Weed Management by In Situ Cover Crops and Anaerobic Soil Disinfestation in Plasticulture
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Location and Set Up
2.2. Cover Crops Seeding, Growth and Termination
2.3. Termination of Cover Crops and Initiation of Anaerobic Soil Disinfestation
2.4. Tomato Transplantation and Management
2.5. Data Collection
2.6. Statistical Analysis
3. Results and Discussion
3.1. Weather Conditions at the Field Experimental Site
3.2. Cover Crop Biomass and Plant Population
3.3. Soil Redox Potential
3.4. Weed Control
3.5. Tomato Crop Performance and Yield
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USDA-NASS, The United States Department of Agriculture-National Agricultural Statistics Service. 2020. Available online: https://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Organic_Production/pdf/2019_Organic_Executive_Briefing.pdf (accessed on 2 July 2022).
- Benaragama, D.; Shirtliffe, S.J.; Johnson, E.N.; Duddu, H.S.; Syrovy, L.D. Does yield loss due to weed competition differ between organic and conventional cropping systems? Weed Res. 2016, 56, 274–283. [Google Scholar] [CrossRef]
- Brown, B.; Hoshide, A.K.; Gallandt, E.R. An economic comparison of weed management systems used in small-scale organic vegetable production. Org. Agric. 2018, 9, 53–63. [Google Scholar] [CrossRef]
- Ampong-Nyarko, K.; De Datta, S.K. A Handbook for Weed Control in Rice; IRRI: Manila, Philippines, 1991. [Google Scholar]
- Morales-Payan, J.P.; Santos, B.M.; Stall, W.M.; Bewick, T.A. Effects of purple nutsedge (Cyperus rotundus) on tomato (Lycopersicon esculentum) and bell pepper (Capsicum annuum) vegetative growth and fruit yield. Weed Technol. 1997, 11, 672–676. [Google Scholar] [CrossRef]
- Pimentel, D.; McLaughlin, L.; Zepp, A.; Lakitan, B.; Kraus, T.; Kleinman, P.; Vancini, F.; Roach, W.J.; Graap, E.; Keeton, W.S.; et al. Environmental and economic impacts of reducing U.S. agricultural pesticide use. In The Pesticide Question; Springer: Boston, MA, USA, 1993; pp. 223–278. [Google Scholar]
- Katan, J.; Gamliel, A. Plant Health Management: Soil solarization. Encycl. Agric. Food Syst. 2014, 4, 460–471. [Google Scholar]
- Achmon, Y.; Fernández-Bayo, J.D.; Hernandez, K.; McCurry, D.G.; Harrold, D.R.; Su, J.; Dahlquist-Willard, R.M.; Stapleton, J.J.; VanderGheynst, J.S.; Simmons, C.W. Weed seed inactivation in soil mesocosms via biosolarization with mature compost and Tomato Processing Waste amendments. Pest Manag. Sci. 2016, 73, 862–873. [Google Scholar] [CrossRef]
- Shrestha, U.; Augé, R.M.; Butler, D.M. A meta-analysis of the impact of anaerobic soil disinfestation on pest suppression and yield of horticultural crops. Front. Plant Sci. 2016, 7, 1254. [Google Scholar] [CrossRef]
- Singh, G.; Ward, B.K.; Wechter, W.P.; Katawczik, M.L.; Farmaha, B.S.; Suseela, V.; Cutulle, M.A. Assessment of agro-industrial wastes as a carbon source in anaerobic disinfestation of soil contaminated with weed seeds and phytopathogenic bacterium (Ralstonia solanacearum) in tomato (Solanum lycopersicum). ACS Agric. Sci. Technol. 2022, 2, 769–779. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Mazzola, M. Carbon source-dependent effects of anaerobic soil disinfestation on soil microbiome and suppression of Rhizoctonia Solani AG-5 and Pratylenchus penetrans. Phytopathology 2016, 106, 1015–1028. [Google Scholar] [CrossRef]
- Testen, A.L.; Miller, S.A. Carbon source and soil origin shape soil microbiomes and tomato soilborne pathogen populations during anaerobic soil disinfestation. Phytobiomes J. 2018, 2, 138–150. [Google Scholar] [CrossRef]
- Creamer, N.G.; Bennett, M.A.; Stinner, B.R.; Cardina, J.; Regnier, E.E. Mechanisms of weed suppression in cover crop-based production systems. HortScience 1996, 31, 410–413. [Google Scholar] [CrossRef]
- Adler, M.J.; Chase, C.A. Comparison of the allelopathic potential of leguminous summer cover crops: Cowpea, Sunn Hemp, and Velvetbean. HortScience 2007, 42, 289–293. [Google Scholar] [CrossRef]
- Weston, L.A. Utilization of allelopathy for weed management in Agroecosystems. Agron. J. 1996, 88, 860–866. [Google Scholar] [CrossRef]
- Kelton, J.; Price, A.J.; Mosjidis, J. Allelopathic weed suppression through the use of cover crops. Weed Control 2012, 2, 953–978. [Google Scholar]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Butler, D.M.; Rosskopf, E.N.; Kokalis-Burelle, N.; Albano, J.P.; Muramoto, J.; Shennan, C. Exploring warm-season cover crops as carbon sources for anaerobic soil disinfestation (ASD). Plant Soil 2011, 355, 149–165. [Google Scholar] [CrossRef]
- Vecchia, L.; Di Gioia, F.; Ferrante, A.; Hong, J.C.; White, C.; Rosskopf, E.N. Integrating cover crops as a source of carbon for anaerobic soil disinfestation. Agronomy 2020, 10, 1614. [Google Scholar] [CrossRef]
- Vegetable Crop Handbook—Alabama Cooperative Extension System. Available online: https://www.aces.edu/wp-content/uploads/2019/12/2020_SEVG_final_web.pdf (accessed on 10 September 2022).
- Kelley, W.; Boyhan, G.; Harrison, K.; Sumner, P.; Langston, D.; Sparks, A.; Culpepper, S.; Hurst, W.; Fonsah, E. Commercial Tomato Production Handbook: Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Commercial-tomato-production-handbook-Kelley-Boyhan/dbe48a962a87e8483e37f25fbdfc36387fed174f (accessed on 1 September 2022).
- U.S. Department of Agriculture. 1997 United States Standards for Grades for Fresh Tomato Agr. Mkt. Serv. 7 CFR 51. Available online: https://www.ams.usda.gov/sites/default/files/media/Tomato_Standard%5B1%5D.pdf (accessed on 10 August 2022).
- Besançon, T.E.; Wasacz, M.H.; Heckman, J.R. Weed Suppression, Nitrogen Availability, and Cabbage Production Following sunn Hemp or Sorghum-Sudangrass. Available online: https://journals.ashs.org/horttech/view/journals/horttech/31/4/article-p439.xml (accessed on 10 September 2022).
- Wang, G.; Ngouajio, M.; Warncke, D.D. Nutrient cycling, weed suppression, and onion yield following brassica and sorghum sudangrass cover crops. HortTechnology 2008, 18, 68–74. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Malik, M.S.; Jha, P.; Riley, M.B. Suppression of digitaria sanguinalis and Amaranthus palmeri using autumn-sown glucosinolate-producing cover crops in organically grown bell pepper. Weed Res. 2007, 47, 425–432. [Google Scholar] [CrossRef]
- Treadwell, D.D.; Creamer, N.G.; Schultheis, J.R.; Hoyt, G.D. Cover crop management affects weeds and yield in organically managed Sweetpotato Systems. Weed Technol. 2007, 21, 1039–1048. [Google Scholar] [CrossRef]
- Price, A.J.; Norsworthy, J.K. Cover crops for weed management in southern reduced-tillage vegetable cropping systems. Weed Technol. 2013, 27, 212–217. [Google Scholar] [CrossRef]
- Singh, G.; Wechter, W.P.; Farmaha, B.S.; Cutulle, M. Integration of halosulfuron and anaerobic soil disinfestation for weed control in tomato. HortTechnology 2022, 32, 401–414. [Google Scholar] [CrossRef]
- Fiedler, S.; Vepraskas, M.J.; Richardson, J.L. Soil redox potential: Importance, field measurements, and observations. Adv. Agron. 2007, 94, 1–54. [Google Scholar]
- Guo, H.; Di Gioia, F.; Zhao, X.; Ozores-Hampton, M.; Swisher, M.E.; Hong, J.; Kokalis-Burelle, N.; DeLong, A.N.; Rosskopf, E.N. Optimizing anaerobic soil disinfestation for fresh market tomato production: Nematode and weed control, yield, and fruit quality. Sci. Hortic. 2017, 218, 105–116. [Google Scholar] [CrossRef]
- Khadka, R.B.; Marasini, M.; Rawal, R.; Testen, A.L.; Miller, S.A. Effects of anaerobic soil disinfestation carbon sources on soilborne diseases and weeds of okra and eggplant in Nepal. Crop Prot. 2020, 135, 104846. [Google Scholar] [CrossRef]
- McCarty, D.G.; Eichler Inwood, S.E.; Ownley, B.H.; Sams, C.E.; Wszelaki, A.L.; Butler, D.M. Field evaluation of carbon sources for anaerobic soil disinfestation in tomato and Bell Pepper production in Tennessee. HortScience 2014, 49, 272–280. [Google Scholar] [CrossRef]

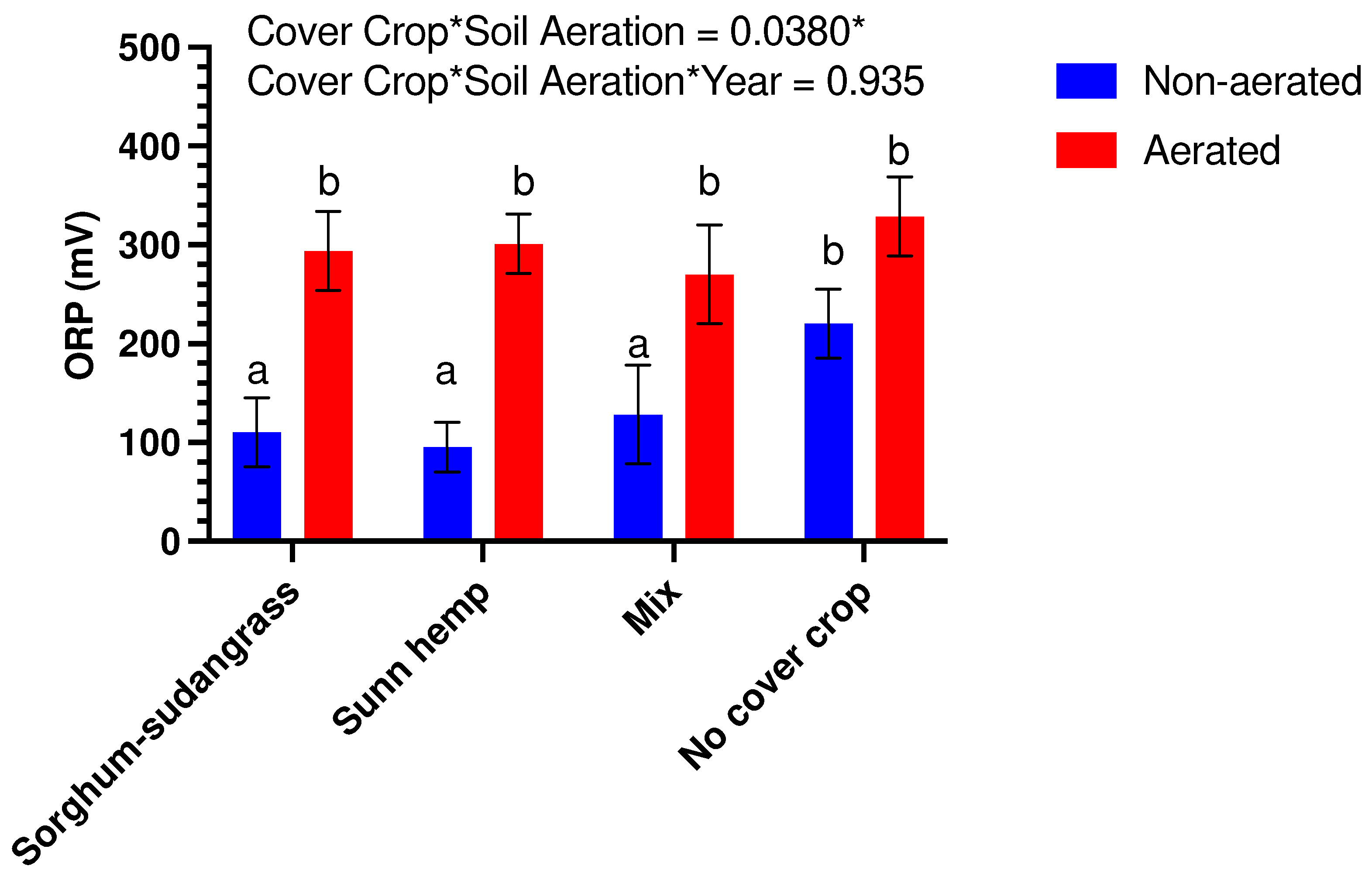
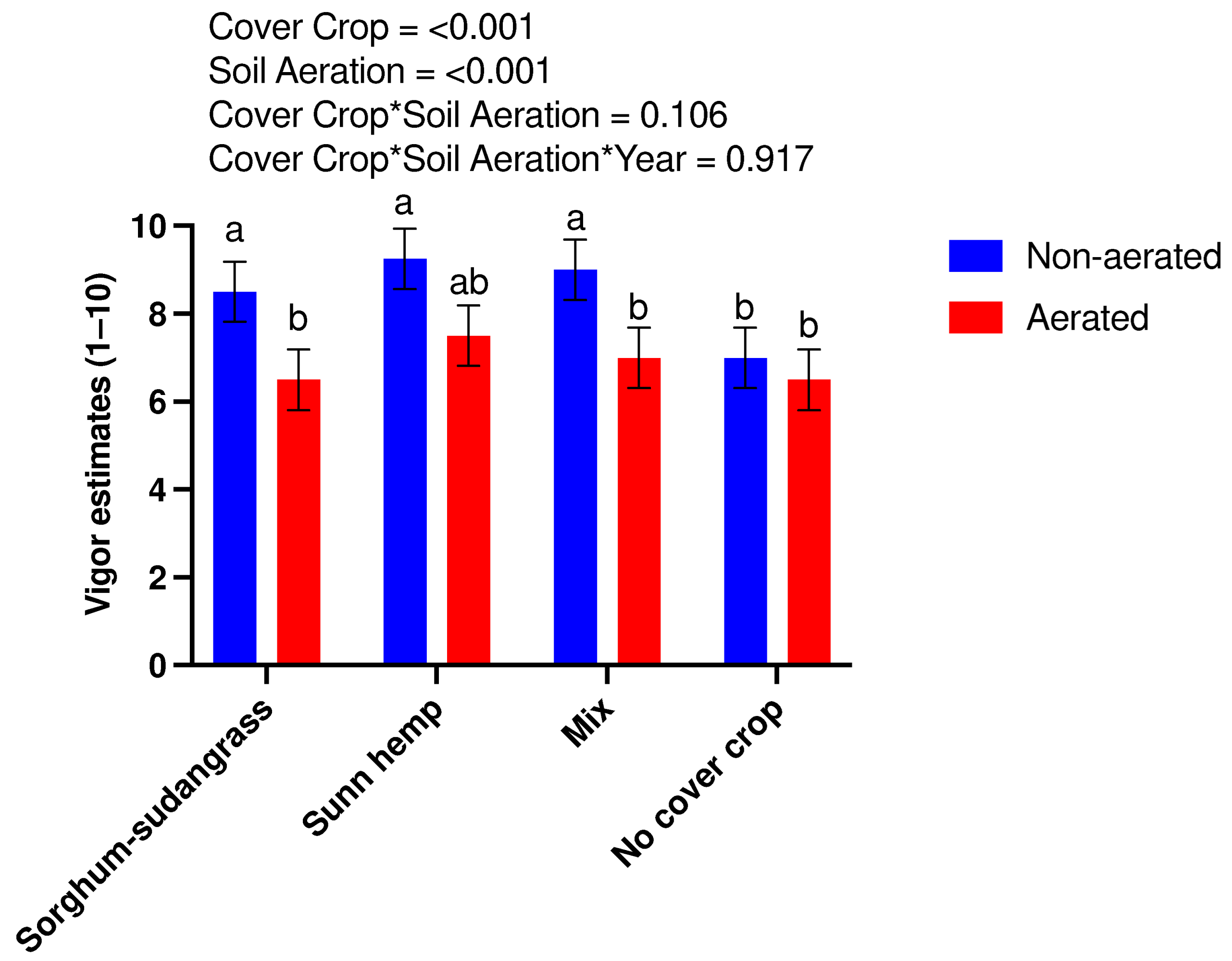
| Cover Crop | Fresh Biomass (kg ha−1) | Dry Biomass (kg ha−1) | Plant Population (Plants m−2) |
|---|---|---|---|
| Sorghum-sudangrass | 56,472 ± 4134 a | 11,357 ± 657 a | 65 ± 3 a |
| Sunn hemp | 39,232 ± 2631 b | 8466 ± 840 b | 69 ± 4 a |
| Sorghum-sudangrass + Sunn hemp | 42,282 ± 3004 b | 8507 ± 645 b | 68 ± 6 a |
| p Values | |||
| Cover crop | <0.001 * | <0.001 * | 0.12 |
| Cover crop * Year | 0.673 | 0.737 | 0.971 |
| Cover Crop | Soil Aeration | Weed Population Per Plot (6 × 1.2 m) | |||||
|---|---|---|---|---|---|---|---|
| Yellow Nutsedge | Grasses | ||||||
| 0 DAT | 45 DAT | 90 DAT | 0 DAT | 45 DAT | 90 DAT | ||
| Sorghum-sudangrass | Non-Aerated | 1 C | 5 D | 12 E | 0 C | 3 A | 8 A |
| Aerated | 10 B | 12 BC | 21 BC | 1 BC | 4 A | 10 A | |
| Sunn hemp | Non-Aerated | 1 C | 5 D | 14 DE | 0 C | 5 A | 11 A |
| Aerated | 14 B | 17 B | 24 B | 3 AB | 6 A | 12 A | |
| Sorghum-sudangrass + sunn hemp | Non-Aerated | 0 C | 6 D | 13 E | 0 C | 5 A | 9 A |
| Aerated | 10 B | 11 C | 18 CD | 1 C | 4 A | 10 A | |
| No Cover crop | Non-Aerated | 1 C | 7 CD | 16 CDE | 0 C | 5 A | 10 A |
| Aerated | 21 A | 26 A | 32 A | 3 AB | 5 A | 11 A | |
| p Values | |||||||
| Cover Crop | 0.039 * | 0.005 * | 0.003 * | 0.1549 | 0.485 | 0.363 | |
| Soil Aeration | <0.001 * | <0.001 * | <0.001 * | 0.005 * | 0.461 | 0.390 | |
| Cover crop * Soil Aeration | 0.057 | 0.0091 * | 0.0715 | 0.1549 | 0.616 | 0.959 | |
| Cover Crop | Soil Aeration | Weed Population Per Plot (6 × 1.2 m) | |||||
|---|---|---|---|---|---|---|---|
| Yellow Nutsedge | Grasses | ||||||
| 0 DAT | 45 DAT | 90 DAT | 0 DAT | 45 DAT | 90 DAT | ||
| Sorghum-sudangrass | Non-Aerated | 1 C | 4 C | 9 D | 1 BC | 2 B | 4 A |
| Aerated | 10 B | 11 B | 19 BC | 2 ABC | 4 A | 7 A | |
| Sunn hemp | Non-Aerated | 2 C | 3 C | 17 C | 1 BC | 2 B | 5A |
| Aerated | 11 B | 15 B | 21 BC | 3 A | 4 A | 6 A | |
| Sorghum-sudangrass + sunn hemp | Non-Aerated | 3 C | 7 C | 10 D | 1 BC | 5 A | 5 A |
| Aerated | 10 B | 14 B | 21 BC | 3 AB | 5 A | 6 A | |
| No Cover crop | Non-Aerated | 9 B | 13 B | 22 B | 2 AB | 4 A | 7 A |
| Aerated | 20 A | 23 A | 30 A | 3 AB | 5 A | 5 A | |
| p Values | |||||||
| Cover Crop | <0.001 * | <0.0001 * | <0.001 * | 0.199 | 0.001 * | 0.991 | |
| Soil Aeration | <0.001 * | <0.001 * | <0.001 * | 0.005 * | 0.002 * | 0.173 | |
| Cover crop * Soil Aeration | 0.459 | 0.458 | 0.176 | 0.241 | 0.465 | 0.288 | |
| Cover Crop | Soil Aeration | Marketable Yield (t ha−1) | |
|---|---|---|---|
| 2020 | 2021 | ||
| Sorghum-sudangrass | Non-Aerated | 15.56 a | 11.99 ab |
| Aerated | 10.27 b | 7.29 b | |
| Sunn hemp | Non-Aerated | 18.66 a | 12.87 a |
| Aerated | 11.11 b | 7.02 b | |
| Sorghum-sudangrass + sunn hemp | Non-Aerated | 15.34 a | 18.26 a |
| Aerated | 9.82 b | 9.85 b | |
| No Cover crop | Non-Aerated | 11.22 b | 7.24 b |
| Aerated | 10.87 b | 6.45 b | |
| p Values | |||
| Year | 0.002 * | ||
| Cover Crop | 0.430 | 0.051 | |
| Soil Aeration | 0.007 * | 0.012 * | |
| Cover Crop * Soil Aeration | 0.456 | 0.529 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, G.; Ward, B.; Levi, A.; Cutulle, M. Weed Management by In Situ Cover Crops and Anaerobic Soil Disinfestation in Plasticulture. Agronomy 2022, 12, 2754. https://doi.org/10.3390/agronomy12112754
Singh G, Ward B, Levi A, Cutulle M. Weed Management by In Situ Cover Crops and Anaerobic Soil Disinfestation in Plasticulture. Agronomy. 2022; 12(11):2754. https://doi.org/10.3390/agronomy12112754
Chicago/Turabian StyleSingh, Gursewak, Brian Ward, Amnon Levi, and Matthew Cutulle. 2022. "Weed Management by In Situ Cover Crops and Anaerobic Soil Disinfestation in Plasticulture" Agronomy 12, no. 11: 2754. https://doi.org/10.3390/agronomy12112754
APA StyleSingh, G., Ward, B., Levi, A., & Cutulle, M. (2022). Weed Management by In Situ Cover Crops and Anaerobic Soil Disinfestation in Plasticulture. Agronomy, 12(11), 2754. https://doi.org/10.3390/agronomy12112754





