression, and genomic imprinting [148]. High-resolution DNA methylation profiling in Arabidopsis has shown that DNA methylation is widespread in repetitive DNA sequences and pericentromeric transposons [149]. In plants, DNA methylation is mediated by plant DNA methyltransferases, such as DDM1 [150]. An HDA6-dependent, RNA-directed DNA methylation mechanism has been identified [108]. In vegetative structures, the repressor of silencing 1 (ROS1) has been shown to play a critical role in DNA demethylation [151]. Although most plant viruses have RNA genomes, the genomes of Geminiviridae family members are single-stranded DNA. Virus infection in plants is fought partly by a defense mechanism involving DNA methylation of the geminivirus viral genome in plant guard cells [152]. Viral DNA methylation has also been detected in tomato plants. Some infectious viral strains carry the beta satellite encoding βC1, a repressor of viral genome silencing [153]. Resistant strains of tomato plants have developed the ability to polyubiquitinate βC1, targeting βC1 for proteasome-mediated degradation. Apart from βC1, various viral effectors have been shown to repress transcriptional silencing [154]. Plants initiate alterations in DNA methylation throughout the pathogen attack [155]. However, mutant plants defective in DNA methylation may protect against viral infections by upregulating SA-responsive genes [146].
4.4. Non-Coding RNAs (ncRNA)
Non-coding RNAs, either as long ncRNAs (lncRNAs) or processed into small non-coding RNAs (sncRNA), are a group of RNAs that do not encode functional proteins and were initially thought to regulate gene expression only at the post-transcriptional level. However, many recent studies have shown that the most common regulatory RNAs are miRNAs, endogenous small interfering RNA (siRNAs), piRNAs, and lncRNAs. There is increasing evidence that regulatory ncRNAs play an important role in epigenetic control [156]. Recently, it has been discovered that various ncRNAs participate directly or indirectly in various epigenetic phenomena that control different phenotypes within cloned cells and the specific determination of various physiological processes. ncRNAs can regulate the production of defense markers, hormone biosynthesis, signal transduction, or RNA-mediated silencing (Figure 6 and Table 1).
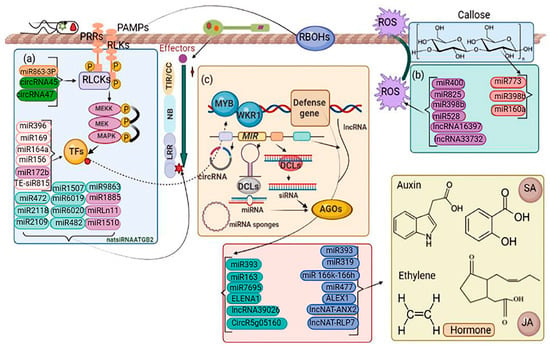
Figure 6.
Interfering ncRNAs in plant immunity. Membrane PRRs and cytoplasmic NLRs are essential receptors of the plant immune response. Membrane PRRs mainly contain receptor-like kinases (RLKs) and receptor-like proteins (RLPs). Both are commonly involved in PTI. NLRs are another type of plant safety receptor commonly involved in ETI. Pattern recognition receptors (PRRs) and NLRs are regulated at transcriptional, post-transcriptional, and post-translational levels. Downstream signaling components of these receptors include kinases, transcription factors, etc. A variety of proteins and several plant ncRNAs directly target the expression of signaling components to regulate reaction immunity. (a) Noncoding RNAs (ncRNAs) regulate immune signaling components. PRRs and NLRs, such as FLS2 and RPS5, are immunological receptors that mediate pathogen recognition. RLCKs and TFs implicated in immunological signal transduction, such as SpRLK and WRKY45, are regulated by other ncRNAs. (b) ncRNAs that affect plant immunity by modulating numerous biological processes such as ROS accumulation, callose deposition, defense-related gene expression, and plant hormone regulation, either directly or indirectly. (c) In immunity, ncRNAs play a coordinated role. PhasiRNAs can be triggered by miRNAs that target NLR genes. Target mimics and miRNA/siRNA precursors are the major functions of lncRNAs. CircRNAs may operate as miRNA decoys, increasing the production of miRNA-targeted mRNAs. PAMPs, pathogen-associated molecular patterns; PRRs, pattern recognition receptors; RLKs, receptor-like kinases; RLCKs, receptor-like cytosolic kinases; TFs, transcription factors; P, phosphorylation; ROS, reactive oxygen species; DCL, DICER-LIKE; AGO, ARGONAUTE; SGS3, SUPPRESSOR OF GENE SILENCING 3; and RDR6, RNA-dependent RNA polymerase 6. Dark purple ovals are involved in ROS accumulation, including miR400, miR825, miR3985, miR528, IncRNA16397, and IncRNA33732. Dark pink ovals are involved in callose deposition, including miR773, miR398b, and miR160a. Blue ovals, including miR393, miR319, miR166k-166h, miR477, ALEX1, IncNATANX2, and IncNAT-RLP1, are involved in regulating hormones. Turquoise blue ovals, including miR393, miR163, miR7695, ELENA1, and circRg05610, are involved in PATHOGENESIS-RELATED (PR) gene expressions. Light pink, including miR863-3P, is involved in the miRNA biosynthesis pathway. Dark blue ovals, including circRNA45 and circRNA47, are involved in regulating receptor-like kinase. Pale pink ovals, including miR396, miR169, miR156, miR164a, and TE-siR815, are involved in transcription factors. Light purple ovals, including miR1726, miR1885, miRLn11, and miR1510, are involved in regulating immune receptors. Light green ovals, including miR472, miR2118, miR2109, miR1507, miR6019, miR6020, miR482, and miR9863, are involved in triggering phasiRNA production.

Table 1.
ncRNAs involved in plant immune reactions.
Moreover, the joint function among ncRNAs, including lncRNAs, siRNAs, miRNAs, and circular RNAs (circRNAs), plays an essential role in plant immunity. Recent studies have shown that various ncRNAs change their expression levels under abiotic and biotic stresses. Interaction of these ncRNAs usually results in the co-regulation of the target gene expressions to modulate plant immunity (Figure 6). Through the complicated interactions that occur between lncRNAs, siRNAs, miRNAs, and circRNAs, a variety of immune responses may be coordinated either synergistically or antagonistically [186].
4.4.1. MicroRNAs
MicroRNAs are produced from precursors called primary microRNAs (pri-miRNAs). DICER-LIKE 1 (DCL1) sequentially cleaves most pri-miRNAs, stabilized in nuclear foci known as dicing bodies. miRNAs mediate PTGS (post-transcriptional gene silencing) through translational inhibition or mRNA degradation [187]. The mutation of DCL1 in different plants results in pleiotropic developmental effects or lethal embryos, mainly because of the significant reduction in the miRNA levels [188]. These findings indicate the importance of miRNAs in the developmental processes of plants. MiRNAs also seem to regulate the epigenomic mechanism because some of these molecules regulate genes involved in chromatin rearrangement. For example, miR773 targets DNA methyltransferase 2 (MET2) transcripts, and after infection with pathogens, the miRNA levels are reduced, leading to the increased accumulation of MET2 required for a proper immune response. The overexpression of miR773 suppresses MET2 and weakens PAMP-triggered immunity [174]. After the miRNAs are processed and exported to the cytoplasm, these transcripts are loaded into one of the several ARGONAUTE (AGO) complexes (see section Plant Argonautes) present in the plant cell (10 paralogs in Arabidopsis) [189]. Various miRNAs have been found to guide the R gene cleavage, thereby strictly controlling the expression of the R gene to regulate the immune response in Solanaceae, Leguminosae, Arabidopsis, etc. Most of these miRNAs trigger the production of phased secondary small interfering RNAs (phasiRNAs) from their nucleotide-binding leucine-rich repeat (NLR) targets [186]. Some miRNAs suppress NLR genes through mRNA cleavage rather than by initiating phasiRNA production to inhibit further NLR genes [162]. These facts indicate that various plant species may utilize a widely conserved immunity mechanism in which miRNAs directly target NLR genes to fine tune the immune response. Some siRNAs and miRNAs directly target signal transduction regulators to modulate immunity. Moreover, many siRNAs and miRNAs target transcription factors to modulate the transcription of genes involved in defense mechanisms. After pathogen infection, the ncRNAs mediate transcriptional reprogramming via direct targeting of transcription factors to regulate various immune responses [186].
4.4.2. Small Interfering RNAs
Small RNAs are non-protein-coding RNAs that are 18–30 nucleotides long and regulate gene expression [22]. Small RNAs are an epigenetic mechanism modulating plant immunity according to various biological stresses. Plants have developed mechanisms to silence foreign gene expressions as defense mechanisms against viral attacks [190].
The vast majority of plant viruses are RNA viruses. A double-stranded RNA (dsRNA) molecule is produced from the viral genome by a viral replicase that catalyzes the synthesis of a complementary RNA molecule using an RNA template. Viral dsRNA can be modified by plant dicer-like proteins, triggering the assembly of small RNA complexes that promote viral genome silencing via degradation of dsRNA replication intermediates and viral transcripts or inhibition of their translation.
Similarly, single-stranded RNA viral genomes initiate silencing mechanisms after being converted to dsRNA molecules by plant RNA-dependent RNA polymerases (RDR). In plant viruses with a single-stranded DNA genome (e.g., Geminivirus), the viral transcripts are converted to dsRNA by the plant RDR2, generating viral small RNAs resembling heterochromatic small RNAs and thereby silencing the viral genome at the transcriptional level [191]. Upon recognizing viruses and other plant pathogens, defense mechanisms initiate ROS production along with small RNAs to regulate the levels of plant hormones, such as SA and JA [34].
SiRNA precursors are long dsRNAs that can be produced by various mechanisms, such as inverted sequence folding, partial complementarity between unrelated transcripts, hybridization of antisense and sense sequences, a lncRNA, and the activity of RDRs. The processing of siRNAs mainly occurs through the actions of DCL2, DCL3, and DCL4, resulting in various types of siRNAs that can be classified into subgroups [189].
4.4.3. Long Non-Coding RNAs
LncRNAs, which are longer than 200 nt, represent another type of non-coding regulatory RNA in the cytoplasm or nucleus. Unlike miRNAs and siRNAs, lncRNAs can function without being processed and cleaved by DCR proteins. Among their multiple functions, lncRNAs have been reported to be involved in modulating mRNA stability and translation, miRNAs and protein hijack, and chromatin modification at different levels [192]. Sometimes, miRNAs can be hijacked by “target mimicry”. This process, also known as miRNA kidnapping, was first described in plants. It occurs through partial complementary sequences when lncRNAs act as decoys for miRNAs by blocking the interaction between a miRNA and its target. Moreover, in animals, lncRNAs involved in the sequestration of miRNAs, known as competing for endogenous RNAs (ceRNAs), have been reported. This mechanism in different organisms indicates its significance in regulating various genetic networks in the eukaryotic cell. For instance, regulating miR399 through miRNA kidnapping is a significant regulator of plant phosphate homeostasis. MiR399 guides the degradation of the PHO2 mRNA and promotes the expression of two phosphate transporters in roots, enhancing phosphate uptake [189].
In plants, disease resistance is usually conferred by a diverse intracellular system of NLR innate immune receptors, which detect pathogen proteins and their effects on the host [193]. These receptors represent valuable agronomic traits that plant breeders rely on to maximize yields in the face of devastating pathogens. PRRs and NLRs are regulated at different transcription levels: post-transcription and post-translation. Transcription factors and kinases are among the downstream signaling components of these receptors.
- Circular RNAs
CircRNA, a new type of endogenous ncRNA, has been studied and characterized in various species. These ncRNAs are commonly generated by thousands of genes and have important functions in regulating gene expression at multiple levels in eukaryotic cells [172]. Based on the spatial- and/or tissue-specific expression patterns, plant circRNAs have been found to function during flowering, fiber development, and fruit coloration [194,195]. The expression of plant circRNAs is also affected by biotic and abiotic stimuli, including pathogen invasion [196,197]. For instance, circRNA45 and circRNA47 in tomato function as positive immunity regulators against Phytophthora infestans by regulating the expression of SpRLK and miR477-3P [198]. CircRNAs are the elaborate regulators of immune signaling that modulate plant immunity [186].
4.5. RNA-Binding Proteins (RBPs)
To modify transcription and translation, RNA-binding proteins (RBPs) frequently phase separate RNAs into condensates [199]. From synthesis to decomposition, RBPs are essential regulators of RNA fate. Therefore, changes in the RBPome coordinate RNA metabolism and gene expression reprogramming during plants’ responses to several signals, including pathogen attacks [200]. The number of RBPs characterized in plants is limited, and their contribution to plant immunity is even less evaluated. Plant RBPs containing RNA-binding domains have putative roles in RNA processing and are also involved in plant immune responses (Table 2). Many RBPs have been identified through genomic analysis in the model plant Arabidopsis thaliana, among which 50% are specific to plants, most with unknown functions. Studying plant RBPs related to pathogen infection response can extend our understanding of similar RBPs in other taxa. Because of the instability of their mRNA targets, RBPs have traditionally been challenging to study. Next-generation sequencing (NGS) methods such as RNA-seq will significantly enhance our ability to analyze both RBPs and RBP knockout mutants [201].

Table 2.
Several RBPs are involved in plant immunity.
4.6. Plant ARGONAUTES
AGOs are the effector proteins in eukaryotic small RNA-based gene silencing pathways controlling gene expression and transposon activity. Eukaryotes produce many small RNAs, such as siRNA, miRNA, scanRNAs, piRNAs, and 21U-RNAs, that each group associates with different AGO family members, such as AGO PIWI, etc. Small RNA-guided AGO proteins regulate gene expression at various levels, including deletion of internal genomic DNA sequences (in ciliates), inhibition of translation (animals), and RNA cleavage (all eukaryotes), which is sometimes followed by chromatin remodeling and DNA methylation. As the model plant species, Arabidopsis contains 10 AGO proteins belonging to 3 phylogenetic groups [215]. AGOs in plants regulate vital biological processes such as genome structure and integrity, development, response to stress, and pathogen defense. Typical functions of plant AGO–sRNA complexes comprise the translational inhibition or endonucleolytic cleavage of target RNAs and the methylation of target DNAs [216] (Table 3).

Table 3.
The biological pattern of plant ARGONAUTEs.
4.7. RNAi-Based Antiviral Innate Immunity in Plants
According to numerous studies, RNAi is found to be evolutionarily conserved in eukaryotes and to govern all elements of biological events [246]. Non-coding small RNA (ncRNA) is activated by self-complementary or dsRNA and serves as the signal and specificity determinant of gene silencing. Pathogen RNA-derived siRNAs of various sizes are created during pathogen infection to induce RNAi-based antimicrobial immunity and confer host resistance [158,247]. Plant researchers discovered the first RNAi-based antiviral defense agent [12,248] to play an essential role in antiviral immunity in invertebrates [12] and mammals [18,29]. The function of DCLs, AGOs, and RDRs in antiviral immunity was based on discoveries in transgenic silencing and endogenous gene silencing. It is now known that RNAi-based antiviral innate immunity is developed in virtually all eukaryotes to resist invasion by various RNA or DNA viruses. The function of DCLs, AGOs, and RDRs in antiviral immunity was based on discoveries in transgenic silencing and endogenous gene silencing. It is now understood that RNAi-based antiviral innate immunity will be triggered in practically all eukaryotes to resist the aggression of all types of RNA or DNA viruses (Figure 7). Many viruses, particularly pathogenic viruses, evolve viral suppressors of RNAi (VSRs) to attack distinct steps of the RNAi-based antiviral pathway (Figure 7). The predominance of VSR contributes to viral epidemics and blinds us to the importance of RNAi-based antiviral innate immunity. Furthermore, VSR hampered the use of traditional genetic screens to uncover new regulators in the antiviral pathway for decades until recently, when an effective genetic technique to overcome the barrier was devised [249,250].
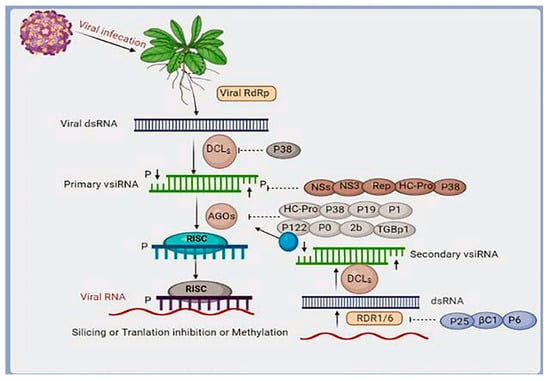
Figure 7.
An RNAi-based antiviral mechanism in Arabidopsis. Following viral infection, the double-stranded viral RNA replication intermediate is recognized and processed by DCL4, DCL2, or DCL3, producing 21, 22, or 24 nucleotide duplex primary viral siRNAs. These viral siRNAs will be uploaded into AGO1 or AGO2 to create RISC and induce RNA virus slicing or translation inhibition via PTGS. In contrast, 24nt viral siRNAs will be uploaded to AGO4, AGO6, or AGO9 to create RISC to induce DNA methylation or histone modification via TGS, hence silencing DNA viruses. Secondary viral siRNAs generated by RDR1/RDR6 or RDR2 amplification are necessary to establish an RNAi-based antiviral defense against RNA viruses or DNA viruses. Some viral suppressors of RNAi (VSRs), such as the NSs of tomato spotted wilt virus and the Hc-Pro of potato virus Y, also connect to long viral dsRNA to prevent DCLs from sensing or processing viral RNAs. Some of the other VSRs might be able to directly attack vsiRNA to suppress the RNAi-based antiviral innate immune response. For instance, P19, a well-known VSR found in tombusviruses, can connect to vsiRNA and sequester it [251]. Some VSRs can also disrupt the antiviral activity of effector AGOs. For instance, the P0 protein of the potato leafroll virus can activate the degradation of AGO1 [252], and the 2b protein of the cytomegalovirus can interrupt the function of AGO1 and AGO4 [253]. P38 of the carmovirus TCV interacts with dsRNAs to block the activities of DCL4 and AGO1 [254]. The Ipomovirus P1 protein inhibits the formation of dsRNA, suppressing the local silencing of RNA and interfering with AGO1 [255]. Tobamovirus P122 is responsible for binding dsRNA, mediating methylation of small RNA, and inhibiting AGO1 [256]. Cucumovirus 2b binds with dsRNA to downregulate the concentration of RDR6 mRNA and interacts with AGO1 and AGO4 to block AGO1. It also binds with dsRNA to inhibit AGO1, which inhibits AGO1 [257]. Begomovirus strain βC1 is responsible for repressing RDR6 expression, which blocks the generation of secondary siRNA [258]. AGO1 interacts with Potexvirus TGBp1 (P25) and promotes the degradation of AGO1 via the proteasome pathway [259]. Caulimovirus P6 inhibits RDR6-mediated biosynthesis of secondary siRNAs [260]. The Rep of Mastrevirus binds to siRNA to block its translation [261].
5. Conclusions
Plants respond to biotic and abiotic stressors by modifying gene expression, protein structure, and metabolic circuits. These physiological responses depend on the perception and transmission of numerous stress cues, which cause multiple defensive mechanisms to be activated. Given the importance of protein phosphorylation and dephosphorylation in signal transduction, protein kinases and phosphatases are essential in plant defense pathways. In general, abiotic stressors cause large-scale alterations in gene expression, which correspond to fundamental plant physiological functions such as photosynthesis, respiration, and metabolic pathways.
However, the processes that control GA biosynthesis are still unknown. Because of GA’s importance in the agricultural economy, the molecular processes controlling plant hormone interactions, particularly between DELLA proteins and other vital members of hormone signaling pathways, have been intensively studied. JA and SA are also required for stress signal transduction in plants. Plant stimulation with JA or SA stimulates various defensive systems via the stearic acid pathway, protecting plants from abiotic stressors. Despite this, little is known about JA and SA’s roles in plant responses to biotic stress. Future research must uncover the processes that allow JA-and SA-mediated signals to be transmitted across plant cells.
ET is another plant hormone that is induced by biotic and abiotic stressors. Despite extensive research into ET signal transduction in plants, little is known about the function of ET-responsive genes. Plant adaptation to abiotic stress requires ET pathway components such as EIN2 and EIN3/EIl1. However, the roles of ET pathway components in stress reactions are unknown. The discovery of the molecular processes underlying ET-mediated stress adaptation is predicted to improve crop yield and have a high agricultural economic value. In conclusion, ET signaling is critical in plant responses to drought and salinity challenges, but its role in other abiotic stresses is unknown.
The ABA signal transduction pathway is critical for plants’ responses to abiotic stresses, and SnRK2 is a vital component of the ABA signal transduction pathway, relating metabolism to stress responses under extreme abiotic stresses. SnRK2 regulates stress signal transduction in response to drought, salt, and osmotic stress. Although the function of SnRK2 in the ABA pathway is well established, its role in ABA-independent responses remains unknown. Furthermore, despite phosphorylation activating SnRK2, the effect of other post-translational modifications on SnRK2 activation and function is unknown. The role of SnRK2 in osmotic stress adaptation is also unknown. Future research must elucidate the mechanisms underlying SnRK2 activation and function in response to various stress signals.
Significant progress has been made in understanding the role of GA metabolism in plants in recent years. The role of GA metabolism in plant growth and development regulation is now relatively well understood. Unexpectedly, the function of GA metabolism in responding to abiotic stress is still largely unknown.
In the promoter regions of genes, many regulatory sequences are typically found. These regulatory sequences make it possible to fine tune the expression of genes in response to a variety of stimuli from the environment. The increased adaptability of plants is largely due to the crosstalk between the various plant hormones. Plants have developed sophisticated mechanisms for responding to stress, which allow them to mount a strong defense and successfully adapt without compromising their ability to continue growing. This system is able to detect stressful conditions such as low temperatures, high temperatures, drought, toxicity from heavy metals, and oxidative stress.
Plant epigenetic regulation of immunity, including histone modifications, DNA methylation, chromatin remodeling, and RNA silencing, has been extensively explored in plants, particularly Arabidopsis. The primary defense mechanism against viruses is mediated by small RNAs, triggering viral genome degradation. However, how plants differentiate new viruses from previously exposed ones is unclear. Future studies are required to assess the role of histone ubiquitination-mediated regulation of gene expression in plant adaptation to stress. Identification of the mechanisms involved in the propagation of epigenetic modifications regulating plant immunity merits further investigation.
Author Contributions
A.M. designed and supervised the project. S.A.-D., B.B., S.K., H.W. and S.S., drafted and wrote the manuscript. L.Y. reviewed and commented. C.X. funded this project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Natural Science Foundation of China (31400347); The Natural Science of the Jiangsu Higher Education Institutions of China (18KJA180007); and Nanjing Key Laboratory of Quality and safety of agricultural products (NJGS2021-16).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the funders of this research, the editors, and the reviewers for the effort and time spent giving helpful comments to improve our work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Dong, X.N. How do plants achieve immunity?: Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Baek, K.H. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Chen, Y.C.; Sattely, E.S.; Mudgett, M.B. An engineered pathway for N-hydroxy-pipecolic acid synthesis enhances systemic acquired resistance in tomato. Sci. Signal. 2019, 12, eaay3066. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Whitaker, V.M.; Hutton, S.F. Mini Review: Potential Applications of Non-host Resistance for Crop Improvement. Front. Plant Sci. 2016, 7, 997. [Google Scholar] [CrossRef] [PubMed]
- Salguero-Linares, J.; Coll, N.S. Plant proteases in the control of the hypersensitive response. J. Exp. Bot. 2019, 70, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; de Vos, R.C.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Liu, G.; Li, Y.; Liu, S.; Yu, C.; Chen, Y.; Zhong, F.; Zhang, J. Genome-Wide Characterization and Abiotic Stresses Expression Analysis of Annexin Family Genes in Poplar. Int. J. Mol. Sci. 2022, 23, 515. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Shabala, L.; Cuin, T.A.; Huang, X.; Zhou, M.; Munns, R.; Shabala, S. Nax loci affect SOS1-like Na+/H+ exchanger expression and activity in wheat. J. Exp. Bot. 2016, 67, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Sato, M.; Glazebrook, J.; Cohen, J.D.; Katagiri, F. Interplay between MAMP-triggered and SA-mediated defense responses. Plant J. 2008, 53, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, A.; Pieterse, C.M.J. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Leon-Reyes, A.; van der Ent, S.; van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Navarro, L.; Jay, F.; Nomura, K.; He, S.Y.; Voinnet, O. Suppression of the microRNA pathway by bacterial effector proteins. Science 2008, 321, 964–967. [Google Scholar] [CrossRef]
- Zhai, K.; Deng, Y.; Liang, D.; Tang, J.; Liu, J.; Yan, B.; Yin, X.; Lin, H.; Chen, F.; Yang, D.; et al. RRM Transcription Factors Interact with NLRs and Regulate Broad-Spectrum Blast Resistance in Rice. Mol. Cell 2019, 74, 996–1009.e7. [Google Scholar] [CrossRef]
- Albert, I.; Hua, C.; Nürnberger, T.; Pruitt, R.N.; Zhang, L. Surface Sensor Systems in Plant Immunity. Plant Physiol. 2020, 182, 1582–1596. [Google Scholar] [CrossRef]
- Wan, W.-L.; Fröhlich, K.; Pruitt, R.N.; Nürnberger, T.; Zhang, L. Plant cell surface immune receptor complex signaling. Curr. Opin. Plant Biol. 2019, 50, 18–28. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Dong, M.; Wu, Z.; Shen, Z.; Xie, Y.; Kong, Z.; Dai, X.; Xu, B. Metabolic reprogramming and AMPKα1 pathway activation by caulerpin in colorectal cancer cells. Int. J. Oncol. 2017, 50, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, A.; Zhang, J.; Sun, W.; Kadkhodaei, S.; Mohammadi, K.; Almasizadehyaghuti, A.; Yin, T.; Zhuge, Q. Plant small RNAs: Definition, classification and response against stresses. Biologia 2018, 73, 285–294. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.M. Plant immunity triggered by microbial molecular signatures. Mol. Plant 2010, 3, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.C.; Kozik, A.; Griego, A.; Kuang, H.H.; Michelmore, R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 2003, 15, 809–834. [Google Scholar] [CrossRef]
- Tsuda, K.; Sato, M.; Stoddard, T.; Glazebrook, J.; Katagiri, F. Network Properties of Robust Immunity in Plants. PLoS Genet. 2009, 5, e1000772. [Google Scholar] [CrossRef]
- Ramirez-Prado, J.S.; Abulfaraj, A.A.; Rayapuram, N.; Benhamed, M.; Hirt, H. Plant Immunity: From Signaling to Epigenetic Control of Defense. Trends Plant Sci. 2018, 23, 833–844. [Google Scholar] [CrossRef]
- Ding, B.; Wang, G.L. Chromatin versus pathogens: The function of epigenetics in plant immunity. Front. Plant Sci. 2015, 6, 675. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Prasad, M. Plant innate immunity: An updated insight into defense mechanism. J. Biosci. 2013, 38, 433–449. [Google Scholar] [CrossRef]
- Habib, H.; Majid, K. Plant protease inhibitors: A defense strategy in plants. BMBR 2007, 2, 68–85. [Google Scholar] [CrossRef]
- Césari, S.; Kanzaki, H.; Fujiwara, T.; Bernoux, M.; Chalvon, V.; Kawano, Y.; Shimamoto, K.; Dodds, P.; Terauchi, R.; Kroj, T. The NB-LRR proteins RGA4 and RGA5 interact functionally and physically to confer disease resistance. EMBO J. 2014, 33, 1941–1959. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Hemetsberger, C. Apoplastic immunity and its suppression by filamentous plant pathogens. New Phytol. 2013, 198, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Erb, M. Plant Biology: Evolution of Volatile-Mediated Plant-Plant Interactions. Curr. Biol. 2019, 29, R873–R875. [Google Scholar] [CrossRef]
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef]
- Ádám, A.L.; Nagy, Z.Á.; Kátay, G.; Mergenthaler, E.; Viczián, O. Signals of Systemic Immunity in Plants: Progress and Open Questions. Int. J. Mol. Sci. 2018, 19, 1146. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Yang, D.-L.; Yang, Y.; He, Z. Roles of plant hormones and their interplay in rice immunity. Mol. Plant 2013, 6, 675–685. [Google Scholar] [CrossRef]
- Dey, S.; Wenig, M.; Langen, G.; Sharma, S.; Kugler, K.G.; Knappe, C.; Hause, B.; Bichlmeier, M.; Babaeizad, V.; Imani, J.; et al. Bacteria-triggered systemic immunity in barley is associated with WRKY and ETHYLENE RESPONSIVE FACTORs but not with salicylic acid. Plant Physiol. 2014, 166, 2133–2151. [Google Scholar] [CrossRef]
- Lenk, M.; Wenig, M.; Bauer, K.; Hug, F.; Knappe, C.; Lange, B.; Timsy; Häußler, F.; Mengel, F.; Dey, S.; et al. Pipecolic Acid Is Induced in Barley upon Infection and Triggers Immune Responses Associated with Elevated Nitric Oxide Accumulation. Mol. Plant Microbe Interact. 2019, 32, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Ramlal, A.; Kumar, K.; Rani, A.; Mishra, V. Signaling Pathways and Downstream Effectors of Host Innate Immunity in Plants. Int. J. Mol. Sci. 2021, 22, 9022. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Manohar, M.; Manosalva, P.; Tian, M.; Moreau, M.; Klessig, D.F. Activation of Plant Innate Immunity by Extracellular High Mobility Group Box 3 and Its Inhibition by Salicylic Acid. PLoS Pathog. 2016, 12, e1005518. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Yan, H.; Li, Y. Flagellin: A unique microbe-associated molecular pattern and a multi-faceted immunomodulator. Cell Mol. Immunol. 2017, 14, 862–864. [Google Scholar] [CrossRef]
- Latrasse, D.; Jégu, T.; Li, H.; de Zelicourt, A.; Raynaud, C.; Legras, S.; Gust, A.; Samajova, O.; Veluchamy, A.; Rayapuram, N.; et al. MAPK-triggered chromatin reprogramming by histone deacetylase in plant innate immunity. Genome Biol. 2017, 18, 131. [Google Scholar] [CrossRef]
- Kozieł, E.; Otulak-Kozieł, K.; Bujarski, J.J. Plant Cell Wall as a Key Player during Resistant and Susceptible Plant-Virus Interactions. Front. Microbiol. 2021, 12, 656809. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Joshi-Saha, A.; Valon, C.; Leung, J. A brand new START: Abscisic acid perception and transduction in the guard cell. Sci. Signal. 2011, 4, re4. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to Environmental Stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef]
- Zhang, J.H.; Jia, W.S.; Yang, J.C.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Barrero, J.M.; Piqueras, P.; Gonzalez-Guzman, M.; Serrano, R.; Rodriguez, P.L.; Ponce, M.R.; Micol, J.L. A mutational analysis of the ABA1 gene of Arabidopsis thaliana highlights the involvement of ABA in vegetative development. J. Exp. Bot. 2005, 56, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zeevaart, J.A. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc. Natl. Acad. Sci. USA 1999, 96, 15354–15361. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, M.; Apostolova, N.; Bellés, J.M.; Barrero, J.M.; Piqueras, P.; Ponce, M.R.; Micol, J.L.; Serrano, R.; Rodríguez, P.L. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 2002, 14, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Boudsocq, M.; Barbier-Brygoo, H.; Lauriere, C. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J. Biol. Chem. 2004, 279, 41758–41766. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Yamamoto, S.; Minami, H.; Kagaya, Y.; Hattori, T. Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 2004, 16, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.M.; Soon, F.F.; Zhou, X.E.; West, G.M.; Kovach, A.; Suino-Powell, K.M.; Chalmers, M.J.; Li, J.; Yong, E.L.; Zhu, J.K.; et al. Structural basis for basal activity and autoactivation of abscisic acid (ABA) signaling SnRK2 kinases. Proc. Natl. Acad. Sci. USA 2011, 108, 21259–21264. [Google Scholar] [CrossRef]
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 2010, 51, 1821–1839. [Google Scholar] [CrossRef]
- Kline, K.G.; Barrett-Wilt, G.A.; Sussman, M.R. In planta changes in protein phosphorylation induced by the plant hormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 15986–15991. [Google Scholar] [CrossRef]
- Wang, P.C.; Dua, Y.Y.; Hou, Y.J.; Zhao, Y.; Hsu, C.C.; Yuan, F.J.; Zhu, X.H.; Tao, W.A.; Song, C.P.; Zhu, J.K. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc. Natl. Acad. Sci. USA 2015, 112, 613–618. [Google Scholar] [CrossRef]
- McLoughlin, F.; Galvan-Ampudia, C.S.; Julkowska, M.M.; Caarls, L.; van der Does, D.; Lauriere, C.; Munnik, T.; Haring, M.A.; Testerink, C. The Snf1-related protein kinases SnRK2.4 and SnRK2.10 are involved in maintenance of root system architecture during salt stress. Plant J. 2012, 72, 436–449. [Google Scholar] [CrossRef]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant 2013, 147, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.R.; Wagner, R.L.; Verhey, S.D.; Walker-Simmons, M.K. The abscisic acid-responsive kinase PKABA1 interacts with a seed-specific abscisic acid response element-binding factor, TaABF, and phosphorylates TaABF peptide sequences. Plant Physiol. 2002, 130, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Negi, J.; Matsuda, O.; Nagasawa, T.; Oba, Y.; Takahashi, H.; Kawai-Yamada, M.; Uchimiya, H.; Hashimoto, M.; Iba, K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 2008, 452, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, M.-X.; Jiang, M.; Wang, Y.-D. Spectroscopic investigation of the interaction between human serum albumin and three organic acids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 2245–2251. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, Z.F.; Quan, R.D.; Li, G.J.; Wang, R.G.; Huang, R.F. An AP2 Domain-Containing Gene, ESE1, Targeted by the Ethylene Signaling Component EIN3 Is Important for the Salt Response in Arabidopsis. Plant Physiol. 2011, 157, 854–865. [Google Scholar] [CrossRef]
- Tudzynski, B.; Mihlan, M.; Rojas, M.C.; Linnemannstons, P.; Gaskin, P.; Hedden, P. Characterization of the final two genes of the gibberellin biosynthesis gene cluster of Gibberella fujikuroi: Des and P450-3 encode GA4 desaturase and the 13-hydroxylase, respectively. J. Biol. Chem. 2003, 278, 28635–28643. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Z.; Wang, Y.; Zheng, L.; Ye, R.; Ji, Y.; Zhao, S.; Ji, S.; Liu, R.; Xu, L.; et al. Viral-inducible Argonaute18 confers broad-spectrum virus resistance in rice by sequestering a host microRNA. eLife 2015, 4, e05733. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Qin, Q.Q.; Yue, J.; Huang, B.Y.; Xu, X.F.; Yan, L.F.; Hou, S.W. The six conserved serine/threonine sites of REPRESSOR OF ga1-3 protein are important for its functionality and stability in gibberellin signaling in Arabidopsis. Planta 2014, 240, 763–779. [Google Scholar] [CrossRef]
- Sun, T.P. Gibberellin-GID1-DELLA: A pivotal regulatory module for plant growth and development. Plant Physiol. 2010, 154, 567–570. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hirai, T.; Yamamoto, E.; Kawamura, M.; Sato, T.; Kitano, H.; Matsuoka, M.; Ueguchi-Tanaka, M. A Rice gid1 Suppressor Mutant Reveals That Gibberellin Is Not Always Required for Interaction between Its Receptor, GID1, and DELLA Proteins. Plant Cell 2010, 22, 3589–3602. [Google Scholar] [CrossRef]
- Lozano-Juste, J.; Leon, J. Nitric Oxide Regulates DELLA Content and PIF Expression to Promote Photomorphogenesis in Arabidopsis. Plant Physiol. 2011, 156, 1410–1423. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chang, M.; Wang, B.; Wu, B. Cloning of a Ca(2+)-ATPase gene and the role of cytosolic Ca2+ in the gibberellin-dependent signaling pathway in aleurone cells. Plant J. 1997, 11, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tian, S.; Hou, L.; Huang, X.; Zhang, X.; Guo, H.; Yang, S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef] [PubMed]
- Catalá, R.; López-Cobollo, R.; Mar Castellano, M.; Angosto, T.; Alonso, J.M.; Ecker, J.R.; Salinas, J. The Arabidopsis 14-3-3 protein RARE COLD INDUCIBLE 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. Plant Cell 2014, 26, 3326–3342. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Chen, D.; Ma, X.; Li, C.; Zhang, W.; Xia, G.; Wang, M. A wheat aminocyclopropane-1-carboxylate oxidase gene, TaACO1, negatively regulates salinity stress in Arabidopsis thaliana. Plant Cell Rep. 2014, 33, 1815–1827. [Google Scholar] [CrossRef]
- Desikan, R.; Last, K.; Harrett-Williams, R.; Tagliavia, C.; Harter, K.; Hooley, R.; Hancock, J.T.; Neill, S.J. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 2006, 47, 907–916. [Google Scholar] [CrossRef]
- Yang, X.J.; Seto, E. The Rpd3/Hda1 family of lysine deacetylases: From bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 2008, 9, 206–218. [Google Scholar] [CrossRef]
- Ji, Y.S.; Guo, H.W. From Endoplasmic Reticulum (ER) to Nucleus: EIN2 Bridges the Gap in Ethylene Signaling. Mol. Plant 2013, 6, 11–14. [Google Scholar] [CrossRef]
- Guo, H.; Ecker, J.R. Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 2003, 115, 667–677. [Google Scholar] [CrossRef]
- Binder, B.M.; Walker, J.M.; Gagne, J.M.; Emborg, T.J.; Hemmann, G.; Bleecker, A.B.; Vierstra, R.D. The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell 2007, 19, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Novillo, F.; Medina, J.; Salinas, J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc. Natl. Acad. Sci. USA 2007, 104, 21002–21007. [Google Scholar] [CrossRef] [PubMed]
- Onate-Sanchez, L.; Anderson, J.P.; Young, J.; Singh, K.B. AtERF14, a member of the ERF family of transcription factors, plays a nonredundant role in plant defense. Plant Physiol. 2007, 143, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sonbol, F.-M.; Huot, B.; Gu, Y.; Withers, J.; Mwimba, M.; Yao, J.; He, S.Y.; Dong, X. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 13099. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. Intervention of Phytohormone Pathways by Pathogen Effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef]
- Dong, C.J.; Li, L.; Shang, Q.M.; Liu, X.Y.; Zhang, Z.G. Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 2014, 240, 687–700. [Google Scholar] [CrossRef]
- Hu, Y.R.; Jiang, L.Q.; Wang, F.; Yu, D.Q. Jasmonate Regulates the INDUCER OF CBF EXPRESSION-C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 Cascade and Freezing Tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, L.; Wang, X.G.; Hou, Y.J.; Gao, J.H.; Wang, P.C.; Duan, C.G.; Zhu, X.H.; Zhu, J.K. The ABA Receptor PYL8 Promotes Lateral Root Growth by Enhancing MYB77-Dependent Transcription of Auxin-Responsive Genes. Sci. Signal. 2014, 7, ra53. [Google Scholar] [CrossRef]
- Choi, K.; Park, C.; Lee, J.; Oh, M.; Noh, B.; Lee, I. Arabidopsis homologs of components of the SWR1 complex regulate flowering and plant development. Development 2007, 134, 1931–1941. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Wang, C.; He, J.; Liao, H.; Duan, Y.; Zhu, Z.; Guo, Y.; Chen, Z.; Gong, Z. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 2012, 24, 2546–2561. [Google Scholar] [CrossRef] [PubMed]
- Shah, J. The salicylic acid loop in plant defense. Curr. Opin. Plant Biol. 2003, 6, 365–371. [Google Scholar] [CrossRef]
- Guo, H.; Ecker, J.R. The ethylene signaling pathway: New insights. Curr. Opin. Plant Biol. 2004, 7, 40–49. [Google Scholar] [CrossRef]
- Yu, M.M.; Shen, L.; Fan, B.; Zhao, D.Y.; Zheng, Y.; Sheng, J.P. The effect of MeJA on ethylene biosynthesis and induced disease resistance to Botrytis cinerea in tomato. Postharvest Biol. Technol. 2009, 54, 153–158. [Google Scholar] [CrossRef]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Zhang, X.; Zhu, Z.; Ji, Y.; He, W.; Jiang, Z.; Li, M.; Guo, H. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 2012, 22, 915–927. [Google Scholar] [CrossRef]
- Bastian, R.; Dawe, A.; Meier, S.; Ludidi, N.; Bajic, V.B.; Gehring, C. Gibberellic acid and cGMP-dependent transcriptional regulation in Arabidopsis thaliana. Plant Signal. Behav. 2010, 5, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; El-kereamy, A.; Zhu, T.; Beatty, P.H.; Good, A.G.; Bi, Y.M.; Rothstein, S.J. The APETALA-2-Like Transcription Factor OsAP2-39 Controls Key Interactions between Abscisic Acid and Gibberellin in Rice. PLoS Genet. 2010, 6, e1001098. [Google Scholar] [CrossRef]
- Zentella, R.; Zhang, Z.L.; Park, M.; Thomas, S.G.; Endo, A.; Murase, K.; Fleet, C.M.; Jikumaru, Y.; Nambara, E.; Kamiya, Y.; et al. Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 2007, 19, 3037–3057. [Google Scholar] [CrossRef]
- Luger, K.; Mader, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jeong, J.C.; Zhu, Y.; Sokolchik, I.; Miyazaki, S.; Zhu, J.K.; Hasegawa, P.M.; Bohnert, H.J.; Shi, H.; Yun, D.J.; et al. Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc. Natl. Acad. Sci. USA 2008, 105, 4945–4950. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Ding, A.B.; Zhong, X.H. Functions and mechanisms of plant histone deacetylases. Sci. China Life Sci. 2020, 63, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.M.; Tian, L.; Chen, Z.J. Arabidopsis thaliana histone deacetylase 1 (AtHD1) is localized in euchromatic regions and demonstrates histone deacetylase activity in vitro. Cell Res. 2006, 16, 479–488. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhang, L.; Duan, J.; Miki, B.; Wu, K.Q. HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell 2005, 17, 1196–1204. [Google Scholar] [CrossRef]
- Song, C.P.; Galbraith, D.W. AtSAP18, an orthologue of human SAP18, is involved in the regulation of salt stress and mediates transcriptional repression in Arabidopsis. Plant Mol. Biol. 2006, 60, 241–257. [Google Scholar] [CrossRef]
- Kim, K.C.; Lai, Z.; Fan, B.; Chen, Z. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell 2008, 20, 2357–2371. [Google Scholar] [CrossRef]
- Aufsatz, W.; Stoiber, T.; Rakic, B.; Naumann, K. Arabidopsis histone deacetylase 6: A green link to RNA silencing. Oncogene 2007, 26, 5477–5488. [Google Scholar] [CrossRef][Green Version]
- Mengel, A.; Ageeva, A.; Georgii, E.; Bernhardt, J.; Wu, K.Q.; Durner, J.; Lindermayr, C. Nitric Oxide Modulates Histone Acetylation at Stress Genes by Inhibition of Histone Deacetylases. Plant Physiol. 2017, 173, 1434–1452. [Google Scholar] [CrossRef]
- Vaillant, I.; Paszkowski, J. Role of histone and DNA methylation in gene regulation. Curr. Opin. Plant Biol. 2007, 10, 528–533. [Google Scholar] [CrossRef]
- Turck, F.; Roudier, F.; Farrona, S.; Martin-Magniette, M.L.; Guillaume, E.; Buisine, N.; Gagnot, S.; Martienssen, R.A.; Coupland, G.; Colot, V. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 2007, 3, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Bernatavichute, Y.V.; Zhang, X.Y.; Cokus, S.; Pellegrini, M.; Jacobsen, S.E. Genome-Wide Association of Histone H3 Lysine Nine Methylation with CHG DNA Methylation in Arabidopsis thaliana. PLoS ONE 2008, 3, e3156. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Bernatavichute, Y.V.; Cokus, S.; Pellegrini, M.; Jacobsen, S.E. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol. 2009, 10, R62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Clarenz, O.; Cokus, S.; Bernatavichute, Y.V.; Pellegrini, M.; Goodrich, J.; Jacobsen, S.E. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 2007, 5, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Kariola, T.; Palva, E.T. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006, 46, 477–491. [Google Scholar] [CrossRef]
- Alvarez-Venegas, R.; Al Abdallat, A.; Guo, M.; Alfano, J.R.; Avramova, Z. Epigenetic control of a transcription factor at the cross section of two antagonistic pathways. Epigenetics 2007, 2, 106–113. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef]
- Abbasi, S.; Sadeghi, A.; Safaie, N. Streptomyces alleviate drought stress in tomato plants and modulate the expression of transcription factors ERF1 and WRKY70 genes. Sci. Hortic.-Amst. 2020, 265, 109206. [Google Scholar] [CrossRef]
- Weake, V.M.; Workman, J.L. Histone ubiquitination: Triggering gene activity. Mol. Cell 2008, 29, 653–663. [Google Scholar] [CrossRef]
- Dhawan, R.; Luo, H.L.; Foerster, A.M.; AbuQamar, S.; Du, H.N.; Briggs, S.D.; Scheid, O.M.; Mengiste, T. HISTONE MONOUBIQUITINATION1 Interacts with a Subunit of the Mediator Complex and Regulates Defense against Necrotrophic Fungal Pathogens in Arabidopsis. Plant Cell 2009, 21, 1000–1019. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.H.; Yang, D.L.; Shi, Z.Y.; Dong, H.S.; Hua, J. Monoubiquitination of Histone 2B at the Disease Resistance Gene Locus Regulates Its Expression and Impacts Immune Responses in Arabidopsis. Plant Physiol. 2014, 165, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Menard, R.; Verdier, G.; Ors, M.; Erhardt, M.; Beisson, F.; Shen, W.H. Histone H2B Monoubiquitination is Involved in the Regulation of Cutin and Wax Composition in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Feng, H.; Zhang, X.Y.; Zhang, C.J.; Wang, T.; Dong, J.L. An Arabidopsis E3 ligase HUB2 increases histone H2B monoubiquitination and enhances drought tolerance in transgenic cotton. Plant Biotechnol. J. 2019, 17, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Pardal, A.J.; Piquerez, S.J.M.; Dominguez-Ferreras, A.; Frungillo, L.; Mastorakis, E.; Reilly, E.; Latrasse, D.; Concia, L.; Gimenez-Ibanez, S.; Spoel, S.H.; et al. Immunity onset alters plant chromatin and utilizes EDA16 to regulate oxidative homeostasis. PLoS Pathog. 2021, 17, e1009572. [Google Scholar] [CrossRef]
- Kang, H.; Liu, Y.; Fan, T.; Ma, J.; Ma, J.; Wu, D.; Heitz, T.; Heitz, T.; Shen, W.-H.; Shen, W.-H.; et al. Arabidopsis CHROMATIN REMODELING 19 acts as a transcriptional repressor and contributes to plant pathogen resistance. Plant Cell 2022, 34, 1100–1116. [Google Scholar] [CrossRef]
- Wang, S.; Wu, X.-M.; Liu, C.-H.; Shang, J.-Y.; Gao, F.; Guo, H.-S. Verticillium dahliae chromatin remodeling facilitates the DNA damage repair in response to plant ROS stress. PLoS Pathog. 2020, 16, e1008481. [Google Scholar] [CrossRef]
- Flaus, A.; Martin, D.M.; Barton, G.J.; Owen-Hughes, T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res 2006, 34, 2887–2905. [Google Scholar] [CrossRef]
- Knizewski, L.; Ginalski, K.; Jerzmanowski, A. Snf2 proteins in plants: Gene silencing and beyond. Trends Plant Sci. 2008, 13, 557–565. [Google Scholar] [CrossRef]
- Zhang, D.D.; Gao, S.J.; Yang, P.; Yang, J.; Yang, S.G.; Wu, K.Q. Identification and Expression Analysis of Snf2 Family Proteins in Tomato (Solanum lycopersicum). Int. J. Genom. 2019, 2019, 5080935. [Google Scholar] [CrossRef]
- Krogan, N.J.; Keogh, M.C.; Datta, N.; Sawa, C.; Ryan, O.W.; Ding, H.M.; Haw, R.A.; Pootoolal, J.; Tong, A.; Canadien, V.; et al. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol. Cell 2003, 12, 1565–1576. [Google Scholar] [CrossRef]
- Mizuguchi, G.; Shen, X.T.; Landry, J.; Wu, W.H.; Sen, S.; Wu, C. ATP-Driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 2004, 303, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.S.; Amasino, R.M. PIE1, an ISWI family gene, is required for FLC activation and floral repression in Arabidopsis. Plant Cell 2003, 15, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Roberts, D.N.; Cairns, B.R. Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 2005, 123, 219–231. [Google Scholar] [CrossRef]
- Venkatasubrahmanyam, S.; Hwang, W.W.; Meneghini, M.D.; Tong, A.H.Y.; Madhani, H.D. Genome-wide, as opposed to local, antisilencing is mediated redundantly by the euchromatic factors Set1 and H2A.Z. Proc. Natl. Acad. Sci. USA 2007, 104, 16609–16614. [Google Scholar] [CrossRef]
- Guillemette, B.; Gaudreau, L. Reuniting the contrasting functions of H2A.Z. Biochem. Cell Biol. 2006, 84, 528–535. [Google Scholar] [CrossRef]
- Zilberman, D.; Coleman-Derr, D.; Ballinger, T.; Henikoff, S. Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature 2008, 456, 125–129. [Google Scholar] [CrossRef]
- Kwon, C.S.; Wagner, D. Unwinding chromatin for development and growth: A few genes at a time. Trends Genet. 2007, 23, 403–412. [Google Scholar] [CrossRef]
- Walley, J.W.; Rowe, H.C.; Xiao, Y.M.; Chehab, E.W.; Kliebenstein, D.J.; Wagner, D.; Dehesh, K. The Chromatin Remodeler SPLAYED Regulates Specific Stress Signaling Pathways. PLoS Pathog. 2008, 4, e1000237. [Google Scholar] [CrossRef]
- Johnson, K.C.M.; Xia, S.; Feng, X.; Li, X. The Chromatin Remodeler SPLAYED Negatively Regulates SNC1-Mediated Immunity. Plant Cell Physiol. 2015, 56, 1616–1623. [Google Scholar] [CrossRef]
- Peirats-Llobet, M.; Han, S.K.; Gonzalez-Guzman, M.; Jeong, C.W.; Rodriguez, L.; Belda-Palazon, B.; Wagner, D.; Rodriguez, P.L. A Direct Link between Abscisic Acid Sensing and the Chromatin-Remodeling ATPase BRAHMA via Core ABA Signaling Pathway Components. Mol. Plant 2016, 9, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Prado, J.S.; Piquerez, S.J.M.; Bendahmane, A.; Hirt, H.; Raynaud, C.; Benhamed, M. Modify the Histone to Win the Battle: Chromatin Dynamics in Plant-Pathogen Interactions. Front. Plant Sci. 2018, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, S.; Kobayashi, A.; Kawabe, A.; Mathieu, O.; Miura, A.; Kakutani, T. Bursts of retrotransposition reproduced in Arabidopsis. Nature 2009, 461, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Furci, L.; Jain, R.; Stassen, J.; Berkowitz, O.; Whelan, J.; Roquis, D.; Baillet, V.; Colot, V.; Johannes, F.; Ton, J. Identification and characterisation of hypomethylated DNA loci controlling quantitative resistance in Arabidopsis. eLife 2019, 8, e40655. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Sun, Q.; Zhang, W.; Ding, Y.; Yang, D.L.; Shi, Z.; Hua, J. The Arabidopsis Chromatin-Remodeling Factor CHR5 Regulates Plant Immune Responses and Nucleosome Occupancy. Plant Cell Physiol. 2017, 58, 2202–2216. [Google Scholar] [CrossRef]
- Lopez Sanchez, A.; Stassen, J.H.; Furci, L.; Smith, L.M.; Ton, J. The role of DNA (de)methylation in immune responsiveness of Arabidopsis. Plant J. 2016, 88, 361–374. [Google Scholar] [CrossRef]
- Wilkinson, S.W.; Mageroy, M.H.; Lopez Sanchez, A.; Smith, L.M.; Furci, L.; Cotton, T.E.A.; Krokene, P.; Ton, J. Surviving in a Hostile World: Plant Strategies to Resist Pests and Diseases. Annu. Rev. Phytopathol. 2019, 57, 505–529. [Google Scholar] [CrossRef]
- Akimoto, K.; Katakami, H.; Kim, H.J.; Ogawa, E.; Sano, C.M.; Wada, Y.; Sano, H. Epigenetic inheritance in rice plants. Ann. Bot. 2007, 100, 205–217. [Google Scholar] [CrossRef]
- Lister, R.; O’Malley, R.C.; Tonti-Filippini, J.; Gregory, B.D.; Berry, C.C.; Millar, A.H.; Ecker, J.R. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 2008, 133, 523–536. [Google Scholar] [CrossRef]
- Jeddeloh, J.A.; Stokes, T.L.; Richards, E.J. Maintenance of genomic methylation requires a SWI2/SNF2-like protein. Nat. Genet. 1999, 22, 94–97. [Google Scholar] [CrossRef]
- Bewick, A.J.; Schmitz, R.J. Gene body DNA methylation in plants. Curr. Opin. Plant Biol. 2017, 36, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Blevins, T.; Rajeswaran, R.; Shivaprasad, P.V.; Beknazariants, D.; Si-Ammour, A.; Park, H.S.; Vazquez, F.; Robertson, D.; Meins, F.; Hohn, T.; et al. Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res. 2006, 34, 6233–6246. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Xie, Y.; Raja, P.; Li, S.Z.; Wolf, J.N.; Shen, Q.T.; Bisaro, D.M.; Zhou, X.P. Suppression of Methylation-Mediated Transcriptional Gene Silencing by beta C1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection. PLoS Pathog. 2011, 7, e1002329. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, D.; Li, L.; Zhang, Q.; Wang, S.; Huang, H. Identification of Circular RNAs in Kiwifruit and Their Species-Specific Response to Bacterial Canker Pathogen Invasion. Front. Plant Sci. 2017, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Lepere, G.; Jay, F.; Wang, J.Y.; Bapaume, L.; Wang, Y.; Abraham, A.L.; Penterman, J.; Fischer, R.L.; Voinnet, O.; et al. Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc. Natl. Acad. Sci. USA 2013, 110, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-W.; Huang, K.; Yang, C.; Kang, C.-S. Non-coding RNAs as regulators in epigenetics. Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef]
- Boccara, M.; Sarazin, A.; Thiebeauld, O.; Jay, F.; Voinnet, O.; Navarro, L.; Colot, V. The Arabidopsis miR472-RDR6 Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-Transcriptional Control of Disease Resistance Genes. Public Libr. Sci. 2014, 10, e1003883. [Google Scholar] [CrossRef]
- Nie, P.; Chen, C.; Yin, Q.; Jiang, C.; Guo, J.; Zhao, H.; Niu, D. Function of miR825 and miR825* as Negative Regulators in Bacillus cereus AR156-elicited Systemic Resistance to Botrytis cinerea in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 5032. [Google Scholar] [CrossRef]
- Li, F.; Pignatta, D.; Bendix, C.; Brunkard, J.O.; Cohn, M.M.; Tung, J.; Sun, H.; Kumar, P.; Baker, B. MicroRNA regulation of plant innate immune receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1790–1795. [Google Scholar] [CrossRef]
- He, X.-F.; Fang, Y.-Y.; Feng, L.; Guo, H.-S. Characterization of conserved and novel microRNAs and their targets, including a TuMV-induced TIR-NBS-LRR class R gene-derived novel miRNA in Brassica. FEBS Lett. 2008, 582, 2445–2452. [Google Scholar] [CrossRef]
- Yao, S.; Yang, Z.; Yang, R.; Huang, Y.; Guo, G.; Kong, X.; Lan, Y.; Zhou, T.; Wang, H.; Wang, W.; et al. Transcriptional Regulation of miR528 by OsSPL9 Orchestrates Antiviral Response in Rice. Mol. Plant 2019, 12, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Yan, Q.; Gan, S.; Xue, D.; Dou, D.; Guo, N.; Xing, H. Overexpression of gma-miR1510a/b suppresses the expression of a NB-LRR domain gene and reduces resistance to Phytophthora sojae. Gene 2017, 621, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Jiang, N.; Meng, J.; Yang, G.; Liu, W.; Zhou, X.; Ma, N.; Hou, X.; Luan, Y. LncRNA33732-respiratory burst oxidase module associated with WRKY1 in tomato-Phytophthora infestans interactions. Plant J. 2019, 97, 933–946. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Gao, S.; Wang, W.-C.; Katiyar-Agarwal, S.; Huang, H.-D.; Raikhel, N.; Jin, H. Arabidopsis Argonaute 2 regulates innate immunity via miRNA393(*)-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol. Cell 2011, 42, 356–366. [Google Scholar] [CrossRef]
- Chow, H.T.; Ng, D.W.-K. Regulation of miR163 and its targets in defense against Pseudomonas syringae in Arabidopsis thaliana. Sci. Rep. 2017, 7, 46433. [Google Scholar] [CrossRef]
- Kurihara, Y.; Watanabe, Y. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc. Natl. Acad. Sci. USA 2004, 101, 12753–12758. [Google Scholar] [CrossRef]
- Campo, S.; Peris-Peris, C.; Siré, C.; Moreno, A.B.; Donaire, L.; Zytnicki, M.; Notredame, C.; Llave, C.; San Segundo, B. Identification of a novel microRNA (miRNA) from rice that targets an alternatively spliced transcript of the Nramp6 (Natural resistance-associated macrophage protein 6) gene involved in pathogen resistance. New Phytol. 2013, 199, 212–227. [Google Scholar] [CrossRef]
- Sánchez-Sanuy, F.; Peris-Peris, C.; Tomiyama, S.; Okada, K.; Hsing, Y.-I.; San Segundo, B.; Campo, S. Osa-miR7695 enhances transcriptional priming in defense responses against the rice blast fungus. BMC Plant Biol. 2019, 19, 563. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Sun, H.-X.; Park, B.S.; Huang, C.-H.; Yeh, S.-D.; Jung, C.; Chua, N.-H. ELF18-INDUCED LONG-NONCODING RNA Associates with Mediator to Enhance Expression of Innate Immune Response Genes in Arabidopsis. Plant Cell 2017, 29, 1024–1038. [Google Scholar] [CrossRef]
- Hou, X.; Cui, J.; Liu, W.; Jiang, N.; Zhou, X.; Qi, H.; Meng, J.; Luan, Y. LncRNA39026 Enhances Tomato Resistance to Phytophthora infestans by Decoying miR168a and Inducing PR Gene Expression. Phytopathology 2020, 110, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-H.; Stephen, S.; Taylor, J.; Helliwell, C.A.; Wang, M.-B. Long noncoding RNAs responsive to Fusarium oxysporum infection in Arabidopsis thaliana. New Phytol. 2014, 201, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Quan, W.; Li, G.-B.; Hu, X.-H.; Wang, Q.; Wang, H.; Li, X.-P.; Luo, X.; Feng, Q.; Hu, Z.-J.; et al. circRNAs Are Involved in the Rice-Magnaporthe oryzae Interaction. Plant Physiol. 2020, 182, 272–286. [Google Scholar] [CrossRef]
- Salvador-Guirao, R.; Hsing, Y.-I.; San Segundo, B. The Polycistronic miR166k-166h Positively Regulates Rice Immunity via Post-transcriptional Control of EIN2. Front. Plant Sci. 2018, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Zhang, J.; Wu, L.; Qi, Y.; Zhou, J.-M. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiol. 2010, 152, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D.G. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Z.; Wu, K.; Yang, L.; Li, Y.; Yang, Z.; Shi, S.; Liu, X.; Zhao, S.; Yang, Z.; et al. Suppression of Jasmonic Acid-Mediated Defense by Viral-Inducible MicroRNA319 Facilitates Virus Infection in Rice. Mol. Plant 2016, 9, 1302–1314. [Google Scholar] [CrossRef]
- Hu, G.; Hao, M.; Wang, L.; Liu, J.; Zhang, Z.; Tang, Y.; Peng, Q.; Yang, Z.; Wu, J. The Cotton miR477-CBP60A Module Participates in Plant Defense Against Verticillium dahlia. Mol. Plant Microbe Interact. 2020, 33, 624–636. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.-F.; Feng, Y.-Z.; He, H.; Lian, J.-P.; Yang, Y.-W.; Lei, M.-Q.; Zhang, Y.-C.; Chen, Y.-Q. Transcriptional landscape of pathogen-responsive lncRNAs in rice unveils the role of ALEX1 in jasmonate pathway and disease resistance. Plant Biotechnol. J. 2020, 18, 679–690. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M.; Li, N.; Wang, H.; Qiu, P.; Pei, L.; Xu, Z.; Wang, T.; Gao, E.; Liu, J.; et al. Long noncoding RNAs involve in resistance to Verticillium dahliae, a fungal disease in cotton. Plant Biotechnol. J. 2018, 16, 1172–1185. [Google Scholar] [CrossRef]
- Niu, D.; Lii, Y.E.; Chellappan, P.; Lei, L.; Peralta, K.; Jiang, C.; Guo, J.; Coaker, G.; Jin, H. miRNA863-3p sequentially targets negative immune regulator ARLPKs and positive regulator SERRATE upon bacterial infection. Nat. Commun. 2016, 7, 11324. [Google Scholar] [CrossRef]
- Yu, G.L.; Katagiri, F.; Ausubel, F.M. Arabidopsis mutations at the RPS2 locus result in loss of resistance to Pseudomonas syringae strains expressing the avirulence gene avrRpt2. Mol. Plant Microbe Interact. 1993, 6, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiao, X.; Kong, X.; Hamera, S.; Wu, Y.; Chen, X.; Fang, R.; Yan, Y. A Signaling Cascade from miR444 to RDR1 in Rice Antiviral RNA Silencing Pathway. Plant Physiol. 2016, 170, 2365–2377. [Google Scholar] [CrossRef]
- Harvey, J.J.W.; Lewsey, M.G.; Patel, K.; Westwood, J.; Heimstädt, S.; Carr, J.P.; Baulcombe, D.C. An antiviral defense role of AGO2 in plants. PLoS ONE 2011, 6, e14639. [Google Scholar] [CrossRef]
- Diao, P.; Zhang, Q.; Sun, H.; Ma, W.; Cao, A.; Yu, R.; Wang, J.; Niu, Y.; Wuriyanghan, H. miR403a and SA Are Involved in NbAGO2 Mediated Antiviral Defenses Against TMV Infection in Nicotiana benthamiana. Genes 2019, 10, 526. [Google Scholar] [CrossRef]
- Garcia-Ruiz, H.; Carbonell, A.; Hoyer, J.S.; Fahlgren, N.; Gilbert, K.B.; Takeda, A.; Giampetruzzi, A.; Garcia Ruiz, M.T.; McGinn, M.G.; Lowery, N.; et al. Roles and programming of Arabidopsis ARGONAUTE proteins during Turnip mosaic virus infection. PLoS Pathog. 2015, 11, e1004755. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Fang, Y.; Chen, L.; Wang, J.; Chen, X. Role of non-coding RNAs in plant immunity. Plant Commun. 2021, 2, 100180. [Google Scholar] [CrossRef]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015, 16, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Nodine, M.D.; Bartel, D.P. MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes Dev. 2010, 24, 2678–2692. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Prado, J.S.; Ariel, F.; Benhamed, M.; Crespi, M. Plant Epigenetics: Non-coding RNAs as Emerging Regulators. In Plant Epigenetics; Rajewsky, N., Jurga, S., Barciszewski, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 129–147. ISBN 978-3-319-55520-1. [Google Scholar]
- Waterhouse, P.M.; Wang, M.B.; Lough, T. Gene silencing as an adaptive defence against viruses. Nature 2001, 411, 834–842. [Google Scholar] [CrossRef]
- Raja, P.; Sanville, B.C.; Buchmann, R.C.; Bisaro, D.M. Viral genome methylation as an epigenetic defense against geminiviruses. J. Virol. 2008, 82, 8997–9007. [Google Scholar] [CrossRef]
- Ariel, F.; Romero-Barrios, N.; Jégu, T.; Benhamed, M.; Crespi, M. Battles and hijacks: Noncoding transcription in plants. Trends Plant Sci. 2015, 20, 362–371. [Google Scholar] [CrossRef] [PubMed]
- van de Weyer, A.-L.; Monteiro, F.; Furzer, O.J.; Nishimura, M.T.; Cevik, V.; Witek, K.; Jones, J.D.G.; Dangl, J.L.; Weigel, D.; Bemm, F. A Species-Wide Inventory of NLR Genes and Alleles in Arabidopsis thaliana. Cell 2019, 178, 1260–1272.e14. [Google Scholar] [CrossRef]
- Tong, W.; Yu, J.; Hou, Y.; Li, F.; Zhou, Q.; Wei, C.; Bennetzen, J.L. Circular RNA architecture and differentiation during leaf bud to young leaf development in tea (Camellia sinensis). Planta 2018, 248, 1417–1429. [Google Scholar] [CrossRef]
- Tan, J.; Zhou, Z.; Niu, Y.; Sun, X.; Deng, Z. Identification and Functional Characterization of Tomato CircRNAs Derived from Genes Involved in Fruit Pigment Accumulation. Sci. Rep. 2017, 7, 8594. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, J.; Luo, M.; Li, H.; Chen, Q.; Wang, L.; Song, S.; Zhao, L.; Xu, W.; Zhang, C.; et al. Characterization and Cloning of Grape Circular RNAs Identified the Cold Resistance-Related Vv-circATS1. Plant Physiol. 2019, 180, 966–985. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Izadpanah, K.; Peters, J.R.; Dietzgen, R.G.; Mitter, N. Detection and profiling of circular RNAs in uninfected and maize Iranian mosaic virus-infected maize. Plant Sci. 2018, 274, 402–409. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Meng, J.; Zhang, M.; Luan, Y.-S. Identification of tomato circular RNAs responsive to Phytophthora infestans. Gene 2020, 746, 144652. [Google Scholar] [CrossRef]
- Zhou, G.; Niu, R.; Zhou, Y.; Luo, M.; Peng, Y.; Wang, H.; Wang, Z.; Xu, G. Proximity editing to identify RNAs in phase-separated RNA binding protein condensates. Cell Discov. 2021, 7, 72. [Google Scholar] [CrossRef]
- Bach Pages, M.; Preston, G.; van der Hoorn, R.; Castello Palomares, A. Uncovering the Role of RNA-Binding Proteins in Plant Immunity. Ph.D. Thesis, University of Oxford, Oxford, UK, 2019. [Google Scholar]
- Woloshen, V.; Huang, S.; Li, X. RNA-Binding Proteins in Plant Immunity. J. Pathog. 2011, 2011, 278697. [Google Scholar] [CrossRef]
- Zhang, S.; Mehdy, M.C. Binding of a 50-kD Protein to a U-Rich Sequence in an mRNA Encoding a Proline-Rich Protein That Is Destabilized by Fungal Elicitor. Plant Cell 1994, 6, 135–145. [Google Scholar] [CrossRef][Green Version]
- Rezaei, A.; Mahdian, S.; Hashemi-Petroudi, S.H.; Goodwin, P.H.; Babaeizad, V.; Rahimian, H. Correction to: Nonhost resistance EST profiling of wheat interacting with Blumeria graminis f. sp. hordei identifies genes for durable resistance to powdery mildew. Eur. J. Plant Pathol. 2022, 162, 1005. [Google Scholar] [CrossRef]
- Petitot, A.-S.; Blein, J.-P.; Pugin, A.; Suty, L. Cloning of two plant cDNAs encoding a β-type proteasome subunit and a transformer-2-like SR-related protein: Early induction of the corresponding genes in tobacco cells treated with cryptogein. Plant Mol. Biol. PMB Int. J. Mol. Biol. Mol. Genet. Biochem. 1997, 35, 261–269. [Google Scholar] [CrossRef]
- Zhou, X.-J.; Lu, S.; Xu, Y.-H.; Wang, J.-W.; Chen, X.-Y. A cotton cDNA (GaPR-10) encoding a pathogenesis-related 10 protein with in vitro ribonuclease activity. Plant Sci. 2002, 162, 629–636. [Google Scholar] [CrossRef]
- Staiger, D.; Zecca, L.; Wieczorek Kirk, D.A.; Apel, K.; Eckstein, L. The circadian clock regulated RNA-binding protein AtGRP7 autoregulates its expression by influencing alternative splicing of its own pre-mRNA. Plant J. 2003, 33, 361–371. [Google Scholar] [CrossRef]
- Peng, S.; Guo, D.; Guo, Y.; Zhao, H.; Mei, J.; Han, Y.; Guan, R.; Wang, T.; Song, T.; Sun, K.; et al. CONSTITUTIVE EXPRESSER OF PATHOGENESIS-RELATED GENES 5 is an RNA-binding protein controlling plant immunity via an RNA processing complex. Plant Cell 2022, 34, 1724–1744. [Google Scholar] [CrossRef]
- Piron, F.; Nicolaï, M.; Minoïa, S.; Piednoir, E.; Moretti, A.; Salgues, A.; Zamir, D.; Caranta, C.; Bendahmane, A. An induced mutation in tomato eIF4E leads to immunity to two potyviruses. PLoS ONE 2010, 5, e11313. [Google Scholar] [CrossRef] [PubMed]
- Germain, H.; Qu, N.; Cheng, Y.T.; Lee, E.; Huang, Y.; Dong, O.X.; Gannon, P.; Huang, S.; Ding, P.; Li, Y.; et al. MOS11: A new component in the mRNA export pathway. PLoS Genet. 2010, 6, e1001250. [Google Scholar] [CrossRef] [PubMed]
- Ellendorff, U.; Fradin, E.F.; de Jonge, R.; Thomma, B.P.H.J. RNA silencing is required for Arabidopsis defence against Verticillium wilt disease. J. Exp. Bot. 2009, 60, 591–602. [Google Scholar] [CrossRef]
- Vélez-Bermúdez, I.C.; Schmidt, W. Chromatin enrichment for proteomics in plants (ChEP-P) implicates the histone reader ALFIN-LIKE 6 in jasmonate signalling. BMC Genom. 2021, 22, 845. [Google Scholar] [CrossRef]
- Monaghan, J.; Xu, F.; Xu, S.; Zhang, Y.; Li, X. Two putative RNA-binding proteins function with unequal genetic redundancy in the MOS4-associated complex. Plant Physiol. 2010, 154, 1783–1793. [Google Scholar] [CrossRef]
- Qi, Y.; Tsuda, K.; Joe, A.; Sato, M.; Le Nguyen, V.; Glazebrook, J.; Alfano, J.R.; Cohen, J.D.; Katagiri, F. A putative RNA-binding protein positively regulates salicylic acid-mediated immunity in Arabidopsis. Mol. Plant Microbe Interact. 2010, 23, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, T.; Wang, H.; Liu, J.; Chen, Y.; Shim, W.B.; Ma, Z. The RNA binding protein FgRbp1 regulates specific pre-mRNA splicing via interacting with U2AF23 in Fusarium. Nat. Commun. 2021, 12, 2661. [Google Scholar] [CrossRef] [PubMed]
- Vaucheret, H. Plant ARGONAUTES. Trends Plant Sci. 2008, 13, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, A. (Ed.) Plant Argonaute Proteins; Springer: New York, NY, USA, 2017; ISBN 978-1-4939-7164-0. [Google Scholar]
- Agorio, A.; Vera, P. ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell 2007, 19, 3778–3790. [Google Scholar] [CrossRef]
- Morel, J.-B.; Godon, C.; Mourrain, P.; Béclin, C.; Boutet, S.; Feuerbach, F.; Proux, F.; Vaucheret, H. Fertile hypomorphic ARGONAUTE (ago1) mutants impaired in post-transcriptional gene silencing and virus resistance. Plant Cell 2002, 14, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Dzianott, A.; Sztuba-Solińska, J.; Bujarski, J.J. Mutations in the antiviral RNAi defense pathway modify Brome mosaic virus RNA recombinant profiles. Mol. Plant Microbe Interact. 2012, 25, 97–106. [Google Scholar] [CrossRef]
- Takeda, A.; Iwasaki, S.; Watanabe, T.; Utsumi, M.; Watanabe, Y. The mechanism selecting the guide strand from small RNA duplexes is different among argonaute proteins. Plant Cell Physiol. 2008, 49, 493–500. [Google Scholar] [CrossRef]
- Carbonell, A.; Fahlgren, N.; Garcia-Ruiz, H.; Gilbert, K.B.; Montgomery, T.A.; Nguyen, T.; Cuperus, J.T.; Carrington, J.C. Functional analysis of three Arabidopsis ARGONAUTES using slicer-defective mutants. Plant Cell 2012, 24, 3613–3629. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Jovel, J.; Udomporn, P.; Wang, Y.; Wu, Q.; Li, W.-X.; Gasciolli, V.; Vaucheret, H.; Ding, S.-W. The 21-nucleotide, but not 22-nucleotide, viral secondary small interfering RNAs direct potent antiviral defense by two cooperative argonautes in Arabidopsis thaliana. Plant Cell 2011, 23, 1625–1638. [Google Scholar] [CrossRef]
- Olmedo-Monfil, V.; Durán-Figueroa, N.; Arteaga-Vázquez, M.; Demesa-Arévalo, E.; Autran, D.; Grimanelli, D.; Slotkin, R.K.; Martienssen, R.A.; Vielle-Calzada, J.-P. Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 2010, 464, 628–632. [Google Scholar] [CrossRef]
- Singh, M.; Goel, S.; Meeley, R.B.; Dantec, C.; Parrinello, H.; Michaud, C.; Leblanc, O.; Grimanelli, D. Production of viable gametes without meiosis in maize deficient for an ARGONAUTE protein. Plant Cell 2011, 23, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, N.; Baulcombe, D.C. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl. Acad. Sci. USA 2005, 102, 11928–11933. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ba, Z.; Gao, M.; Wu, Y.; Ma, Y.; Amiard, S.; White, C.I.; Rendtlew Danielsen, J.M.; Yang, Y.-G.; Qi, Y. A role for small RNAs in DNA double-strand break repair. Cell 2012, 149, 101–112. [Google Scholar] [CrossRef]
- Oliver, C.; Santos, J.L.; Pradillo, M. On the role of some ARGONAUTE proteins in meiosis and DNA repair in Arabidopsis thaliana. Front. Plant Sci. 2014, 5, 177. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Zhang, H.; Arikit, S.; Huang, K.; Nan, G.-L.; Walbot, V.; Meyers, B.C. Spatiotemporally dynamic, cell-type-dependent premeiotic and meiotic phasiRNAs in maize anthers. Proc. Natl. Acad. Sci. USA 2015, 112, 3146–3151. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, L.; Pi, L.; Liu, Q.; Ling, Q.; Wang, H.; Poethig, R.S.; Huang, H. Genetic interaction between the AS1-AS2 and RDR6-SGS3-AGO7 pathways for leaf morphogenesis. Plant Cell Physiol. 2006, 47, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Adenot, X.; Elmayan, T.; Lauressergues, D.; Boutet, S.; Bouché, N.; Gasciolli, V.; Vaucheret, H. DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr. Biol. 2006, 16, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Ito, M.; Kamiya, N.; Sato, Y.; Matsuoka, M. OsPNH1 regulates leaf development and maintenance of the shoot apical meristem in rice. Plant J. 2002, 30, 189–201. [Google Scholar] [CrossRef]
- Douglas, R.N.; Wiley, D.; Sarkar, A.; Springer, N.; Timmermans, M.C.P.; Scanlon, M.J. ragged seedling2 Encodes an ARGONAUTE7-like protein required for mediolateral expansion, but not dorsiventrality, of maize leaves. Plant Cell 2010, 22, 1441–1451. [Google Scholar] [CrossRef]
- Oliver, C.; Santos, J.L.; Pradillo, M. Accurate Chromosome Segregation at First Meiotic Division Requires AGO4, a Protein Involved in RNA-Dependent DNA Methylation in Arabidopsis thaliana. Genetics 2016, 204, 543–553. [Google Scholar] [CrossRef]
- Nonomura, K.-I.; Morohoshi, A.; Nakano, M.; Eiguchi, M.; Miyao, A.; Hirochika, H.; Kurata, N. A germ cell specific gene of the ARGONAUTE family is essential for the progression of premeiotic mitosis and meiosis during sporogenesis in rice. Plant Cell 2007, 19, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.R.; Okada, T.; Hu, Y.; Scholefield, A.; Taylor, J.M.; Koltunow, A.M.G. Somatic small RNA pathways promote the mitotic events of megagametogenesis during female reproductive development in Arabidopsis. Development 2012, 139, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, T.A.; Howell, M.D.; Cuperus, J.T.; Li, D.; Hansen, J.E.; Alexander, A.L.; Chapman, E.J.; Fahlgren, N.; Allen, E.; Carrington, J.C. Specificity of ARGONAUTE7-miR390 interaction and dual functionality in TAS3 trans-acting siRNA formation. Cell 2008, 133, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.; Sun, H.; Poethig, R.S. The Arabidopsis heterochronic gene ZIPPY is an ARGONAUTE family member. Curr. Biol. 2003, 13, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, H.; Itoh, J.; Hayashi, K.; Hibara, K.; Satoh-Nagasawa, N.; Nosaka, M.; Mukouhata, M.; Ashikari, M.; Kitano, H.; Matsuoka, M.; et al. The small interfering RNA production pathway is required for shoot meristem initiation in rice. Proc. Natl. Acad. Sci. USA 2007, 104, 14867–14871. [Google Scholar] [CrossRef]
- Zhou, Y.; Honda, M.; Zhu, H.; Zhang, Z.; Guo, X.; Li, T.; Li, Z.; Peng, X.; Nakajima, K.; Duan, L.; et al. Spatiotemporal sequestration of miR165/166 by Arabidopsis Argonaute10 promotes shoot apical meristem maintenance. Cell Rep. 2015, 10, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yao, X.; Pi, L.; Wang, H.; Cui, X.; Huang, H. The ARGONAUTE10 gene modulates shoot apical meristem maintenance and establishment of leaf polarity by repressing miR165/166 in Arabidopsis. Plant J. 2009, 58, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Dolata, J.; Bajczyk, M.; Bielewicz, D.; Niedojadlo, K.; Niedojadlo, J.; Pietrykowska, H.; Walczak, W.; Szweykowska-Kulinska, Z.; Jarmolowski, A. Salt Stress Reveals a New Role for ARGONAUTE1 in miRNA Biogenesis at the Transcriptional and Posttranscriptional Levels. Plant Physiol. 2016, 172, 297–312. [Google Scholar] [CrossRef]
- Ye, R.; Chen, Z.; Lian, B.; Rowley, M.J.; Xia, N.; Chai, J.; Li, Y.; He, X.-J.; Wierzbicki, A.T.; Qi, Y. A Dicer-Independent Route for Biogenesis of siRNAs that Direct DNA Methylation in Arabidopsis. Mol. Cell 2016, 61, 222–235. [Google Scholar] [CrossRef]
- Montgomery, T.A.; Yoo, S.J.; Fahlgren, N.; Gilbert, S.D.; Howell, M.D.; Sullivan, C.M.; Alexander, A.; Nguyen, G.; Allen, E.; Ahn, J.H.; et al. AGO1-miR173 complex initiates phased siRNA formation in plants. Proc. Natl. Acad. Sci. USA 2008, 105, 20055–20062. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, F.; Wang, R.; Zhou, X.; Sze, S.-H.; Liou, L.W.; Barefoot, A.; Dickman, M.; Zhang, X. Arabidopsis Argonaute10 specifically sequesters miR166/165 to regulate shoot apical meristem development. Cell 2011, 145, 242–256. [Google Scholar] [CrossRef]
- Earley, K.; Smith, M.R.; Weber, R.; Gregory, B.; Poethig, R. An endogenous F-box protein regulates ARGONAUTE1 in Arabidopsis thaliana. Silence 2010, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small RNAs–Big Players in Plant-Microbe Interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Ding, S.-W. Small RNA-based antimicrobial immunity. Nat. Rev. Immunol. 2019, 19, 31–44. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, J.; Wang, X.; Zhan, B.; Li, W.; Ding, S.-W. Lipid flippases promote antiviral silencing and the biogenesis of viral and host siRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 1377–1382. [Google Scholar] [CrossRef]
- Várallyay, É.; Oláh, E.; Havelda, Z. Independent parallel functions of p19 plant viral suppressor of RNA silencing required for effective suppressor activity. Nucleic Acids Res. 2014, 42, 599–608. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Q.; Zhao, T.; Xiang, H.; Zhang, X.; Wu, Z.; Zhou, C.; Zhang, X.; Wang, Y.; Zhang, Y.; et al. Interaction between Brassica yellows virus silencing suppressor P0 and plant SKP1 facilitates stability of P0 in vivo against degradation by proteasome and autophagy pathways. New Phytol. 2019, 222, 1458–1473. [Google Scholar] [CrossRef]
- Nemes, K.; Gellért, Á.; Balázs, E.; Salánki, K. Alanine scanning of cucumber mosaic virus (CMV) 2b protein identifies different positions for cell-to-cell movement and gene silencing suppressor activity. PLoS ONE 2014, 9, e112095. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Stupina, V.A.; Gao, F.; Szarko, C.R.; Kuhlmann, M.M.; Yuan, X.; Shi, K.; Simon, A.E. Requirement for Host RNA-Silencing Components and the Virus-Silencing Suppressor when Second-Site Mutations Compensate for Structural Defects in the 3’ Untranslated Region. J. Virol. 2015, 89, 11603–11618. [Google Scholar] [CrossRef] [PubMed]
- Burgyán, J.; Havelda, Z. Viral suppressors of RNA silencing. Trends Plant Sci. 2011, 16, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Csorba, T.; Bovi, A.; Dalmay, T.; Burgyán, J. The p122 subunit of Tobacco Mosaic Virus replicase is a potent silencing suppressor and compromises both small interfering RNA- and microRNA-mediated pathways. J. Virol. 2007, 81, 11768–11780. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Y.; Zhao, J.-H.; Liu, S.-W.; Wang, S.; Duan, C.-G.; Guo, H.-S. CMV2b-AGO Interaction Is Required for the Suppression of RDR-Dependent Antiviral Silencing in Arabidopsis. Front. Microbiol. 2016, 7, 1329. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, C.; Li, Z.; Zhou, X. Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLoS Pathog. 2014, 10, e1003921. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.-H.; Chen, I.-H.; Baulcombe, D.C.; Tsai, C.-H. The silencing suppressor P25 of Potato virus X interacts with Argonaute1 and mediates its degradation through the proteasome pathway. Mol. Plant Pathol. 2010, 11, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Song, X.; Xie, C.; Huo, Y.; Zhang, F.; Chen, X.; Geng, Y.; Fang, R. Rice yellow stunt rhabdovirus protein 6 suppresses systemic RNA silencing by blocking RDR6-mediated secondary siRNA synthesis. Mol. Plant Microbe Interact. 2013, 26, 927–936. [Google Scholar] [CrossRef]
- Watanabe, K.; Ugaki, M. Mastrevirus Rep and RepA Proteins Suppress de novo Transcriptional Gene Silencing. Int. J. Mol. Sci. 2021, 22, 11462. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).