Growth, Yield, and Grain Quality of Barley (Hordeum vulgare L.) Grown across South Korean Farmlands with Different Temperature Distributions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area
2.2. Growth and Yield of Barley in Various Areas with Different Temperature Distributions
2.3. Meteorological Factors in Different Cultivation Areas
2.4. Main Constituents and Amino Acid Composition in Barley Seeds Grown in Different Cultivation Areas
2.5. Chemical Composition of the Soils and Mineral Content of the Plants Grown in Different Cultivation Areas
2.6. Statistical Analysis
3. Results and Discussion
3.1. Growth and Yield of Barley in Various Areas with Different Temperature Distributions
3.2. Meteorological Factors in Different Cultivation Areas
3.3. Main Constituents and Amino Acid Composition in Barley Seeds Harvested in Different Cultivation Areas
3.4. Chemical Composition in Soils and Mineral Contents in Plants Grown in Different Cultivation Areas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, MA, UK, 2013. [Google Scholar]
- UNFCCC. United Nations Framework Collection on Climate Change. In Copenhagen Accord; UNFCCC: Copenhagen, Denmark, 2010. [Google Scholar]
- Farooqi, A.B.; Khan, A.H.; Mir, H. Climate change perspective in Pakistan. Pak. J. Meteorol. 2005, 2, 11–21. [Google Scholar]
- Högy, P.; Poll, C.; Marhan, S.; Kandeler, E.; Fangmeier, A. Impacts of temperature increase and change in precipitation pattern on crop yield and yield quality of barley. Food Chem. 2013, 136, 1470–1477. [Google Scholar] [CrossRef]
- Yau, S.K.; Ryan, J. Differential impacts of climate variability on yields of rainfed barley and legumes in semi-arid Mediterranean conditions. Arch. Agron. Soil Sci. 2013, 59, 1659–1674. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Fraizier, A.G.; Longman, R.J.; Dacks, R.S.; Walton, M.M.; Tong, E.J.; Sanchez, J.J.; Kaiser, L.R.; Stender, Y.O.; Anderson, J.M.; et al. The projected timing of climate departure from recent variability. Nature 2013, 502, 183–188. [Google Scholar] [CrossRef]
- Newton, A.C.; Flavell, A.J.; George, T.S.; Leat, P.; Mullholland, B.; Ramsay, L.; RevoredoGiha, C.; Russell, J.; Steffenson, B.J.; Swanston, J.S.; et al. Crops that feed the world 4. Barley: A resilient crop? Strengths and weaknesses in the context of food security. Food Secur. 2011, 3, 141–178. [Google Scholar] [CrossRef]
- Ko, J.; Ng, C.T.; Jeong, S.; Kim, J.H.; Lee, B.; Kim, H.Y. Impacts of regional climate change on barley yield and its geographical variation in South Korea. Int. Agrophys. 2019, 33, 81–96. [Google Scholar] [CrossRef]
- Brisson, N.; Gate, P.; Gouache, D.; Charmet, G.; Oury, F.-X.; Huard, F. Why are wheat yields stagnating in Europe? A comprehensive data analysis for France. Field Crops Res. 2010, 119, 201–212. [Google Scholar] [CrossRef]
- Dawson, I.K.; Russell, J.; Powell, W.; Steffenson, B.; Thomas, W.T.B.; Waugh, R. Barley: A translational model for adaptation to climate change. New Phytol. 2015, 206, 913–931. [Google Scholar] [CrossRef]
- Schierhorn, F.; Hofmann, M.; Adrian, I.; Bobojonov, H.; Müller, D. Spatially varying impacts of climate change on wheat and barley yields in Kazakhstan. J. Arid Environ. 2020, 178, 104–164. [Google Scholar] [CrossRef]
- Hakala, K.; Jauhiainen, L.; Rajala, A.A.; Jalli, M.; Kujala, M.; Laine, A. Different responses to weather events may change the cultivation balance of spring barley and oats in the future. Field Crops Res. 2020, 259, 107956. [Google Scholar] [CrossRef]
- Hossain, A.; da Silva, J.A.T.; Lozovskaya, M.V.; Zvolinsky, V.P. High temperature combined with drought affect rainfed spring wheat and barley in South-Eastern Russia: I. Phenology and growth. Saudi J. Biol. Sci. 2012, 19, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term Climate Change: Projections, Commitments and Irreversibility. In Climate Change 2013—The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; Volume 12, pp. 1029–1136. [Google Scholar]
- Wang, Y.; Frei, M. Stressed food–The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Passarella, V.S.; Savin, R.; Slafer, G.A. Are temperature effects on weight and quality of barley grains modified by resource availability? Aust. J. Agric. Res. 2008, 59, 510–516. [Google Scholar] [CrossRef]
- Savin, R.; Nicolas, M.E. Effects of short periods of drought and high temperature on grain growth and starch accumulation of two malting barley cultivars. Aust. J. Plant Physiol. 1996, 23, 201–210. [Google Scholar] [CrossRef]
- Araya, A.; Hoogenboom, G.; Luedeling, E.; Hadgu, K.M.; Kisekka, I.; Martorano, L.G. Assessment of maize growth and yield using crop models under present and future climate in southwestern Ethiopia. Agric. For. Meteorol. 2015, 214, 252–265. [Google Scholar] [CrossRef]
- Araya, A.; Kisekka, I.; Lin, X.; Vara Prasad, P.V.; Gowda, P.H.; Rice, C.; Andales, A. Evaluating the impact of future climate change on irrigated corn production in Kansas. J. Clim. Risk Manag. 2017, 17, 139–154. [Google Scholar] [CrossRef]
- Asseng, S.; Foster, I.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Chang. Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2014, 5, 143–147. [Google Scholar] [CrossRef]
- Araya, A.; Prasad, P.V.V.; Zambreski, Z.; Gowda, P.H.; Ciampitti, I.A.; Girma, A. Spatial analysis of the impact of climate factors and adaptation strategies on productivity of wheat in Ethiopia. Sci. Total Environ. 2020, 731, 139094. [Google Scholar] [CrossRef]
- Araya, A.; Prasad, P.V.V.; Gowda, P.H.; Djanaguiraman, M.; Kassa, A.H. Potential impacts of climate change factors and agronomic adaptation strategies on wheat yields in central highlands of Ethiopia. Clim. Chang. 2020, 159, 461–479. [Google Scholar] [CrossRef]
- Araya, A.; Prasad, P.V.V.; Gowda, P.H.; Zambreski, Z.; Ciampitti, I.A. Management options for mid-century maize (Zea mays L.) in Ethiopia. Sci. Total Environ. 2021, 758, 143–635. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, T.J.; Birkved, M.; Saxe, H.; Wenzel, H.; Hauschild, M.Z. Environmental impacts of barley cultivation under current and future climatic conditions. J. Clean. Prod. 2017, 140, 644–653. [Google Scholar] [CrossRef]
- Gammans, M.; M′erel, P.; Ortiz-Bobea, A. Negative impacts of climate change on cereal yields: Statistical evidence from France. Environ. Res. Lett. 2017, 12, 54007. [Google Scholar] [CrossRef]
- Bunce, J.A. Responses of stomatal conductance to light, humidity and temperature in winter wheat and barley grown at three concentrations of carbon dioxide in the field. Glob. Chang. Biol. 2000, 6, 371–382. [Google Scholar] [CrossRef]
- Chloupeka, O.; Hrstkovaa, P.; Schweigert, P. Yield and its stability, crop diversity, adaptability and response to climate change, weather and fertilization over 75 years in the Czech Republic in comparison to some European countries. Field Crops Res. 2004, 85, 167–190. [Google Scholar] [CrossRef]
- Olesen, J.E.; Trnka, M.; Kersebaum, K.C.; Skjelvåg, A.O.; Seguin, B.; Peltonen-Sainio, P.; Rossi, F.; Kozyra, J.; Micale, F. Impacts and adaptation of European crop production systems to climate change. Eur. J. Agron. 2011, 34, 96–112. [Google Scholar] [CrossRef]
- Trnka, M.; Dubrovsky, M.; Zalud, Z. Climate change impacts and adaptation strategies in spring barley production in the Czech Republic. Clim. Chang. 2004, 64, 227–255. [Google Scholar] [CrossRef]
- Tubiello, F.N.; Donatelli, M.; Rosenzweig, C.; Stockle, C.O. Effects of climate change and elevated CO2 on cropping systems: Model predictions at two Italian locations. Eur. J. Agron. 2000, 13, 179–189. [Google Scholar] [CrossRef]
- Tao, F.; Rötter, R.P.; Palosuo, T.; Díaz-Ambrona, C.G.H.; Mínguez, M.I.; Semenov, M.A.; Kersebaum, K.C.; Nendel, C.; Cammarano, D.; Hoffmann, H.; et al. Designing future barley ideotypes using a crop model ensemble. Eur. J. Agron. 2017, 82, 144–162. [Google Scholar] [CrossRef]
- Choi, D.H.; Yun, S.H. Agroclimatic zone and characters of the area subject to climatic disaster in Korea. Korean J. Crop Sci. 1989, 34 (Suppl. S2), 13–33. [Google Scholar]
- Choi, D.H.; Kim, K.S. Agricultural Meteorology (Shingo): For Environment and Science Farming; Hyangmunsa: Seoul, Korea, 2001; pp. 45–46. [Google Scholar]
- Cha, J.H.; Kim, K.S. Agriculture, Forestry and Meteorology; Sunjin Culture: Seoul, Korea, 1989; pp. 201–307. [Google Scholar]
- Holden, N.M.; Brereton, A.J.; Fealy, R.; Sweeney, J. Possible change in Irish climate and its impact on barley and potato yields. Agric. For. Meteorol. 2003, 116, 181–196. [Google Scholar] [CrossRef]
- Shim, K.M.; Yun, S.H.; Jung, Y.S.; Lee, J.T.; Hwang, K.H. Impact of recent weather variation on yield components and growth stages of winter barley in Korea. Korean J. Agric. For. Meteorol. 2002, 4, 38–48. [Google Scholar]
- Shim, K.M.; Min, S.H.; Lee, D.B.; Kim, G.Y.; Jeong, H.C.; Lee, S.B.; Kang, K.K. Simulation of the effects of the A1B climate change scenario on the potential yield of winter naked barley in Korea. Korean J. Agric. For. Meteorol. 2011, 13, 192–203. [Google Scholar] [CrossRef][Green Version]
- Rural Development Administration (RDA). Methods of Soil and Plant Analysis; Sammi Press: Seoul, Korea, 2000; pp. 1–202. [Google Scholar]
- AOAC. Official Methods of Analysis, 13th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990; pp. 190–209. [Google Scholar]
- Jang, S.J.; Kuk, Y.I. Effects of biostimulants on primary and secondary substance contents in lettuce plants. Sustainability. 2021, 13, 2441. [Google Scholar] [CrossRef]
- Ohara, I.; Shujiro, A. Comparison of protein precipitants for the determination of free amino acids in plasma. Agric. Biol. Chem. 1979, 43, 1473–1478. [Google Scholar]
- Kononova, M.M. Soil Organic Matter: Its Nature, Its Role in Soil Formation and in Soil Fertility; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Cox, M.S. The Lancaster soil test method as an alternative to the Mehlich 3 soil test method. Soil Sci. 2001, 166, 484–489. [Google Scholar] [CrossRef]
- Statistical Analysis System (SAS). SAS/STAT User’s Guide, 7th ed.; Electronic Version; Statistical Analysis System Institute: Cary, NC, USA, 2000. [Google Scholar]
- Alberdi, M.; Corcuera, L.J. Cold acclimation in plants. Phytochemistry 1991, 30, 3177–3184. [Google Scholar]
- Fowler, D.B.; Carles, R.J. Growth, development, and cold tolerance of fall acclimated cereal grains. Crop Sci. 1979, 19, 915–922. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, H.J.; Roh, S.W.; Hwangbo, H.; Kuk, Y.I. Evaluation of cultivation limit area for different types of barley owing to climate change based on cultivation status and area of certified seed request. Korean J. Crop Sci. 2022, 67, 95–110. [Google Scholar]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef]
- Clausen, S.K.; Frenck, G.; Linden, L.G.; Mikkelsen, T.N.; Lunde, C.; Jørgensen, R.B. Effects of single and multifactor treatments with elevated temperature, CO2 and ozone on oilseed rape and barley. J. Agron. Crop Sci. 2011, 197, 442–453. [Google Scholar] [CrossRef]
- Wheeler, T.R.; Batts, G.R.; Ellis, R.H.; Hadley, P.; Morison, J.I.L. Growth and yield of winter wheat (Triticum aestivum) crops in response to CO2 and temperature. J. Agric. Sci. 1996, 127, 37–48. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z. Impacts of drought and/or heat stress on physiological, development, growth and yield process of crop plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; American Society of Agronomy: Madison, WI, USA, 2008; Volume 1, pp. 301–355. [Google Scholar]
- Prasad, P.V.V.; Bhemanahalli, R.; Jagadish, S.V.K. Field crops and the fear of heat stress: Opportunities, challenges and future directions. Field Crops Res. 2017, 200, 114–121. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Djanaguiraman, M. Response of floret fertility and individual grain weight of wheat to high temperature stress: Sensitive stages and thresholds for temperature and duration. Funct. Plant Biol. 2014, 41, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Djanaguiraman, M.; Perumal, R.; Ciampitti, I.A. Impact of high temperature stress on floret fertility and individual grain weight of grain sorghum: Sensitive stages and thresholds for temperature and duration. Front. Plant Sci. 2015, 6, 820. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H. Seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agric. For. Meteorol. 2006, 139, 237–251. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature. Field Crop Res. 2006, 95, 398–411. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Z.; Sun, L.; Chen, B.; Tubiello, F.N.; Xu, Y. The impacts of increased heat stress events on wheat yield under climate change in China. Clim. Chang. 2017, 140, 605–620. [Google Scholar] [CrossRef]
- Reinhardt, D.; Jansen, G.; Seddig, S.; Eichler-Löbermann, B. Temperature stress during flowering time affects yield and quality parameters of waxy barley. Appl. Agric. For. Res. 2013, 1, 79–84. [Google Scholar]
- Savin, R.; Stone, P.J.; Nicolas, M.E. Responses if grain growth and malting quality of barley to short periods of high temperature in field studies using portable chambers. Aust. J. Agric. Res. 1996, 47, 465–477. [Google Scholar] [CrossRef]
- Nuttall, J.G.; O’leary, G.J.; Panozzo, J.F.; Walker, C.K.; Barlow, K.M.; Fitzgerald, G.J. Models of grain quality in wheat—A review. Field Crops Res. 2017, 202, 136–145. [Google Scholar] [CrossRef]
- Araya, A.; Kisekka, I.; Girma, A.; Hadgu, K.M.; Tegebu, F.N.; Kassa, A.H.; Ferreira-Filho, H.R.; Beltrao, N.E.; Afewerk, A.; Abadi, B. The challenges and opportunities for wheat production under future climate in northern Ethiopia. (Cambridge). J. Agric. Sci. 2017, 55, 379–393. [Google Scholar] [CrossRef]
- Kebede, B.; Korecha, D.; Mamo, G.; Dandesa, D.; Yibrah, M. Modeling climate change and its impacts on food barley (Horduem vulgare L.) production using different climate change scenarios in Lemubilbilo district, Oromia regional state, Ethiopia. Internat. J. Res. Environ. 2019, 5, 33–40. [Google Scholar]
- Ha, Y.W. Barley, Ruderal Development Administration. Geomogmunhasa 2000, 81–82. [Google Scholar]
- Williams, M.; Shewry, P.R.; Lawlor, D.W.; Harwood, J.L. The effects of elevated temperature and atmospheric carbon dioxide concentration on the quality of grain lipids in wheat (Triticum aestivum L.) grown at two levels of nitrogen application. Plant Cell Environ. 1995, 18, 999–1009. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, V.; Pawar, S.K.; Singh, P.K.; Kaur, A.; Sharma, D. Abiotic Stress and Wheat Grain Quality: A Comprehensive Review. In Wheat Production in Changing Environments; Springer: New York, NY, USA, 2017; pp. 63–87. [Google Scholar]
- Carr, S.J.; Ritchie, G.S.P.; Porter, W.M. A soil test for aluminium toxicity in acidic subsoils of yellow earths in Western Australia. Aus. J. Agric. Res. 1991, 42, 875–892. [Google Scholar] [CrossRef][Green Version]
- Scott, B.J.; Conyers, M.K.; Poile, G.J.; Cullis, B.R. Subsurface acidity and liming affect yield of cereals. Aust. J. Agric. Res. 1997, 48, 843–854. [Google Scholar] [CrossRef]
- Young, K. Barley: Soil; Climatic Requirements. In Soil Guide: A Handbook for Understanding and Managing Agricultural Soils; Bulletin 4343; Moore, G., Ed.; Agriculture Western Australia: South Perth, Australia, 1998. [Google Scholar]
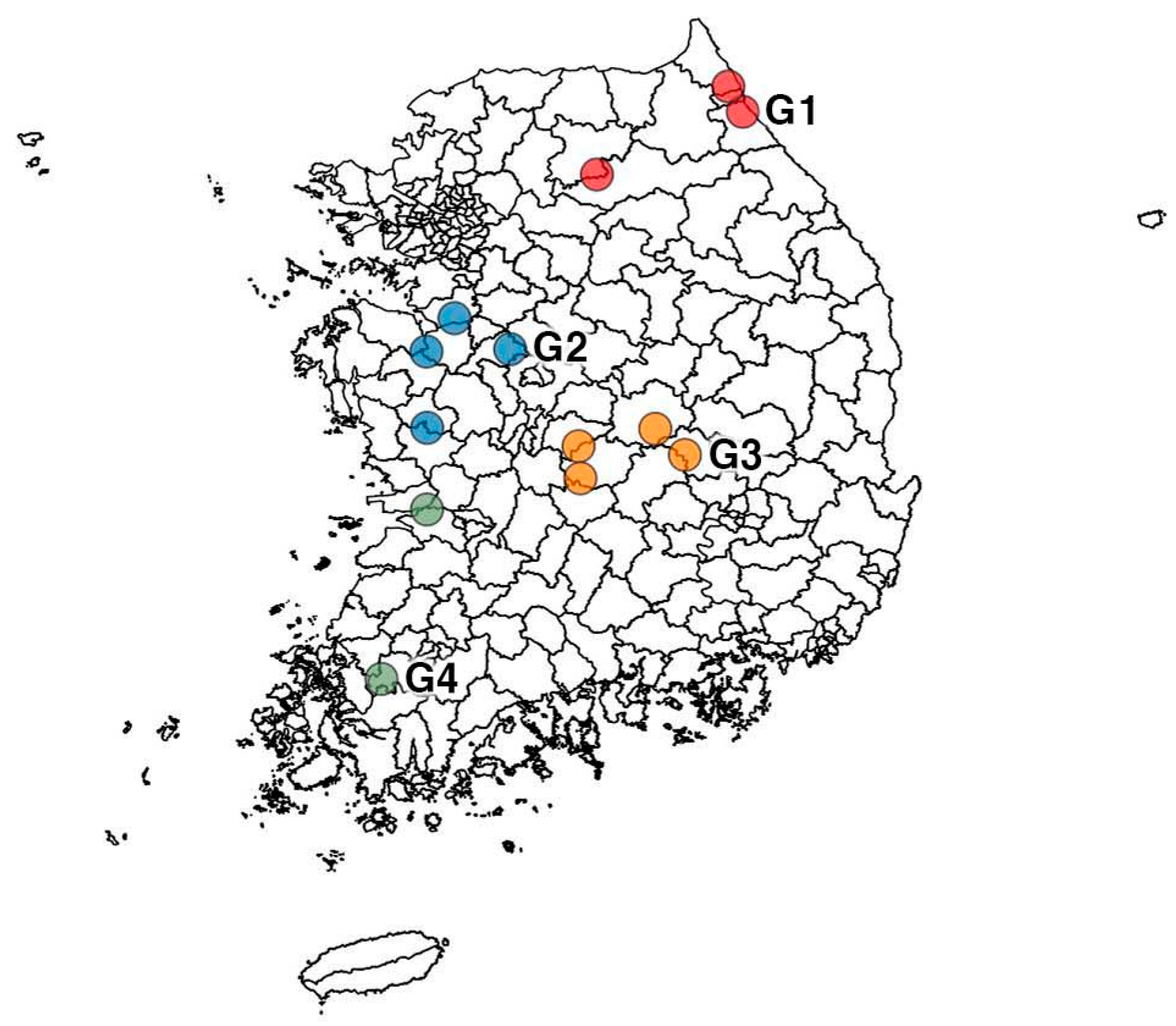

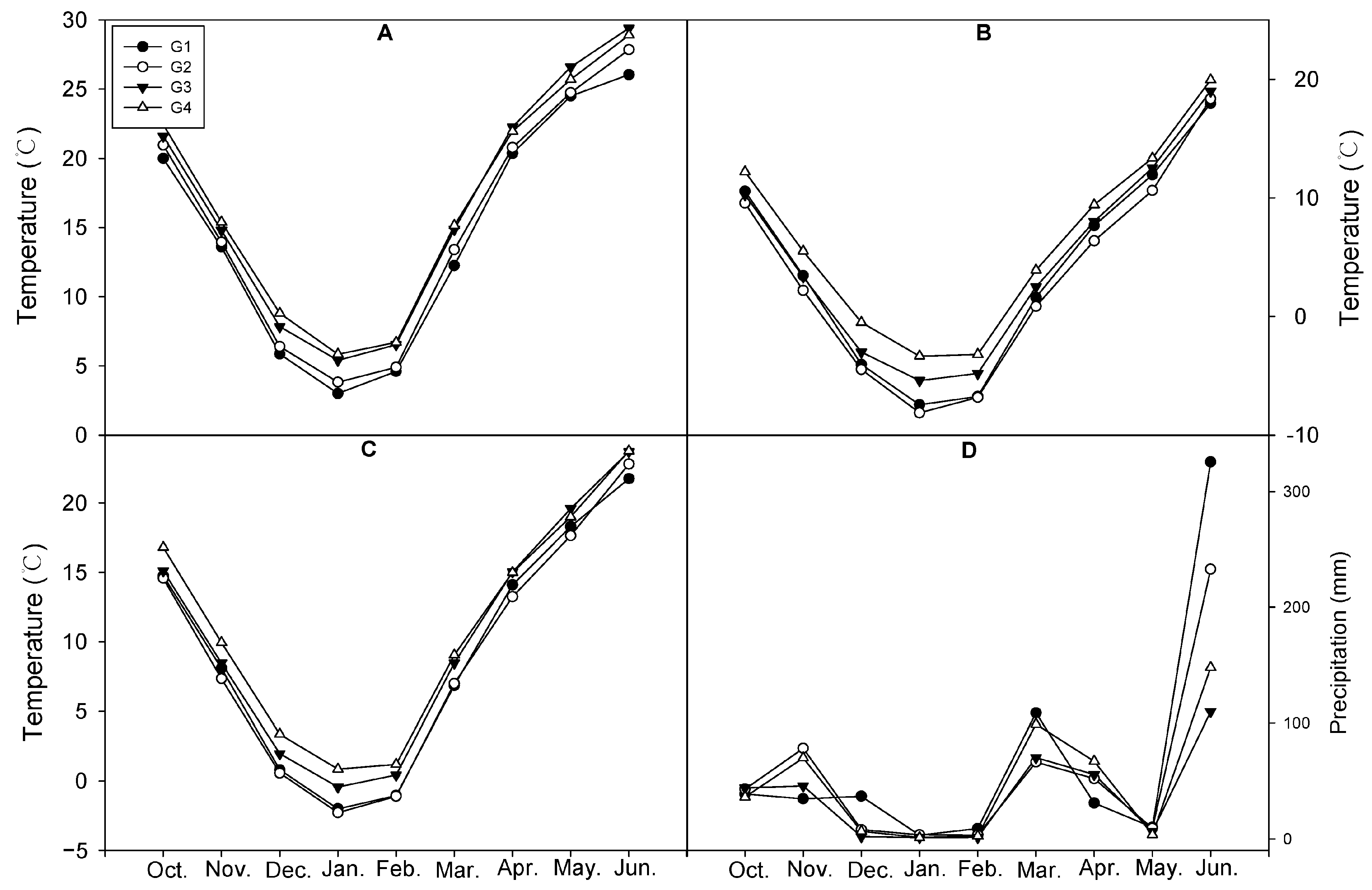
| Group | Area | Tiller Number (m2) | Plant Height (cm) | Dried Aboveground Plant Part (g/m2) |
|---|---|---|---|---|
| G1 | Sokcho 1 | 0 (NA *) g** | 0 (NA) f | 0 (NA) g |
| Sokcho 2 | 295 f | 4.7 e | 6.2 f | |
| Chuncheon | 290 f | 4.7 e | 6.7 f | |
| Average | 195.0 | 3.1 | 4.3 | |
| G2 | Pyeongtaek | 395 e | 7.6 b,c | 5.6 f |
| Cheonan | 400 d,e | 4.1 e | 6.2 f | |
| Asan | 410 d | 8.4 b | 5.5 f | |
| Cheonyang | 485 c | 7.9 b | 17.6 c | |
| Average | 422.5 | 7.0 | 8.7 | |
| G3 | Youngdong 1 | 510 b | 19.5 a | 23.3 a |
| Youngdong 2 | 480 c | 6.6 c,d | 20.8 b | |
| Gumi | 630 a | 8.0 b | 18.7 c | |
| Sangju | 521 b | 6.4 d | 11.5 e | |
| Average | 535.2 | 10.1 | 18.5 | |
| G4 | Gimje | 390 e | 7.3 b,c,d | 15.0 d |
| Naju | 320 f | 4.1 e | 5.68 f | |
| Average | 355.0 | 5.7 | 10.3 |
| Group | Area | Tiller Number (m2) | Plant Height (cm) | Dried Aboveground Plant Part (g/m2) | SPAD Value |
|---|---|---|---|---|---|
| G1 | Sokcho 1 | 10 i* | 3.0 g | 1.2 h | 41.0 e,f |
| Sokcho 2 | 311 h | 4.8 e,f | 15.6 g | 44.7 c,d,e | |
| Chuncheon | 334 g | 5.3 e,f | 16.2 g | 40.4 e,f | |
| Average | 218.3 | 4.3 | 11.0 | 42.0 | |
| G2 | Pyeongtaek | 340 g | 8.4 d | 25.5 e | 42.7 d,e,f |
| Cheonan | 580 e | 4.7 e,f | 20.0 f | 38.7 f | |
| Asan | 720 c | 10.4 c | 72.0 b | 48.0 b,c | |
| Cheonyang | 720 c | 10.2 c | 54.0 c | 51.2 a,b | |
| Average | 590.0 | 8.4 | 42.8 | 45.15 | |
| G3 | Youngdong 1 | 870 b | 14.6 a | 78.3 b | 48.3 b,c |
| Youngdong 2 | 420 f | 8.0 d | 33.6 d | 46.9 b,c,d | |
| Gumi | 430 f | 10.1 c | 17.2 f,g | 50.9 a,b | |
| Sangju | 430 f | 6.1 e | 15.6 g | 44.8 c,d,e | |
| Average | 537.5 | 9.7 | 36.1 | 47.7 | |
| G4 | Gimje | 960 a | 9.9 c | 52.3 c | 54.5 a |
| Naju | 640 d | 12.9 b | 89.6 a | 44.9 c,d,e | |
| Average | 800.0 | 11.4 | 70.9 | 49.7 |
| Group | Area | Tiller Number (m2) | Plant Height (cm) | Dried Aboveground Plant Part (g/m2) | SPAD Value |
|---|---|---|---|---|---|
| G1 | Sokcho 1 | 65 h* | 28.3 g | 59.4 h | 44.6 c,d |
| Sokcho 2 | 438 c | 54.6 d | 488.4 d | 48.1 b,c | |
| Chuncheon | 439 c | 50.5 d,e | 274.4 f,g | 44.9 c,d | |
| Average | 314.0 | 44.4 | 274.0 | 45.8 | |
| G2 | Pyeongtaek | 392 e | 46.7 f | 260.0 g | 56.2 a |
| Cheonan | 386 e | 44.6 e,f | 295.3 f | 49.2 b,c | |
| Asan | 633 a | 55.4 d | 541.2 c | 48.1 b,c | |
| Cheonyang | 642 a | 63.6 b,c | 869.9 a | 41.0 e | |
| Average | 513.2 | 52.5 | 491.6 | 48.6 | |
| G3 | Youngdong 1 | 651 a | 69.2 b | 725.9 b | 44.4 c,d |
| Youngdong 2 | 415 d | 55.1 d | 549.9 c | 45.5 c,d | |
| Gumi | 315 f | 40.4 f | 298.3 f,g | 52.1 a,b | |
| Sangju | 250 g | 30.3 g | 81.3 h | 48.7 b,c | |
| Average | 407.7 | 48.7 | 413.8 | 47.6 | |
| G4 | Gimje | 415 d | 57.9 c,d | 392. e | 37.9 e |
| Naju | 521 b | 84.8 a | 547.1 c | 47.9 b,c | |
| Average | 468.0 | 71.3 | 469.6 | 42.9 |
| Group | Area | Culm Length (cm) | Panicle Length (cm) | Panicle Number (m2) | Spikelet Number/Panicle | Ripening Rate (%) | Weight (g/L) | 1000 Seed Weight (g) | Yield (kg/ha) |
|---|---|---|---|---|---|---|---|---|---|
| G1 | Sokcho 1 | 64.2 f* | 3.8 a | 63 h | 54.3 a,b,c | 95.3 a,b,c,d | 731.2 f | 21.2 f | 2420 j |
| Sokcho 2 | 84.3 b | 3.2 a,b | 210 g | 47.3 b,c,d | 95.5 a,b,c,d | 755.8 e | 23.7 d,e,f | 3273 g | |
| Chuncheon | 70.5 c,d,e,f | 3.4 a,b | 347 e | 40.5 c,d | 93.4 d | 745.9 e,f | 22.4 e,f | 2660 h,i | |
| Average | 73.0 | 3.4 | 206.6 | 47.3 | 94.7 | 744.3 | 22.4 | 2784 | |
| G2 | Pyeongtaek | 75.6 c | 3.8 a,b | 386 c | 52.8 a,b,c,d | 94.3 c,d | 790.9 c,d | 26.2 c,d | 3847 f |
| Cheonan | 87.3 b | 3.9 a | 472 b | 50 a,b,c,d | 97 a,b,c | 804.4 b,c | 28.2 b | 3913 e,f | |
| Asan | 65.9 e,f | 3.3 a,b | 310 f | 53.3 a,b,c | 95.9 a,b,c,d | 740.7 e,f | 23.5 d,e,f | 2493 j | |
| Cheonyang | 75.2 c,d | 3.5 a,b | 374 c,d | 50.8 a,b,c,d | 95.2 a,b,c,d | 806.9 b,c | 25.4 c,d,e | 4153 c,d | |
| Average | 76.0 | 3.6 | 385.5 | 51.7 | 95.6 | 785.7 | 25.8 | 3601 | |
| G3 | Youngdong 1 | 87.2 b | 3.6 a,b | 376 c,d | 61.5 a,b | 94.5 c,d | 807.5 b,c | 22.6 e,f | 4193 b,c |
| Youngdong 2 | 85.5 b | 3.5 a,b | 370 d | 52.5 a,b,c,d | 98.1 a,b | 806.8 b,c | 27.8 b,c | 4020 d,e | |
| Gumi | 73.1 c,d,e | 3.6 a,b | 322 f | 52.8 a,b,c,d | 96.6 a,b,c,d | 746.7 e,f | 30.1 b | 2533 i,j | |
| Sangju | 68.1 c,d,e,f | 2.8 b | 346 e | 38.7 d | 95.1 b,c,d | 780.8 d | 26.4 c,d | 2707 h | |
| Average | 78.4 | 3.3 | 353.5 | 51.3 | 96.0 | 785.4 | 26.7 | 3363 | |
| G4 | Gimje | 97.4 a | 4.2 a | 480 b | 60.2 a,b | 95.3 b,c,d | 818.4 a,b | 35.2 a | 4320 b |
| Naju | 67.2 d,e,f | 4.0 a | 520 a | 62.2 a | 98.4 a | 831 a | 25.8 a | 4520 a | |
| Average | 82.3 | 4.1 | 500.0 | 61.2 | 96.8 | 824.7 | 30.5 | 4420 |
| Group | Area | Culm Length (cm) | Panicle Length (cm) | Panicle Number (m2) | Spikelet Number/Panicle | Ripening Rate (%) | Weight (g/L) | 1000 Seed Weight (g) | Yield (kg/ha) |
|---|---|---|---|---|---|---|---|---|---|
| G1 | Goseong | 56.8 d,e* | 3.1 d–f | 310 d | 48.0 c | 96.0 a | 795.4 a | 33.6 a | 2913 e |
| Hoengseong 1 | 54.5 e | 3.3 b–f | 183 f | 39.6 e | 92.9 a | 819.5 a | 30.7 b | 1847 h | |
| Hoengseong 2 | 58.9 b–d | 4.3 a | 201 e | 52.8 b | 94.3 a | 830.0 a | 31.3 a,b | 2280 g | |
| Average | 56.7 | 3.6 | 231.3 | 46.8 | 94.4 | 815.0 | 31.9 | 2347 | |
| G2 | Pyeongtaek | 58.2 c–e | 2.9 e,f | 160 g | 37.2 e,f | 93.5 a | 798.1 a | 34.1 a | 1623 i |
| Cheonan | 60.4 a–d | 3.6 b–e | 125 h | 43.8 d | 93.4 a | 832.0 a | 31.9 a,b | 1533 i | |
| Asan | 61.9 a–c | 3.9 a–c | 173 f,g | 46.2 c,d | 96.1 a | 817.5 a | 30.1 b | 2370 g | |
| Cheongyang | 62.8 a,b | 3.7 a–d | 215 e | 48.0 c | 92.9 a | 806.1 a | 33.8 a | 2533 f | |
| Average | 60.8 | 3.5 | 168.3 | 43.8 | 94.0 | 813.4 | 32.5 | 2015 | |
| G3 | Yeongdong 1 | 64 a | 4.0 a,b | 475 b | 60.0 a | 95.2 a | 772.3 a | 30.1 b | 4097 b |
| Sangju | 60 a–d | 2.8 f | 320 d | 35.4 f | 96.6 a | 792.2 a | 33.9 a | 3123 d | |
| Average | 62.4 | 3.4 | 397.5 | 47.7 | 95.9 | 782.3 | 32.0 | 3610 | |
| G4 | Gimje | 60.9 a–d | 3.2 c–f | 380 c | 39.0 e | 95.1 a | 818.8 a | 29.9 b | 3303 c |
| Naju | 54.6 e | 3.4 b–f | 556 a | 47.4 c | 96.6 a | 834.1 a | 24.5 c | 4380 a | |
| Average | 57.8 | 3.3 | 468.0 | 43.2 | 95.9 | 826.5 | 27.2 | 3842 |
| Group | Area | December | February | April |
|---|---|---|---|---|
| G1 | Sokcho 1 | - | 40.5 | 27.3 |
| Sokcho 2 | - | - | - | |
| Chuncheon | 42.3 | 43.5 | 30.5 | |
| Average | 42.3 | 42.0 | 28.9 | |
| G2 | Pyeongtaek | - | 38.6 | 53.2 |
| Cheonan | 37.1 | 32.5 | 37.3 | |
| Asan | - | 35.6 | 29.6 | |
| Cheonyang | - | - | 36.3 | |
| Average | 37.1 | 35.5 | 39.10 | |
| G3 | Youngdong 1 | 36.0 | 31.5 | 31.2 |
| Youngdong 2 | 31.5 | 32.5 | 35.6 | |
| Gumi | - | 35.0 | 34.8 | |
| Sangju | 38.8 | 35.2 | 42.1 | |
| Average | 35.4 | 33.55 | 35.92 | |
| G4 | Gimje | 37.5 | 39.6 | 45.7 |
| Naju | 32.3 | 38.9 | 31.3 | |
| Average | 34.90 | 39.25 | 38.50 |
| Group | Area | Crude Protein | Crude Fat | Moisture | Crude Ash | Total Carbohydrate |
|---|---|---|---|---|---|---|
| G1 | Sokcho 2 | 12.22 a* | 1.87 b | 8.95 b | 2.06 d | 74.89 i |
| Chuncheon | 8.60 e | 1.56 e | 8.30 h | 2.44 a | 79.10 c | |
| Average | 10.41 | 1.72 | 8.63 | 2.25 | 77.00 | |
| G2 | Pyeongtaek | 12.42 a | 1.91 b | 9.01 a | 1.39 h,i | 75.27 h |
| Asan | 11.55 b | 1.83 c | 8.70 e | 2.29 b | 75.63 g | |
| Cheonan | 12.20 a | 1.46 f | 8.17 i | 1.79 f | 76.38 f | |
| Cheonyang | 11.18 c | 2.04 a | 8.64 f | 2.30 b | 75.83 g | |
| Average | 11.64 | 1.78 | 8.50 | 2.13 | 75.95 | |
| G3 | Youngdong 1 | 8.91 e | 1.15 g | 8.78 d | 1.36 i | 79.81 b |
| Sangju | 10.46 d | 1.70 d | 8.90 c | 1.89 e | 77.05 e | |
| Gumi | 10.24 d | 2.03 a | 8.67 e,f | 1.43 h | 77.62 d | |
| Average | 9.87 | 1.63 | 8.78 | 1.56 | 78.16 | |
| G4 | Gimje | 10.95 c | 1.67 d | 8.49 g | 2.21 c | 76.68 f |
| Naju | 7.84 f | 1.66 d | 8.46 g | 1.62 g | 80.42 a | |
| Average | 9.40 | 1.67 | 8.48 | 1.92 | 78.55 |
| Group | Area | Amino Acid | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASP | Thr | Ser | Glu | Gly | Ala | Val | Ile | Leu | Tyr | Phe | Lys | His | Arg | Pro | Cys | Met | ||
| G1 | Sokcho 2 | 0.71 b* | 0.44 ab | 0.48 a | 2.93 b | 0.50 a–c | 0.49 ab | 0.60 b–d | 0.40 b | 0.84 ab | 0.35 a | 0.63 b | 0.46 a–c | 0.27 a,b | 0.58 a,b | 1.33 b | 0.23 ab | 0.21 a–c |
| Chuncheon | 0.48 d | 0.28 f | 0.29 e | 1.59 f | 0.34 e | 0.31 f | 0.41 h | 0.27 e | 0.52 g | 0.18 f | 0.37 g | 0.32 f | 0.17 e | 0.38 d | 0.69 g | 0.17 e | 0.16 e | |
| Average | 0.60 | 0.36 | 0.39 | 2.26 | 0.42 | 0.40 | 0.51 | 0.34 | 0.68 | 0.27 | 0.50 | 0.39 | 0.22 | 0.48 | 1.01 | 0.20 | 0.19 | |
| G2 | Pyeongtaek | 0.65 b,c | 0.40 bc | 0.43 b | 2.71 e | 0.48 a–d | 0.46 b,c | 0.60 b,c | 0.40 b | 0.79 b,c | 0.30 b,c | 0.60 b,c | 0.44 a–c | 0.26 b | 0.54 b,c | 1.27 b,c | 0.23 a,b | 0.20 a–c |
| Asan | 0.63 c | 0.37 cd | 0.41 b,c | 2.48 d | 0.44 d | 0.40 d,e | 0.53 e | 0.36 c,d | 0.70 d,e | 0.26 de | 0.53 d,e | 0.39 d,e | 0.23 c,d | 0.49 c | 1.13 d | 0.25 a | 0.22 a | |
| Cheonan | 0.78 a | 0.45 a | 0.49 a | 3.24 a | 0.53 a | 0.54 a | 0.66 a | 0.47 a | 0.87 a | 0.32 ab | 0.71 a | 0.48 a | 0.29 a | 0.62 a | 1.51 a | 0.20 b–d | 0.20 a–c | |
| Cheonyang | 0.70 b,c | 0.41 a–c | 0.41 bc | 2.51 c,d | 0.52 a,b | 0.50 a,b | 0.61 b | 0.40 b | 0.77 b–d | 0.28 cd | 0.58 b–d | 0.48 a,b | 0.27 b | 0.57 a,b | 1.16 c,d | 0.21 b–d | 0.20 b,c | |
| Average | 0.69 | 0.41 | 0.44 | 2.74 | 0.49 | 0.48 | 0.60 | 0.41 | 0.78 | 0.29 | 0.61 | 0.45 | 0.26 | 0.56 | 1.27 | 0.22 | 0.21 | |
| G3 | Youngdong 1 | 0.53 d | 0.32 e | 0.34 de | 1.78 f | 0.37 e | 0.36 e,f | 0.43 g,h | 0.28 e | 0.57 f,g | 0.24e | 0.41 f,g | 0.35 e,f | 0.19 e | 0.42 d | 0.82 f | 0.18 d,e | 0.16 e |
| Sangju | 0.66 b,c | 0.38 c,d | 0.40 bc | 2.45 d | 0.47 b–d | 0.45 b–d | 0.55 d,e | 0.38 b,c | 0.73 c–e | 0.28cd | 0.57 b–d | 0.44 a–d | 0.26 b,c | 0.54 b,c | 1.33 b | 0.22 a,b | 0.21 a,b | |
| Gumi | 0.66 b,c | 0.38 c,d | 0.40 bc | 2.21 e | 0.45 c,d | 0.43 c,d | 0.52 e,f | 0.34 d | 0.70 e | 0.29 cd | 0.49 e | 0.43 b–d | 0.23 c,d | 0.52 b,c | 0.98 e | 0.21 b,c | 0.19 b–d | |
| Average | 0.62 | 0.36 | 0.38 | 2.15 | 0.43 | 0.41 | 0.50 | 0.33 | 0.67 | 0.27 | 0.49 | 0.41 | 0.23 | 0.49 | 1.04 | 0.20 | 0.19 | |
| G4 | Gimje | 0.68 b,c | 0.39 c,d | 0.42 bc | 2.33 d,e | 0.49 a–d | 0.46 b,c | 0.56 c–e | 0.38 b,c | 0.72 c–e | 0.28 cd | 0.56 c,d | 0.44 a–d | 0.25 b,c | 0.56 a,b | 1.12 d | 0.21 b,c | 0.19 c,d |
| Naju | 0.64 b,c | 0.35 d,e | 0.37 cd | 1.76 f | 0.44 d | 0.43 c,d | 0.47 f,g | 0.30 e | 0.60 f | 0.25 de | 0.43 f | 0.42 c,d | 0.22 d | 0.52 b,c | 0.74 f,g | 0.18 c–e | 0.1 d,e | |
| Average | 0.66 | 0.37 | 0.40 | 2.05 | 0.47 | 0.45 | 0.52 | 0.34 | 0.66 | 0.27 | 0.50 | 0.43 | 0.24 | 0.54 | 0.93 | 0.20 | 0.18 | |
| Group | Area | pH (1.5) | EC (ds/m) | OM (g/Kg) | Av.P2O5 (mg/Kg) | Exchangeable base (cmol+/Kg) | CEC (cmol+/Kg) | Zn | Mn | Cu | B | Mo | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Ca | Mg | (mg/kg) | |||||||||||
| G1 | Sokcho 1 | 6.54 c* | 0.32 j | 25 g | 511 b | 0.90 c | 6.62 b | 1.52 h | 15.77 a | 28.53 a | 82.83 e | 3.23 d,e,f | 0.73 f | 0.01 a.b |
| Sokcho 2 | 6.7 b | 0.37 i | 38 a | 1247 a | 0.91 c | 8.68 a | 2.27 c | 13.52 c | 25.96 b | 74.95 f | 2.43 f | 0.82 d | 0.01 a,b | |
| Chuncheon | 5.36 g | 1.5 c | 28 e | 162 g | 0.50 e | 3.88 i | 0.81 j | 7.54 i | 3.29 h | 71.92 g | 4.29 b,c | 0.52 i | 0.01 a,b | |
| Average | 6.20 | 0.73 | 30.33 | 640.00 | 0.77 | 6.39 | 1.53 | 12.28 | 19.26 | 76.57 | 3.32 | 0.69 | 0.01 | |
| G2 | Pyeongtaek | 4.23 i | 3.21 a | 15 k | 34 j | 0.73 d | 3.45 j | 4.01 a | 12.95 d | 6.93 f | 127.62 b | 3.24 d,e,f | 0.50 i | 0.01 b |
| Cheonan | 6.92 a | 0.57 f,g | 36 b | 391 d | 0.73 d | 6.27 d | 1.80 f | 9.22 h | 10.31 e | 141.89 a | 5.48 a | 0.95 b | 0.01 b | |
| Asan | 5.44 g | 0.60 f | 31 d | 87 h | 0.21 g | 5.64 e | 1.87 e | 9.76 g | 3.72 g,h | 61.21 i | 3.84 b,c,d,e | 0.99 a | 0.01 a,b | |
| Cheonyang | 6.25 d | 0.44 h | 17 j | 179 f | 0.48 e | 3.16 k | 1.22 i | 5.36 k | 6.13 f | 85.09 d | 4.60 b | 0.58 h | 0.01 a,b | |
| Average | 5.71 | 1.21 | 24.75 | 172.75 | 0.54 | 4.63 | 2.23 | 9.32 | 6.77 | 103.95 | 4.29 | 0.76 | 0.01 | |
| G3 | Youngdong 1 | 6.88 a | 0.55 g | 34 c | 486 c | 1.32 a | 6.49 c | 1.92 e | 10.23 f | 13.28 d | 129.81 b | 4.05 b,c,d | 0.61 g | 0.01 b |
| Youngdong 2 | 5.13 h | 0.27 k | 19 i | 83 h | 0.14 h,i | 2.82 l | 0.69 k | 5.92 j | 3.99 g,h | 49.33 j | 3.46 c,d,e | 0.29 j | 0.01 a,b | |
| Gumi | 5.89 e | 0.89 e | 23 h | 83 h | 0.11 i | 6.20 d | 1.89 e | 10.35 f | 3.64 g,h | 127.94 b | 2.49 f | 0.81 d | 0.01 b | |
| Sangju | 5.55 f | 1.33 d | 14 l | 71 i | 0.26 f | 4.68 h | 1.63 g | 10.90 e | 4.40 g | 66.45 h | 3.11 e,f | 0.77 e | 0.02 a | |
| Average | 5.86 | 0.76 | 22.50 | 180.75 | 0.46 | 5.05 | 1.53 | 9.35 | 6.33 | 93.38 | 3.28 | 0.62 | 0.01 | |
| G4 | Gimje | 5.39 g | 1.68 b | 27 e,f | 77 h,i | 0.16 h | 5.38 g | 2.06 d | 9.79 g | 2.02 i | 31.07 k | 4.42 b | 0.87 c | 0.01 b |
| Naju | 6.47 c | 0.32 j | 27 f | 225 e | 1.05 b | 5.55 f | 2.39 b | 14.32 b | 24.36 c | 119.00 c | 3.52 c,d,e | 0.84 d | 0.02 a | |
| Average | 5.93 | 1.00 | 27.00 | 151.00 | 0.61 | 5.47 | 2.23 | 12.06 | 13.19 | 75.04 | 3.97 | 0.86 | 0.02 | |
| Group | Area | T-N | P2O5 | K2O | CaO | MgO | Zn | Mn | Cu | B | Mo |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (mg/kg) | ||||||||||
| G1 | Sokcho 1 | 1.54 i* | 1.38 a,b | 3.66 e | 0.25 f | 0.16 d,e,f | 16.97 h | 21.77 i | 3.72 d | 13.02 g | 0.11 e |
| Sokcho 2 | 1.49 j | 1.30 a,b | 3.70 d,e | 0.25 f | 0.15 f,g | 24.18 c | 25.83 h | 3.89 c | 13.06 g | 0.20 c,d | |
| Chuncheon | 2.16 d | 0.24 b | 3.92 c | 0.39 c | 0.14 f,g | 19.71 f | 44.30 d | 3.18 f | 14.09 e,f | 0.13 d,e | |
| Average | 1.73 | 0.97 | 3.76 | 0.30 | 0.15 | 20.29 | 30.63 | 3.60 | 13.39 | 0.15 | |
| G2 | Pyeongtaek | 3.14 b | 0.47 b | 3.73 d,e | 0.19 g | 0.19 c,d | 21.97 e | 163.18 a | 4.49 b | 15.27 c,d | 0.10 e |
| Cheonan | 1.81 g | 0.48 b | 3.83 c,d | 0.24 f | 0.12 g | 11.89 k | 19.07 j | 2.98 g | 13.59 f,g | 0.34 b | |
| Asan | 3.07 c | 0.46 b | 4.85 a | 0.42 b | 0.21 c | 22.79 d | 40.08 e | 3.19 f | 17.51 a | 0.34 b | |
| Cheonyang | 1.26 k | 0.89 a,b | 3.29 g | 0.29 e | 0.16 e,f | 26.30 a | 28.79 g | 4.50 b | 15.47 c | 0.27 b,c | |
| Average | 2.32 | 0.57 | 3.93 | 0.28 | 0.17 | 20.74 | 62.78 | 3.79 | 15.46 | 0.26 | |
| G3 | Youngdong 1 | 1.62 h | 1.13 a,b | 4.17 b | 0.33 d | 0.15 f,g | 11.67 k | 10.81 k | 3.44 e | 16.38 b | 0.23 c |
| Youngdong 2 | 1.96 e | 0.26 b | 2.21 h | 0.25 f | 0.13 g | 12.30 k | 64.31 b | 1.56 i | 14.53 d,e | 0.08 e | |
| Gumi | 4.07 a | 0.58 b | 3.79 c,d,e | 0.69 a | 0.34 b | 24.88 b | 32.86 f | 4.77 a | 14.00 e,f | 0.20 c,d | |
| Sangju | 4.09 a | 2.39 a | 3.79 c,d,e | 0.69 a | 0.37 a | 18.54 g | 46.17 c | 3.57 e | 15.35 c | 0.11 e | |
| Average | 2.94 | 1.09 | 3.49 | 0.49 | 0.25 | 16.85 | 38.54 | 3.34 | 15.07 | 0.16 | |
| G4 | Gimje | 1.82 g | 0.38 b | 3.42 f | 0.39 c | 0.19 c | 13.84 i | 20.95 i | 2.36 h | 16.85 a,b | 0.25 c |
| Naju | 1.88 f | 0.91 a,b | 4.19 b | 0.41 b | 0.18 c,d,e | 13.09 j | 8.51 l | 3.92 c | 17.20 a,b | 0.53 a | |
| Average | 1.85 | 0.65 | 3.81 | 0.40 | 0.19 | 13.47 | 14.73 | 3.14 | 17.02 | 0.39 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-G.; Park, H.-H.; Lee, H.-J.; Kim, H.-K.; Kuk, Y.-I. Growth, Yield, and Grain Quality of Barley (Hordeum vulgare L.) Grown across South Korean Farmlands with Different Temperature Distributions. Agronomy 2022, 12, 2731. https://doi.org/10.3390/agronomy12112731
Kim Y-G, Park H-H, Lee H-J, Kim H-K, Kuk Y-I. Growth, Yield, and Grain Quality of Barley (Hordeum vulgare L.) Grown across South Korean Farmlands with Different Temperature Distributions. Agronomy. 2022; 12(11):2731. https://doi.org/10.3390/agronomy12112731
Chicago/Turabian StyleKim, Ye-Geon, Hyun-Hwa Park, Hyo-Jin Lee, Hee-Kwon Kim, and Yong-In Kuk. 2022. "Growth, Yield, and Grain Quality of Barley (Hordeum vulgare L.) Grown across South Korean Farmlands with Different Temperature Distributions" Agronomy 12, no. 11: 2731. https://doi.org/10.3390/agronomy12112731
APA StyleKim, Y.-G., Park, H.-H., Lee, H.-J., Kim, H.-K., & Kuk, Y.-I. (2022). Growth, Yield, and Grain Quality of Barley (Hordeum vulgare L.) Grown across South Korean Farmlands with Different Temperature Distributions. Agronomy, 12(11), 2731. https://doi.org/10.3390/agronomy12112731






