Limited Influence of Abiotic and Biotic Factors on the Efficacy of Soil Insecticides and Entomopathogenic Nematodes when Managing the Maize Pest Diabrotica v. virgifera (Coleoptera: Chrysomelidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Fields
2.2. Target Pest
2.3. Experimental Design and Treatments
2.3.1. Heterorhabditis Bacteriophora Fluid
2.3.2. Clothianidin Seed Coating
2.3.3. Cypermethrin Microgranules
2.3.4. Tefluthrin Fine Granules
2.3.5. Untreated Control
2.4. Assessment of Abiotic and Biotic Factors
2.5. Assessment of Pest Populations and Root Damage
2.6. Data Analysis
3. Results
3.1. Treatment Efficacies
3.2. Abiotic and Biotic Factors Influencing Treatment Efficacies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Statistics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021.
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops That Feed the World 6. Past Successes and Future Challenges to the Role Played by Maize in Global Food Security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar]
- Jones, P.G.; Thornton, P.K. The Potential Impacts of Climate Change on Maize Production in Africa and Latin America in 2055. Glob. Environ. Chang. 2003, 13, 51–59. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Cairns, J.E.; Zaidi, P.H.; Beyene, Y.; Makumbi, D.; Gowda, M.; Magorokosho, C.; Zaman-Allah, M.; Olsen, M.; Das, A.; et al. Beat the Stress: Breeding for Climate Resilience in Maize for the Tropical Rainfed Environments. Theor. Appl. Genet. 2021, 134, 1729–1752. [Google Scholar] [CrossRef] [PubMed]
- De Groote, H.; Kimenju, S.C.; Munyua, B.; Palmas, S.; Kassie, M.; Bruce, A. Spread and Impact of Fall Armyworm (Spodoptera frugiperda Smith, J.E.) in Maize Production Areas of Kenya. Agric. Ecosyst. Environ. 2020, 292, 106804. [Google Scholar] [CrossRef]
- Meinke, L.J.; Sappington, T.W.; Onstad, D.W.; Guillemaud, T.; Miller, N.J.; Komaromi, J.; Levay, N.; Furlan, L.; Kiss, J.; Toth, F. Western Corn Rootworm (Diabrotica Virgifera Virgifera LeConte) Population Dynamics. Agric. For. Entomol. 2009, 11, 29–46. [Google Scholar] [CrossRef]
- Methods for the Study of Pest Diabrotica; Krysan, J.L., Miller, T.A., Eds.; Springer: New York, NY, USA, 1986; 260p. [Google Scholar]
- Krysan, J.L.; Smith, R.F. Systematics of the Virgifera Species Group of Diabrotica (Coleoptera: Chrysomelidae: Galerucinae). Entomography 1987, 5, 375–484. [Google Scholar]
- Campbell, L.A.; Meinke, L.J. Seasonality and Adult Habitat Use by Four Diabrotica Species at Prairie-Corn Interfaces. Environ. Entomol. 2006, 35, 922–936. [Google Scholar] [CrossRef]
- Miller, N.; Estoup, A.; Toepfer, S.; Bourguet, D.; Lapchin, L.; Derridj, S.; Kim, K.S.; Reynaud, P.; Furlan, L.; Guillemaud, T. Multiple Transatlantic Introductions of the Western Corn Rootworm. Science 2005, 310, 992. [Google Scholar] [CrossRef]
- Szalai, M.; Komáromi, J.P.; Bažok, R.; Barčic´, J.I.; Kiss, J.; Toepfer, S. Generational Growth Rate Estimates of Diabrotica Virgifera Virgifera Populations (Coleoptera: Chrysomelidae). J. Pest Sci. 2011, 84, 133–142. [Google Scholar] [CrossRef]
- CABI. Diabrotica virgifera virgifera. In Invasive Species Compendium; CABI: Wallingford, UK, 2021; Available online: www.cabi.org/isc (accessed on 19 October 2022.).
- Moeser, J.; Hibbard, B.E. A Synopsis on the Nutritional Ecology of Larvae and Adults of Diabrotica Virgifera Virgifera (LeConte) in the New and Old World—Nouvelle Cuisine for the Invasive Maize Pest Diabrotica Virgifera Virgifera in Europe? In Western Corn Rootworm: Ecology and Management; Vidal, S., Kuhlmann, U., Edwards, C.R., Eds.; CABI: Wallingford, UK, 2005; p. 320. [Google Scholar]
- Urías-López, M.A.; Meinke, L.J. Influence of Western Corn Rootworm (Coleoptera: Chrysomelidae) Larval Injury on Yield of Different Types of Maize. J. Econ. Entomol. 2001, 94, 106–111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gyeraj, A.; Szalai, M.; Pálinkás, Z.; Edwards, C.R.; Kiss, J. Effects of Adult Western Corn Rootworm (Diabrotica Virgifera Virgifera LeConte, Coleoptera: Chrysomelidae) Silk Feeding on Yield Parameters of Sweet Maize. Crop Prot. 2021, 140, 105447. [Google Scholar] [CrossRef]
- Baufeld, P.; Enzian, S. Maize growing, maize high-risk areas and potential yield losses due to western corn rootworm (Diabrotica virgifera virgifera) damage in selected European countries. In Western Corn Rootworm: Ecology and Management; Vidal, S., Kuhlmann, U., Edwards, C.R., Eds.; CABI Books 2005; CABI: Wallingford, UK, 2005; p. 285. [Google Scholar] [CrossRef]
- Feusthuber, E.; Mitter, H.; Schönhart, M.; Schmid, E. Integrated Modelling of Efficient Crop Management Strategies in Response to Economic Damage Potentials of the Western Corn Rootworm in Austria. Agric. Syst. 2017, 157, 93–106. [Google Scholar] [CrossRef]
- Falkner, K.; Mitter, H.; Moltchanova, E.; Schmid, E. A Zero-Inflated Poisson Mixture Model to Analyse Spread and Abundance of the Western Corn Rootworm in Austria. Agric. Syst. 2019, 174, 105–116. [Google Scholar] [CrossRef]
- Bažok, R.; Lemi, D.; Chiarini, F. In Europe: Current Status and Sustainable Pest Management. Insects 2021, 12, 195. [Google Scholar] [CrossRef]
- Paddock, K.J.; Robert, C.A.M.; Erb, M.; Hibbard, B.E. Western Corn Rootworm, Plant and Microbe Interactions: A Review and Prospects for New Management Tools. Insects 2021, 12, 171. [Google Scholar] [CrossRef]
- Rozen, K. van Ester, A. Chemical Control of Diabrotica Virgifera Virgifera LeConte. J. Appl. Entomol. 2010, 134, 376–384. [Google Scholar] [CrossRef]
- Veres, A.; Wyckhuys, K.A.G.; Kiss, J.; Tóth, F.; Burgio, G.; Pons, X.; Avilla, C.; Vidal, S.; Razinger, J.; Bazok, R.; et al. An Update of the Worldwide Integrated Assessment (WIA) on Systemic Pesticides. Part 4: Alternatives in Major Cropping Systems. Environ. Sci. Pollut. Res. 2020, 27, 29867–29899. [Google Scholar] [CrossRef]
- Mitchell, S.B.; Ciha, A.J.; Tollefson, J.J. Corn Rootworm Biology and Management. 2010, pp. 1–146. Available online: https://cornrootworm.extension.iastate.edu/management (accessed on 19 October 2022).
- Ball, H.J.; Weekman, G.T. Insecticide Resistance in the Adult Western Corn Rootworm in Nebraska. J. Econ. Entomol. 1962, 55, 439–441. [Google Scholar] [CrossRef]
- Meinke, L.J.; Souza, D.; Siegfried, B.D. The Use of Insecticides to Manage the Western Corn Rootworm, Diabrotica Virgifera Virgifera, Leconte: History, Field-Evolved Resistance, and Associated Mechanisms. Insects 2021, 12, 112. [Google Scholar] [CrossRef]
- Souza, D.; Peterson, J.A.; Wright, R.J.; Meinke, L.J. Field Efficacy of Soil Insecticides on Pyrethroid-Resistant Western Corn Rootworms (Diabrotica Virgifera Virgifera LeConte). Pest Manag. Sci. 2020, 76, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Felsot, A.; Maddox, J.V.; Bruce, W. Enhanced Microbial Degradation of Carbofuran in Soils with Histories of Furadan® Use. Bull. Environ. Contam. Toxicol. 1981, 26, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Felsot, A.S.; Wilson, J.G.; Kuhlman, D.E.; Steffey, K.L. Rapid Dissipation of Carbofuran as a Limiting Factor in Corn Rootworm (Coleoptera: Chrysomelidae) Control in Fields with Histories of Continous Carbofuran Use. J. Econ. Entomol. 1982, 75, 1098–1103. [Google Scholar] [CrossRef]
- Racke, K.D.; Coats, J.R. Enhanced Degradation of Isofenphos by Soil Microorganisms. J. Agric. Food Chem. 1987, 35, 94–99. [Google Scholar] [CrossRef]
- Harris, C.R. Factors Influencing the Biological Activity of Technical Chlordane and Some Related Components in Soil1. J. Econ. Entomol. 1972, 65, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.R. Influence of Soil Type on the Activity of Insecticides in Soil. J. Econ. Entomol. 1966, 59, 1221–1224. [Google Scholar] [CrossRef]
- Jones, E.W. The Influence of Temperature on the Toxicity of Carbon Disulphide to Wireworms1. J. Econ. Entomol. 1933, 26, 887–892. [Google Scholar] [CrossRef]
- Fleming, W.E. Chlordan for Control of Japanese Beetle Larvae. J. Econ. Entomol. 1948, 41, 905–912. [Google Scholar] [CrossRef]
- Harris, C.R. Influence of Temperature on the Biological Activity of Insecticides in Soil1. J. Econ. Entomol. 1971, 64, 1044–1049. [Google Scholar] [CrossRef]
- Hills, T.M.; Peters, D.C. Methods of Applying Insecticides for Controlling Western Corn Rootworm Larvae. J. Econ. Entomol. 1972, 65, 1714–1718. [Google Scholar] [CrossRef]
- Mayo, Z.B. Influence of Planting Dates on the Efficacy of Soil Insecticides Applied to Control Larvae of the Western and Northern Corn Rootworm. J. Econ. Entomol. 1980, 73, 211–212. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Shrestha, R.B.; Jakka, S.R.K.; Dunbar, M.W.; Clifton, E.H.; Paolino, A.R.; Ingber, D.A.; French, B.W.; Masloski, K.E.; Dounda, J.W.; et al. Evidence of Resistance to Cry34/35Ab1 Corn by Western Corn Rootworm (Coleoptera: Chrysomelidae): Root Injury in the Field and Larval Survival in Plant-Based Bioassays. J. Econ. Entomol. 2016, 109, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Field, L.M. Neonicotinoids. Curr. Biol. 2018, 28, R772–R773. [Google Scholar] [CrossRef] [PubMed]
- Möhring, N.; Ingold, K.; Kudsk, P.; Martin-Laurent, F.; Niggli, U.; Siegrist, M.; Studer, B.; Walter, A.; Finger, R. Pathways for Advancing Pesticide Policies. Nat. Food 2020, 1, 535–540. [Google Scholar] [CrossRef]
- Demeter, E.; Lazar, M.V. Növényvédő Szerek Értékesítése, 2019 XIX; Évfolyam 1; Kutatóintézet, N.-A.A., Ed.; NAIK Agrárgazdasági Kutatóintézet: Budapest, Hungary, 2020. [Google Scholar]
- Gaugler, R. Entomopathogenic Nematology; CABI: Wallingford, UK, 2002; ISBN 0851995675. [Google Scholar]
- Ehlers, R.U.; Kuhlmann, U.; Toepfer, S. Field Results on the Use of Heterorhabditis Bacteriophora against the Invasive Maize Pest Diabrotica Virgifera Virgifera. IOBC/WPRS Bulletin 2008, 31, 332–335. [Google Scholar]
- Toepfer, S.; Peters, A.; Ehlers, R.U.; Kuhlmann, U. Comparative Assessment of the Efficacy of Entomopathogenic Nematode Species at Reducing Western Corn Rootworm Larvae and Root Damage in Maize. J. Appl. Entomol. 2008, 132, 337–348. [Google Scholar] [CrossRef]
- Kurtz, B.; Hiltpold, I.; Turlings, T.C.J.; Kuhlmann, U.; Toepfer, S. Comparative Susceptibility of Larval Instars and Pupae of the Western Corn Rootworm to Infection by Three Entomopathogenic Nematodes. BioControl 2009, 54, 255–262. [Google Scholar] [CrossRef]
- Rauch, H.; Steinwender, B.M.; Mayerhofer, J.; Sigsgaard, L.; Eilenberg, J.; Enkerli, J.; Zelger, R.; Strasser, H. Field Efficacy of Heterorhabditis Bacteriophora (Nematoda: Heterorhabditidae), Metarhizium Brunneum (Hypocreales: Clavicipitaceae), and Chemical Insecticide Combinations for Diabrotica Virgifera Virgifera Larval Management. Biol. Control 2017, 107, 1–10. [Google Scholar] [CrossRef]
- Pilz, C.; Toepfer, S.; Knuth, P.; Strimitzer, T.; Heimbach, U.; Grabenweger, G. Persistence of the Entomoparasitic Nematode Heterorhabditis Bacteriophora in Maize Fields. J. Appl. Entomol. 2014, 138, 202–212. [Google Scholar] [CrossRef]
- Toth, S.; Szalai, M.; Kiss, J.; Toepfer, S. Missing Temporal Effects of Soil Insecticides and Entomopathogenic Nematodes in Reducing the Maize Pest Diabrotica Virgifera Virgifera. J. Pest Sci. 2020, 93, 767–781. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Fuzy, E.M. Soil Moisture Effects on Infectivity and Persistence of the Entomopathogenic Nematodes Steinernema Scarabaei, S. Glaseri, Heterorhabditis Zealandica, and H. Bacteriophora. Appl. Soil Ecol. 2007, 35, 128–139. [Google Scholar] [CrossRef]
- Kung, S.P.; Gaugler, R.; Kaya, H.K. Soil Type and Entomopathogenic Nematode Persistence. J. Invertebr. Pathol. 1990, 55, 401–406. [Google Scholar] [CrossRef]
- Grant, J.A.; Villani, M.G. Soil Moisture Effects on Entomopathogenic Nematodes. Environ. Entomol. 2003, 32, 80–87. [Google Scholar] [CrossRef]
- Kung, S.P.; Gaugler, R.; Kaya, H.K. Effects of Soil Temperature, Moisture, and Relative Humidity on Entomopathogenic Nematode Persistence. J. Invertebr. Pathol. 1991, 57, 242–249. [Google Scholar] [CrossRef]
- Levente, V.; Rita, A.; Krisztina, N.; Szabolcs, T.; Stefan, T. Can Heterorhabditis Bacteriophora Nematode Still Control Western Corn Rootworm Larvae When Applied With Low Amounts Of Water? Novenyvedelem 2022, 83, 192–200. [Google Scholar]
- Singh, P.; Moore, R.F. Handbook of Insect Rearing; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Toth, S.; Szalai, M.; Toepfer, S. On Understanding and Manipulating the Hatching Patterns of Diabrotica Virgifera Virgifera Eggs to Improve the Design of Experiments. Entomol. Exp. Appl. 2022, 170, 122–133. [Google Scholar] [CrossRef]
- Toepfer, S.; Kuhlmann, U. Constructing Life-Tables for the Invasive Maize Pest Diabrotica Virgifera Virgifera (Col.; Chrysomelidae) in Europe. J. Appl. Entomol. 2006, 130, 193–205. [Google Scholar] [CrossRef]
- Anonymous. Evaluation of Insecticides: Diabrotica v. Virgifera. EPPO, 1999. PP 1/212 (1: 3 pp). Available online: https://www.eppo.int (accessed on 19 October 2022).
- Toepfer, S.; Toth, S.; Szalai, M. Can the botanical azadirachtin replace phased-out soil insecticides in suppressing the soil insect pest Diabrotica virgifera virgifera? CABI Agric. Biosci. 2021, 2, 28. [Google Scholar] [CrossRef]
- Anonymous. Efficacy Evaluation of Plant Protection Products: Harmonized Classification and Coding of the Uses of Plant Protection Products: PP 1/248(1). EPPO Bull. 2007, 37, 25–28. [Google Scholar] [CrossRef]
- Toepfer, S.; Hatala-Zseller, I.; Ehlers, R.U.; Peters, A.; Kuhlmann, U. The Effect of Application Techniques on Field-Scale Efficacy: Can the Use of Entomopathogenic Nematodes Reduce Damage by Western Corn Rootworm Larvae? Agric. For. Entomol. 2010, 12, 389–402. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; James, G.; Witten, D. An Introduction to Statistical Learning, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 102, p. 618. [Google Scholar]
- Sutter, G.R.; Branson, T.F.; Fisher, J.R.; Elliott, N.C. Effect of Insecticides on Survival, Development, Fecundity, and Sex Ratio in Controlled Infestations of Western Corn Rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1991, 84, 1905–1912. [Google Scholar] [CrossRef]
- Oloumi, S.H.; Steffey, K.L.; Gray, M.E. Planting-Time Versus Cultivation-Time Soil Insecticides for Corn Rootworm Control in Illinois, USA; 1991. 1992. Insecticide and Acaricide Tests 1992, 1, 212. [Google Scholar] [CrossRef]
- Furlan, L.; Canzi, S.; Di Bernardo, A.; Edwards, C.R. The Ineffectiveness of Insecticide Seed Coatings and Planting-Time Soil Insecticides as Diabrotica Virgifera Virgifera LeConte Population Suppressors. J. Appl. Entomol. 2006, 130, 485–490. [Google Scholar] [CrossRef]
- Toepfer, S.; Burger, R.; Ehlers, R.U.; Peters, A.; Kuhlmann, U. Controlling Western Corn Rootworm Larvae with Entomopathogenic Nematodes: Effect of Application Techniques on Plant-Scale Efficacy. J. Appl. Entomol. 2010, 134, 467–480. [Google Scholar] [CrossRef]
- Sutter, G.R.; Branson, T.F.; Fisher, J.R.; Elliott, N.C.; Jackson, J.J. Effect of Insecticide Treatments on Root Damage Ratings of Maize in Controlled Infestations of Western Corn Rootworms (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1989, 82, 1792–1798. [Google Scholar] [CrossRef]
- Schwarz, M.; Christie, D.; Andersch, W.; Kemper, K.; Fellmann, K.; Altmann, R. Control of Corn Rootworms (Diabrotica Spp.) and of Secondary Pests of Corn (Zea Mays) Using Seed Treatments of Clothianidin. In The BCPC Conference: Pests and Diseases, Volumes 1 and 2, Proceedings of An International Conference Held at the Brighton Hilton Metropole Hotel, Brighton, UK, 18–21 November 2002; BCPC: UK, Cambridge, 2002; pp. 59–64. [Google Scholar]
- Ferracini, C.; Blandino, M.; Rigamonti, I.E.; Jucker, C.; Busato, E.; Saladini, M.A.; Reyneri, A.; Alma, A. Chemical-Based Strategies to Control the Western Corn Rootworm, Diabrotica Virgifera Virgifera LeConte. Crop Prot. 2021, 139, 105306. [Google Scholar] [CrossRef]
- Tollefson, J.J. Evaluation of Insecticides and Plant-Incorporated Protectants. In File Number 266-04 of the Department of Entomology; Iowa State University: Ames, IA, USA, 2004. [Google Scholar]
- Tollefson, J.J. Evaluation of Insecticides and Plant-Incorporated Protectants. In File Number 272-05 of the Department of Entomology; Iowa State University: Ames, IA, USA, 2005. [Google Scholar]
- Obermeyer, J.; Obermeyer, J.; Krupke, C.; Bledsoe, L. Rootworm Soil Insecticides: Choices, Considerations, and Efficacy Results. In Pest & Crop; Purdue Cooperative Extension Service 25 (7 December); Purdue University: West Lafayette, IN, USA, 2006; Volume 1–3. [Google Scholar]
- Jeschke, P.; Nauen, R. Neonicotinoids-from Zero to Hero in Insecticide Chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Mahapatra, B.; Adak, T.; Patil, N.K.B.; Pandi, G.G.P.; Gowda, G.B.; Yadav, M.K.; Mohapatra, S.D.; Rath, P.C.; Munda, S.; Jena, M. Effect of Abiotic Factors on Degradation of Imidacloprid. Bull. Environ. Contam. Toxicol. 2017, 99, 475–480. [Google Scholar] [CrossRef]
- Gupta, S.; Gajbhiye, V.T.; Gupta, R.K. Soil Dissipation and Leaching Behavior of a Neonicotinoid Insecticide Thiamethoxam. Bull. Environ. Contam. Toxicol. 2008, 80, 431–437. [Google Scholar] [CrossRef]
- Pang, S.; Lin, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Insights Into the Microbial Degradation and Biochemical Mechanisms of Neonicotinoids. Front. Microbiol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Parte, S.G.; Kharat, A.S. Aerobic Degradation of Clothianidin to 2-Chloro-Methyl Thiazole and Methyl 3-(Thiazole-Yl) Methyl Guanidine Produced by Pseudomonas Stutzeri Smk. J. Environ. Public Health 2019, 3, 4807913. [Google Scholar] [CrossRef] [PubMed]
- Farmer, W.J.; Igue, K.; Spencer, W.F. Effect of Bulk Density on the Diffusion and Volatilization of Dieldrin from Soil. J. Environ. Qual. 1973, 2, 107–109. [Google Scholar] [CrossRef]
- Harris, C.R.; Lichtenstein, E.P. Factors Affecting the Volatilization of Insecticidal Residues from Soils. J. Econ. Entomol. 1961, 54, 1038–1045. [Google Scholar] [CrossRef]
- Spencer Spencer, W.F.; Cliath, M.M.; Farmer, W.J. Vapor Density of Soil-Applied Dieldrin as Related to Soil-Water Content, Temperature, and Dieldrin Concentration. Soil Sci. Soc. Am. Proc 1969, 33, 509–511. [Google Scholar] [CrossRef]
- Guenzi, W.D.; Beard, W.E. Volatilization of Lindane and DDT from Soils. Soil Sci. Soc. Am. J. 1970, 34, 443–447. [Google Scholar] [CrossRef]
- Alford, A.; Krupke, C.H. Translocation of the Neonicotinoid Seed Treatment Clothianidin in Maize. PLoS ONE 2017, 12, e0173836. [Google Scholar]
- Ma, B.L.; Meloche, F.; Wei, L. Agronomic Assessment of Bt Trait and Seed or Soil-Applied Insecticides on the Control of Corn Rootworm and Yield. F. Crop. Res. 2009, 111, 189–196. [Google Scholar] [CrossRef]
- Blandino, M.; Rigamonti, I.; Testa, G.; Saladini, M.A.; Agosti, M.; Alma, A.; Ferracini, C.; Jucker, C.; Reyneri, A. Control of Western Corn Rootworm Damage by Application of Soil Insecticides at Different Maize Planting Times. Crop Prot. 2016, 93, 19–27. [Google Scholar] [CrossRef]
- Modic, Š.; Žigon, P.; Kolmanič, A.; Trdan, S.; Razinger, J. Evaluation of the Field Efficacy of Heterorhabditis Bacteriophora Poinar (Rhabditida: Heterorhabditidae) and Synthetic Insecticides for the Control of Western Corn Rootworm Larvae. Insects 2020, 11, 202. [Google Scholar] [CrossRef]
- Harris, C.R.; Svec, H.J. Toxicological Studies on Cutworms. III. Laboratory Investigations on the Toxicity of Insecticides to the Black Cutworm, with Special Reference to the Influence of Soil Type, Soil Moisture, Method of Application, and Formulation on Insecticide Activity. J. Econ. Entomol. 1968, 61, 965–969. [Google Scholar] [CrossRef]
- Khambay, B.P.S. Pyrethroid Insecticides. Pesticide Outlook 2002, 13, 49–54. [Google Scholar] [CrossRef]
- Galadima, M.; Singh, S.; Pawar, A.; Khasnabis, S.; Dhanjal, D.S.; Anil, A.G.; Rai, P.; Ramamurthy, P.C.; Singh, J. Toxicity, Microbial Degradation and Analytical Detection of Pyrethroids: A Review. Environ. Adv. 2021, 5, 100–105. [Google Scholar] [CrossRef]
- Whiting, S.A.; Strain, K.E.; Campbell, L.A.; Young, B.G.; Lydy, M.J. A Multi-Year Field Study to Evaluate the Environmental Fate and Agronomic Effects of Insecticide Mixtures. Sci. Total Environ. 2014, 497–498, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Sutter, G.R. Comparative Toxicity of Insecticides for Corn Rootworm (Coleoptera: Chrysomelidae) Larvae in a Soil Bioassay. J. Econ. Entomol. 1982, 75, 489–491. [Google Scholar] [CrossRef]
- Bhatt, P.; Huang, Y.; Zhan, H.; Chen, S. Insight into Microbial Applications for the Biodegradation of Pyrethroid Insecticides. Front. Microbiol. 2019, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Koppenhöfer, A.M.; Fuzy, E.M. Effect of Soil Type on Infectivity and Persistence of the Entomopathogenic Nematodes Steinernema Scarabaei, Steinernema Glaseri, Heterorhabditis Zealandica, and Heterorhabditis Bacteriophora. J. Invertebr. Pathol. 2006, 92, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Toepfer, S.; Kurtz, B.; Kuhlmann, U. Influence of Soil on the Efficacy of Entomopathogenic Nematodes in Reducing Diabrotica Virgifera Virgifera in Maize. J. Pest Sci. 2010, 83, 257–264. [Google Scholar] [CrossRef]
- Campos-Herrera, R.; Pathak, E.; El-Borai, F.E.; Stuart, R.J.; Gutiérrez, C.; Rodríguez-Martín, J.A.; Graham, J.H.; Duncan, L.W. Geospatial Patterns of Soil Properties and the Biological Control Potential of Entomopathogenic Nematodes in Florida Citrus Groves. Soil Biol. Biochem. 2013, 66, 163–174. [Google Scholar] [CrossRef]
- Thurston, G.S.; Ni, Y.; Kaya, H.K. Influence of Salinity on Survival and Infectivity of Entomopathogenic Nematodes. J. Nematol. 1994, 26, 345–351. [Google Scholar]
- Kung, S.-P.; Gaugler, R.; Kaya, H.K. Influence of Soil PH and Oxygen on Persistence of Steinernema Spp. J. Nematol. 1990, 22, 440–445. [Google Scholar]
- El-Borai, F.E.; Campos-Herrera, R.; Stuart, R.J.; Duncan, L.W. Substrate Modulation, Group Effects and the Behavioral Responses of Entomopathogenic Nematodes to Nematophagous Fungi. J. Invertebr. Pathol. 2011, 106, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ekmen, Z.I.; Hazir, S.; Cakmak, I.; Ozer, N.; Karagoz, M.; Kaya, H.K. Potential Negative Effects on Biological Control by Sancassania Polyphyllae (Acari: Acaridae) on an Entomopathogenic Nematode Species. Biol. Control 2010, 54, 166–171. [Google Scholar] [CrossRef]
- Tarasco, E.; Santiago Alvarez, C.; Triggiani, O.; Quesada Moraga, E. Laboratory Studies on the Competition for Insect Haemocoel between Beauveria Bassiana and Steinernema Ichnusae Recovered in the Same Ecological Niche. Biocontrol Sci. Technol. 2011, 21, 693–704. [Google Scholar] [CrossRef]
- Ulug, D.; Hazir, S.; Kaya, H.K.; Lewis, E. Natural Enemies of Natural Enemies: The Potential Top-down Impact of Predators on Entomopathogenic Nematode Populations. Ecol. Entomol. 2014, 39, 462–469. [Google Scholar] [CrossRef]
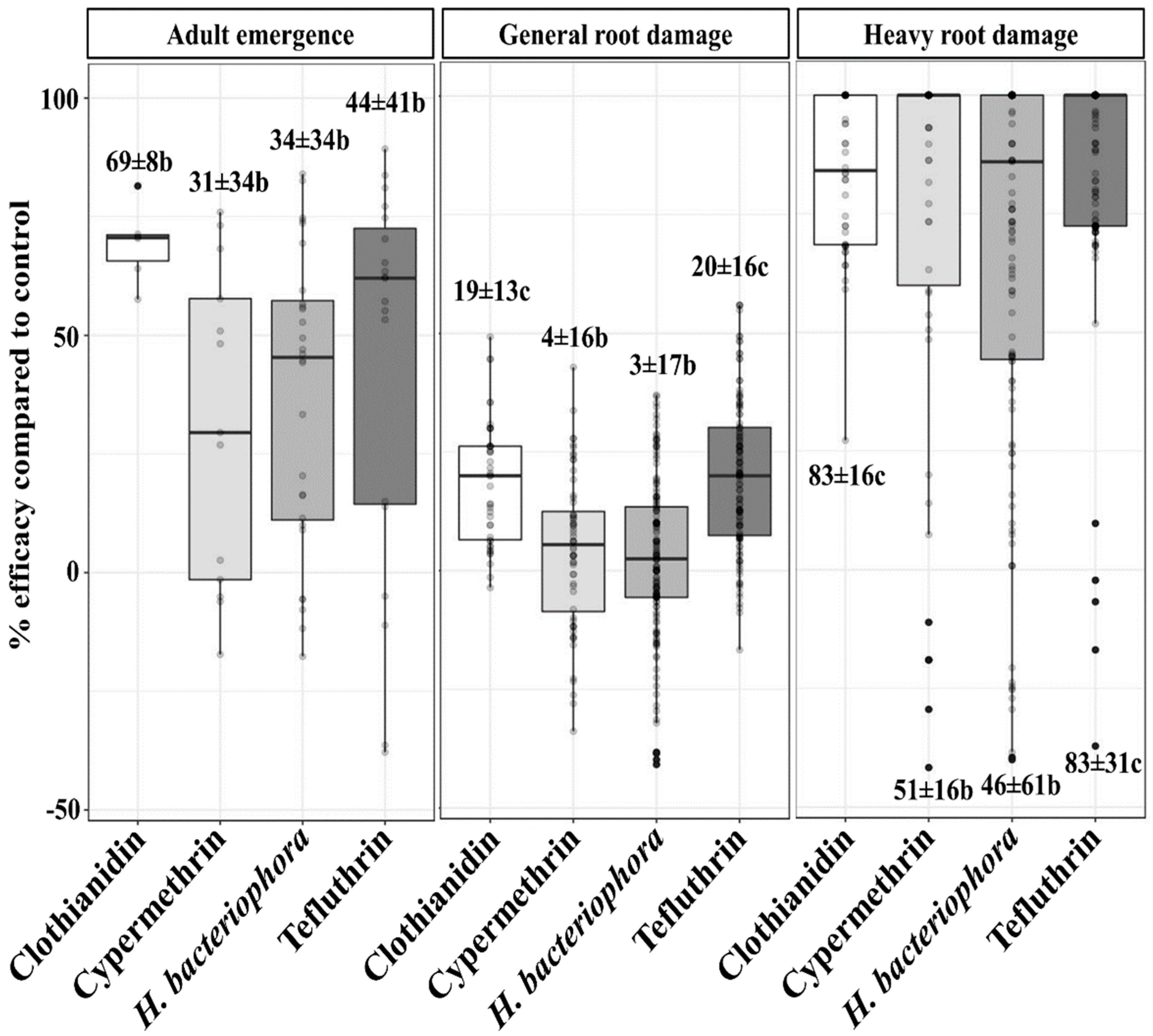
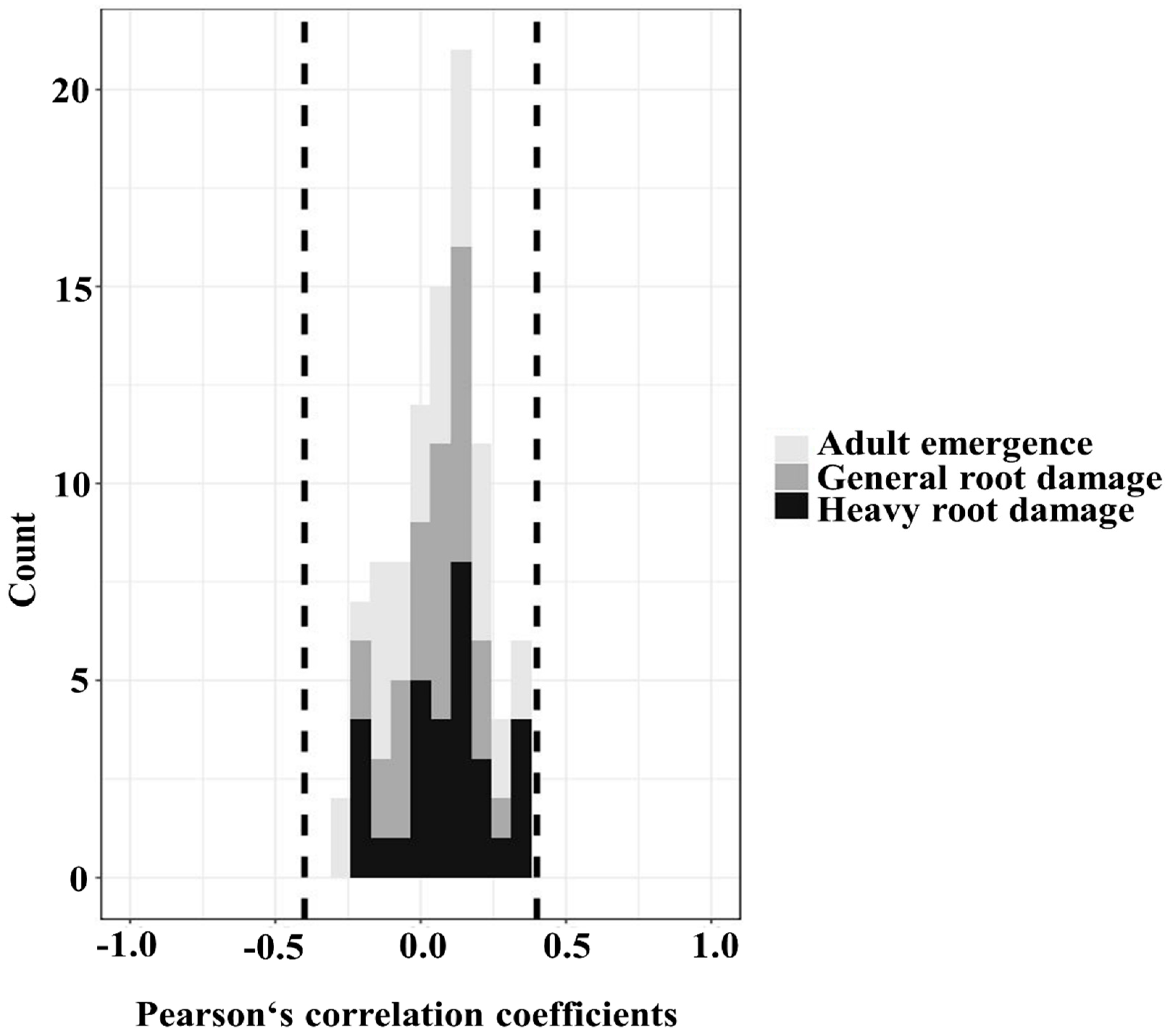
| Factor | Unit | Mean | Standard Deviation | Minimum | Maximum | Range | Shapiro–Wilk Normality Test | Sample Size | Levels of a Factor | |
|---|---|---|---|---|---|---|---|---|---|---|
| W | p | n | Unique Values | |||||||
| Biotic factors | ||||||||||
| Eggs per plant | 406 | 221 | 200 | 1100 | 900 | 0.7 | <0.001 | 482 | 6 | |
| Billion nematodes per ha injected | 1.7 | 0.2 | 1.5 | 1.9 | 0.4 | 0.7 | <0.001 | 282 | 3 | |
| Water per ha injected with nematodes | Liters | 328.7 | 158.4 | 133 | 558 | 425 | 0.6 | <0.001 | 499 | 8 |
| Nematode mortality before application | % | 15 | 8 | 2.9 | 25 | 22.1 | 0.9 | <0.001 | 107 | 9 |
| Nematode mortality after application | % | 23 | 15 | 4 | 57 | 53 | 0.8 | <0.001 | 91 | 14 |
| Nematode virulence before application (1-week bioassay) | % | 41 | 27 | 8 | 90 | 82 | 0.9 | <0.001 | 136 | 14 |
| Nematode virulence after application (1-week bioassay) | % | 38 | 18 | 10 | 76 | 66 | 0.9 | <0.001 | 105 | 9 |
| Maize sowing date | Julian days | 112 | 3 | 108 | 122 | 14 | 0.8 | <0.001 | 530 | 9 |
| Egg infection date | Julian days | 125 | 3 | 122 | 134 | 12 | 0.8 | <0.001 | 451 | 8 |
| Maize density | Plants per ha | 78,000 | 53,760 | 72,000 | 87,000 | 15,000 | 0.8 | <0.001 | 530 | 5 |
| Elevation | m | 90 | 15 | 80 | 150 | 70 | 0.6 | <0.001 | 530 | 11 |
| Natural mortality of adult D. v. virgifera | % | 98.6 | 1.1 | 96.2 | 99.8 | 3.6 | 0.8 | <0.001 | 83 | 25 |
| Abiotic factors | ||||||||||
| Clay content | % m/m | 34 | 7 | 22 | 54 | 32 | 0.9 | <0.001 | 509 | 16 |
| Loam content | % m/m | 29 | 6 | 9 | 39 | 30 | 0.8 | <0.001 | 509 | 16 |
| Sand content | % m/m | 37 | 8 | 24 | 51 | 27 | 0.8 | <0.001 | 509 | 16 |
| Soil bulk density | g/cm3 | 1 | 0.1 | 0.9 | 1.34 | 0.45 | 0.9 | <0.001 | 522 | 11 |
| CaCO3 | % m/m | 5 | 3 | 1 | 12 | 11 | 0.9 | <0.001 | 509 | 16 |
| Soil pH | 7.9 | 0.2 | 7.4 | 8.2 | 0.8 | 0.8 | <0.001 | 509 | 13 | |
| Humus content | % m/m | 2.7 | 0.7 | 1.63 | 3.9 | 2.27 | 0.9 | <0.001 | 509 | 17 |
| Soil moisture in April | w% = grav.% | 16 | 3 | 11.1 | 21 | 9.9 | 0.9 | <0.001 | 476 | 17 |
| Soil moisture in May | w% = grav.% | 21.3 | 7 | 9.9 | 32 | 22.1 | 0.9 | <0.001 | 522 | 19 |
| Soil moisture in June | w% = grav.% | 16.2 | 6.8 | 8 | 29.5 | 21.5 | 0.8 | <0.001 | 420 | 16 |
| Soil moisture in July | w% = grav.% | 12.1 | 4.1 | 7 | 22.9 | 15.9 | 0.9 | <0.001 | 522 | 18 |
| Air temperature in April | °C | 13.3 | 1.5 | 11 | 16.8 | 5.8 | 0.9 | <0.001 | 522 | 9 |
| Air temperature in May | °C | 16.8 | 1.7 | 13.8 | 20.2 | 6.4 | 0.9 | <0.001 | 522 | 9 |
| Air temperature in June | °C | 21.3 | 0.7 | 20 | 23 | 3 | 0.9 | <0.001 | 522 | 9 |
| Air temperature in July | °C | 23.3 | 1.1 | 21.6 | 25 | 3.4 | 0.9 | <0.001 | 490 | 10 |
| Cumulative rainfall in April | mm | 17.6 | 17.6 | 1.4 | 56 | 54.6 | 0.8 | <0.001 | 522 | 11 |
| Cumulative rainfall in May | mm | 66 | 31.4 | 20.3 | 134 | 113.7 | 0.9 | <0.001 | 522 | 11 |
| Cumulative rainfall in June | mm | 36.4 | 30 | 3.3 | 93 | 89.7 | 0.8 | <0.001 | 522 | 9 |
| Cumulative rainfall in July | mm | 45.5 | 32.8 | 14 | 127 | 113 | 0.8 | <0.001 | 490 | 10 |
| Rain around sowing and treatment (±1 day) | mm | 1 | 0.9 | 0 | 1.9 | 1.9 | 0.7 | <0.001 | 393 | 6 |
| Pearson Correlation | Ordinary Least Squares Regression Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Factors | r | p | Model No. | Adjusted R2 | Df | p | β Coefficient | p | 95% CI |
| Pest infestation (Adults that emerged per plant) | ||||||||||
| Clothianidin | CaCO3 soil content | −0.82 | 0.04 | 1 | 0.63 | 3 | 0.1 | −0.8 | 0.5 | −4.5, 2.8 |
| Humus content | −0.85 | 0.02 | −8.8 | 0.3 | −33.2, 15.5 | |||||
| Tefluthrin | Soil moisture in June | −0.82 | <0.001 | 2 | 0.77 | 9 | <0.001 | −2.3 | 0.047 | −4.6, 0.04 |
| Air temperature in June | 0.46 | 0.04 | 15.2 | 0.13 | −5.2, 35.7 | |||||
| Rainfall in July | 0.52 | 0.02 | 0.4 | 0.02 | 0.1, 0.8 | |||||
| Cypermethrin | No factor found | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| H. bacteriophora | No factor found | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| General root damage (1.0 to 6.0 modified Iowa root damage scale) | ||||||||||
| Clothianidin | Soil bulk density | −0.51 | <0.001 | 3 | 0.28 | 40 | <0.001 | −47.2 | <0.001 | −70.2, −24.3 |
| Tefluthrin | No factor found | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Cypermethrin | Clay content | 0.51 | <0.001 | 4 | 0.39 | 35 | <0.001 | 1.2 | 0.2 | −0.8, 3.3 |
| Soil pH | −0.51 | <0.001 | −15.6 | 0.2 | −39, 8 | |||||
| Air temperature in July | 0.54 | <0.001 | 4.7 | 0.4 | −5.9, 15.3 | |||||
| Pest eggs per plant | −0.43 | 0.001 | 0.02 | 0.5 | −0.03, 0.06 | |||||
| H. bacteriophora | No factor found | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Heavy root damage (0.00 to 3.00 Oleson node-injury scale) | ||||||||||
| Clothianidin | Maize sowing date | 0.71 | <0.001 | 5 | 0.55 | 39 | <0.001 | 1.9 | <0.001 | 1.1, 2.8 |
| Soil moisture in July | 0.57 | <0.001 | 1.1 | 0.02 | 0.2, 2.2 | |||||
| Tefluthrin | Sand content | −0.4 | <0.001 | 6 | 0.15 | 109 | <0.001 | −1.4 | <0.001 | −2, −0.8 |
| Cypermethrin | Clay content | 0.41 | 0.001 | 7 | 0.36 | 44 | <0.001 | 4 | 0.02 | 0.6, 7.5 |
| Soil pH | −0.5 | <0.001 | −66.7 | 0.2 | −173, 40 | |||||
| Air temperature in June | 0.47 | <0.001 | 14.7 | 0.6 | −41, 70.6 | |||||
| H. bacteriophora | No factor found | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toth, S.; Toepfer, S.; Szalai, M.; Kiss, J. Limited Influence of Abiotic and Biotic Factors on the Efficacy of Soil Insecticides and Entomopathogenic Nematodes when Managing the Maize Pest Diabrotica v. virgifera (Coleoptera: Chrysomelidae). Agronomy 2022, 12, 2697. https://doi.org/10.3390/agronomy12112697
Toth S, Toepfer S, Szalai M, Kiss J. Limited Influence of Abiotic and Biotic Factors on the Efficacy of Soil Insecticides and Entomopathogenic Nematodes when Managing the Maize Pest Diabrotica v. virgifera (Coleoptera: Chrysomelidae). Agronomy. 2022; 12(11):2697. https://doi.org/10.3390/agronomy12112697
Chicago/Turabian StyleToth, Szabolcs, Stefan Toepfer, Mark Szalai, and Jozsef Kiss. 2022. "Limited Influence of Abiotic and Biotic Factors on the Efficacy of Soil Insecticides and Entomopathogenic Nematodes when Managing the Maize Pest Diabrotica v. virgifera (Coleoptera: Chrysomelidae)" Agronomy 12, no. 11: 2697. https://doi.org/10.3390/agronomy12112697
APA StyleToth, S., Toepfer, S., Szalai, M., & Kiss, J. (2022). Limited Influence of Abiotic and Biotic Factors on the Efficacy of Soil Insecticides and Entomopathogenic Nematodes when Managing the Maize Pest Diabrotica v. virgifera (Coleoptera: Chrysomelidae). Agronomy, 12(11), 2697. https://doi.org/10.3390/agronomy12112697





