Abstract
Sulfur is a necessary nutrient for the proper functioning of living organisms, both plants and animals. However, following pro-environmental activities carried out for many years, there is now a deficit of this element in the soils of many countries. The aim of this model study was to determine the effect of the application of waste elemental sulfur and its mixtures with organic materials (cattle manure, digestate and biochar) on the availability of manganese and zinc in soils. In addition to the standard analysis of variance (ANOVA), the authors propose various methods of advanced statistical analysis, e.g., simplified linear or polynomial regression model development, multiple regression analysis, heatmap statistics and principal component analysis (PCA). The presented findings indicate significant relationships between the soil pH value, S-SO4, Zn and Mn contents, dehydrogenase activity, and experimental duration. The results of regression analysis revealed that the applied materials had a more significant influence on the zinc content than on the manganese content during the incubation period.
1. Introduction
Sustainable development is a multidimensional idea. In addition to aspects of the natural, economic and social synergy of humans with the surrounding world, it also involves institutional, spatial, moral and spiritual aspects related to the quality of life, which are not necessarily treated as material goods. This concept is widely desirable because its main assumption is progress, which enables proper, rational planning and spatial development [1]. Many programs and initiatives are emerging in response to environmental and social challenges, such as climate change, decreased biodiversity, the depletion of natural resources, environmental pollution, increasing inequalities and geopolitical instability. The literature provides a rich overview of issues [2,3,4] that are at the center of current debates on moving toward sustainable development: the bioeconomy, the 2030 Agenda, and the strong paradigm of sustainable development.
The bioeconomy is a concept that promotes new ways of using biological resources. The increasing use of biological resources in society generates a wide range of solutions supporting the concept of sustainable development. The bioeconomy is seen as an essential path to a society without fossil fuels [5,6,7], which will also bring about regional development, new business and employment opportunities, health benefits and cleaner production processes [8,9,10]. In parallel, the 2030 Agenda and Sustainable Development Goals (SDGs), launched by the United Nations in 2015, constitute an action plan to implement the transformation towards sustainable development around the world [11]. In turn, the evolution towards a more circular economy is expected to contribute to the achievement of several SDGs, primarily SDG 12 (sustainable production and consumption patterns) [8] but also SDG 6 (open access to water and sanitation through the sustainable management of water resources), SDG 7 (ensuring access to stable, sustainable and modern energy sources at affordable prices), SDG 8 (promoting stable, sustainable and inclusive economic growth, full-time and productive employment and decent work for all people) and SDG 13 (urgent action to combat climate change and its impacts) in the areas of water, energy, economic growth and climate change, respectively [12,13].
The 2030 EU Biodiversity Strategy calls for a variety of actions that will not only protect different species of living organisms and their habitats but also contribute to carbon sequestration and help meet climate targets [14]. Agriculture is one of the main drivers of climate change, biodiversity loss and land degradation. Agricultural production is closely related to the use of soil resources. In the context of food production, it requires maintaining a high-cultivation culture in soil resources. In the face of the negative nature of the changes taking place in the environment, the principles of soil management must be verified, as it is a non-renewable resource and the main component of natural habitats [14,15,16,17]. Sustainable agricultural practices [18] are the key to maintaining and increasing biodiversity.
The fertilization of arable crops is one of the many important issues of sustainable agriculture. The purpose of agrotechnical measures is to preserve the natural functions of soil and increase its fertility to meet the nutritional needs of plants while reducing threats to the natural environment and humans. Taking the above into account, this objective could require using various types of waste materials to help replenish nutrient resources in the soil [19,20,21,22,23] as an alternative to conventional fertilizers. The subject of the research described in this paper is sulfur waste, formed as a byproduct of the biogas desulfurization process using technology involving solutions of iron (III) complexes with organic ligands. This material is a potentially rich source of sulfur for plants. This element occurs in its elemental form (S0) in the studied material. The use of sulfur waste in the plant production process is consistent with the concept of a circular economy. It allows the reintegration of sulfur into the trophic chain and reduces the consumption of non-renewable resources necessary for the production of traditional mineral fertilizers.
Sulfur belongs to the group of nutrients necessary for the proper functioning of living organisms, both plants and animals. The importance of this element in agricultural production is unquestionable, and the functions it performs in the plant have been widely discussed in the relevant literature [24,25,26]. Sulfur plays a special role in the structure and metabolism of living matter. It regulates the processes of protein biosynthesis and photosynthesis, affecting the nutritional and processing value of crop plants. Sulfur has a protective effect on plants growing under stressful conditions.
Until the industrial revolution, sulfur existed in the atmosphere in negligible quantities, and its primary source in the soil was the deeper layers of the Earth’s crust. Intensive industrial development in the last century led to environmental pollution with sulfur compounds. Excess sulfur poses a threat to the life and health of humans, animals and plants. Environmental measures implemented over many years have resulted in a significant reduction in the emissions of sulfur compounds. Currently, there is a deficit of this element in soils, both in Poland and in many other countries in the world. This is the result of the reduction in sulfur emissions to the atmosphere, the decreased use of natural and organic fertilizers, changes in the structure of the use of mineral fertilizers and the intensification of crops with an increased need for sulfur. Therefore, sulfur resources must be replenished through fertilization.
Before the industrial revolution, this element was present in the atmosphere in small amounts, and its original source in the soil was the deeper layers of the Earth’s crust. The intensive development of industry in the 20th century caused environmental pollution with sulfur compounds. However, following environmental activities carried out for many years, there is now a deficit of this element in the soils of many countries in the world (including Poland) [24,25,27,28].
The mismanagement of fertilization can result in a crop yield reduction, as well as the loss of nutrients (runoff, leaching and volatilization). Considering environmental and economic aspects, this is objectionable, as the proper development of a plant is conditioned by an adequate supply of the necessary macro- and microelements. These elements perform specific functions, and by interacting with each other, they regulate the course of processes taking place in plant cells. Sulfur is an element in sulfur amino acids (cysteine and methionine), which are structural compounds involved in shaping the structures of proteins and enzymes and binding metals to catalyze enzymatic reactions. This element increases the resistance of plants to pest and pathogen attacks. Moreover, sulfur defines the taste, smell and therapeutic properties of plants [29,30,31,32,33,34,35,36]. There is a close relationship between the nutritional conditions of plants and sulfur and nitrogen. Plants adequately supplied with sulfur absorb more nitrogen, so fertilization treatment is more effective, and nitrogen losses are smaller. On the other hand, an insufficient sulfur supply reduces the efficiency of nitrogen utilization, photosynthesis and the production of carbohydrates, fats and proteins and consequently reduces the size and quality of the crop yield [37,38]. Despite the fact that manganese and zinc are needed in only small quantities by plants, these elements are ultimately as critical to growth and development as the other nutrients. This is a result of the specific functions they play in the physiology of plants. Specifically, manganese participates in photosynthesis, respiration, pathogen defense and hormone signaling. Zinc is a component of a wide range of macromolecules and thus participates in growth regulation, protein and carbohydrate synthesis and transformation, gene expression regulation and also the defense against disease. Due to this, a shortage of the mentioned nutrients results in physiological diseases in plants [39,40].
The purpose of this model study was to determine the effect of the application of waste elemental sulfur and its mixtures with organic materials (cattle manure, digestate and biochar) on the availability of manganese and zinc in soils. These elements were selected due to the significant roles they play in plant nutrition. Furthermore, the relationships between the selected soil parameters (soil pH, dehydrogenase activity, and sulfur, manganese and zinc availability) after the introduced treatments were also investigated. Different statistical methods of data analysis can help us advance the interpretation of the obtained results, giving a more complete view of the changes caused by the experimental treatments. The authors employed various methods of advanced statistical analysis in addition to the standard analysis of variance (ANOVA), such as simplified linear or polynomial regression model development, multiple regression analysis, heatmap statistics and principal component analysis (PCA). This allows for the precise exploration of the relationships between variables, which is sometimes difficult to discern when performing only basic calculations and presenting them in a visually legible manner.
2. Materials and Methods
2.1. Properties of the Soil Material
The experiment was set up on two soils: the first was classified into the agronomic category of very light soils (sand), and the second was in the category of heavy soils (silt loam) (Table 1) [41]. The soil material was collected in southern Poland (very light soil: 50°6′48.3″ N 22°40′26.9″ E; heavy soil: 49°57′27.4″ N 19°9′2.7″ E) from a layer of 0–20 cm in the crop field. A representative sample was taken in accordance with the standard PN-R-04031:1997 [42]. The preparation of the soil material for the incubation study included bringing it to an air-dried condition and sifting. Before the experiment, very light and heavy soils were characterized by acidic and very acidic reactions (respectively), low sulfate content and free of contamination with heavy metals.

Table 1.
Selected properties of soil material before establishing the experiment.
Due to the low value of the soil material reaction, a liming treatment was applied one month before starting the incubation. To very light and heavy soils, calcium carbonate (CaCO3) and calcium oxide (CaO; POCH) were applied, respectively. The doses of the introduced liming materials corresponded to 0.75 hydrolytic acidity (in the conversion to CaO, 313 and 873 mg of CaO kg−1 dm of soil were introduced to very light soil and heavy soil, respectively). The soil material was kept under constant humidity (60% of the maximum water capacity) and temperature (25 ± 2 °C) conditions. After the treatment, the pHKCl values of very light and heavy soils indicated slightly acidic (5.78) and acidic reactions (4.95), respectively.
2.2. Design of Incubation Experiment and Used Materials
The incubation experimental design comprised nine treatments for each soil. The study included two sulfur doses for each soil—20 (SI) and 40 mg S kg−1 d.m. of soil (SII) and 30 (SI) and 60 mg S kg−1 d.m. of soil (SII) for very light and heavy soils, respectively. These doses were established after considering sulfate sulfur resources in the soil materials and Polish guidelines describing the estimation of sulfur content in soil environments [43]. Soil with no additions was used as the control object (C). In addition to waste sulfur, manure (M), digestate (D) and biochar (B) were introduced to the soil. The doses of organic materials were calculated so as to introduce the same amount of total carbon with each material. The doses of introduced total carbon amounted to 1000 kg C ha−1 for very light soil and 1500 kg C ha−1 for heavy soil, which corresponded to approximately 100 and 150 kg of introduced nitrogen, respectively. Before soil application, every material was dried and ground. Each treatment was replicated three times (Table 2):

Table 2.
Treatments conducted as part of the research.
In the presented study, the waste constituted a source of sulfur, while manure, digestate and biochar were sources of organic matter. The selected parameters of introduced materials are presented in Table 3. The waste and digestate came from a sewage treatment plant located in central Poland as a byproduct of biogas purification obtained by sewage sludge methane fermentation using technology involving solutions of iron (III) complexes with organic ligands. Manure and biochar were commercially available materials.

Table 3.
Selected properties of materials used in the incubation experiment.
The soil material was incubated in plastic containers. Each container consisted of 280 g of very light soil or 200 g d.m. of heavy soil with additions, according to the experimental design. The soil material was incubated at 25 ± 2 °C, and the soil moisture content was maintained at 60% of the maximum water capacity. The water lost during incubation was periodically restored with deionized water. Soil materials for laboratory analyses were sampled on the day that materials were introduced and 15, 30, 60 and 120 days after their application. Subsequently, they were dried and sieved (1 mm mesh), excluding samples that were intended for analyses to determine the dehydrogenase activity. This determination was made using fresh and sieved samples (2 mm mesh), with the moisture determined by the weight method.
2.3. Methods of Laboratory Analyses
2.3.1. Soil pHKCl
Soil pHKCl was determined potentiometrically in a 1 mol L−1 potassium chloride (KCl) suspension (Chempur, Piekary Śląskie, Poland) (1:2.5 m/v). For measurements, the CPC-502 (Elmetron, Zabrze, Poland) multifunctional device was used. To compute the mean value of pH, the pH values of replicates were converted into hydrogen ion [H+] concentrations. The next step included the calculation of the arithmetic mean and conversion into pH according to the equation: pH = −log[H+].
2.3.2. Available Elements
The available sulfate sulfur (S-SO4), manganese (Mn) and zinc (Zn) were extracted from the soil samples using a 0.03 mol L−1 acetic acid (CH₃COOH) solution (30 min, 40 rpm, m/v 1:10). The element content in the obtained extracts was measured using an Optima 7300 DV inductively coupled plasma optical emission spectrophotometer (ICP-OES method). The emission values of sulfate sulfur, manganese and zinc were determined at 181.975, 257.610 and 206.200 nm in axial mode, respectively. The quantification limits of the method used for sulfate sulfur, manganese and zinc were 0.031, 0.0014 and 0.0059 mg L−1, respectively.
2.3.3. Dehydrogenase Activity
The activity of dehydrogenase (D) was determined via the transformation of colorless, water-soluble 2,3,5-triphenyltetrazolium chloride (TTC) to red water-insoluble 1,3,5-triphenylformazan (TPF) [39]. A sample of soil material (5 g) was placed in a test tube, mixed with 5 mL of 1% TTC and incubated in the dark for 96 h at 30 °C. TTC was prepared in a tris(hydroxymethyl) aminomethane hydrochloride (TRISHCL) buffer, pH 7.4. After incubating the prepared samples, TPF was extracted with 20 mL of methyl alcohol (CH3OH) and then quantified by the colorimetric method at a wavelength of 485 nm on a UV/VIS DU 640 spectrophotometer.
2.3.4. Properties of the Materials Prior to Setting up the Incubation Experiment
To determine the properties of the materials used in the experimental scheme (soils, waste sulfur, manure, digestate and biochar), additional laboratory analyses were carried out before performing the incubation. The granulometric composition of soils was determined by Bouyoucos-Casagrande’s areometric method with Proszynski’s modification [43,44,45]. This method was used because it is adjusted to the procedure used in Poland for the evaluation of sulfur content in soils. The maximum water capacity of the soils was determined by measuring the difference in soil mass before and after moisture conditioning by capillary action. Hydrolytic acidity was determined by the Kappen method after extraction with a 1 mol L−1 sodium acetate solution (CH3COONa) (1 h, 40 rpm, 2:5 m/v). The total concentrations of carbon and nitrogen in all materials (soils, waste sulfur, manure, digestate and biochar) were determined using a Vario MAX cube CNS analyzer. The determination of the total sulfur concentration in soil material included treating samples with 2 mol L−1 magnesium nitrate (Mg(NO3)2) and dry mineralization (12 h, 450 °C). The remains were then dissolved in 31% nitric acid (HNO3). The total sulfur concentration in organic materials (manure, digestate and biochar) was determined after the sample was treated with concentrated HNO3, followed by sulfur binding to Mg(NO3)2, dry mineralization (2 h, 300 °C after that 3 h, 450 °C) and then the dissolution of the residue in a HNO3 solution. The total concentrations of trace elements (Fe, Ni, Pb, Zn, Cu, Cd, Mn and Cr) were determined after samples were incinerated (8 h, 450 °C) and treated with a mixture of concentrated acids (HNO3 and perchloric acid (HClO4), followed by dissolving the residue in hydrochloric acid (HCl). The total concentrations of sulfur and trace elements in the waste sulfur were determined after their digestion in a mixture of concentrated acids: HCl and HNO3 (3:1 v/v) (PN-EN 16964: 2018-03). The concentrations of the extracted elements in the solutions were determined by the ICP-OES method. The total concentration of mercury in the materials (excluding waste sulfur) was measured using an atomic absorption spectrophotometer (AMA-254). The dry matter of the waste sulfur and organic materials (manure, digestate and biochar) was determined by the weight method. The organic matter content in organic materials was assessed by determining the amount of ignition loss (4 h, 500 °C).
2.4. Statistical Analysis
2.4.1. Analysis of Variance (ANOVA)
The results were statistically evaluated using Statistica 13.3. The compliance of the distribution of the determined features with the normal distribution was verified with the Kolmogorov–Smirnov test. The homogeneity of variance was assessed using the Brown–Forsythe test. Differences between treatments, performed separately for the two soil types, were analyzed using ANOVA, followed by Duncan’s post hoc test. Homogeneous groups were determined at a significance level of p ≤ 0.05.
2.4.2. Linear and Polynomial Regression Analysis
Linear regression (General Regression Models module, Statistica 13.3) analysis was performed to show changes in the availability of Mn and Zn in very light and heavy soils over time (from 0 to 120 incubation days). Regression equations were developed separately for the different experimental treatments and types of soil. Analyses were carried out by checking significance at p ≤ 0.05 and determining the coefficient of determination (R2) and the adjusted coefficient of determination (R2adj.). Only significant linear models are shown in the aggregated figures. Additionally, observed versus predicted plots of linear regression models are presented for Mn and Zn content changes over time in the very light soil with SI + B treatment, and the selection criterion was the highest coefficient of determination in both cases. Polynomial regression analysis for the chosen experimental treatments was also performed.
2.4.3. Multiple Regression Analysis
Multiple regression (General Regression Models module, Statistica 13.3) analysis was used to predict the best relationship between dependent variables (Mn and Zn availability in soil) and independent variables (pH, sulfate sulfur content and dehydrogenase activity). The authors performed stepwise regression with backward elimination, which is an automatic computational procedure that attempts to find the best multiple regression model using only statistically significant predictors from a larger set of potential predictive variables. In this procedure, at each step, variables are gradually eliminated from the regression model to find a reduced model that best explains the data. The authors used this method separately for the data obtained for very light and heavy soils over a period from 0 to 120 incubation days. The models were evaluated by the coefficient of determination R2, adjusted coefficient of determination R2adj., standard error of estimation Se and p-value. Observed and predicted values were plotted to visualize the precision of the models. In order to identify the key parameters, Pareto plots were also used.
2.4.4. Principal Component Analysis (PCA)
The data, which were analyzed separately for very light and heavy soils, were subjected to principal component analysis (PCA) with the use of the Statistica 13.3 Mult/Exploratory module. Data were standardized before statistical analysis, and PCA was performed on a correlation matrix. The eigenvalues of the principal components (PCs) were used as the criteria to determine how many PCs should be used. PC1 and PC2, which had eigenvalues of 3.23 and 0.80 (very light soil) and 3.62 and 0.69, respectively, were included in further analyses. The first two principal components together explained 80.58% and 86.22% of the total variance for very light and heavy soils, respectively.
2.4.5. Heatmap Generation
Heatmaps were generated in Statistica 13.3 to detect grouping patterns based on the determined parameters of soil samples, including pH, dehydrogenase activity, and Mn, Zn and S-SO4 contents on different incubation days. Color scales were adopted for each individual case based on dark green (lower values) to dark red (higher values), with corresponding transition colors between these extremes.
3. Results and Discussion
3.1. Availability of Zinc and Manganese in Soils
Soil constitutes a specific environment that constantly undergoes spatial and temporal variations. The processes occurring in this habitat are interrelated, interact with each other and could be a result of the influence of natural and anthropogenic factors.
On the day of the waste sulfur and organic material application, the zinc content in very light soil ranged from 0.388 to 0.808 mg Zn kg−1 d.m., and in heavy soil, it varied from 2.02 to 3.03 mg Zn kg−1 d.m. (Table 4). During incubation, the zinc content in both soils increased. One hundred and twenty days after the materials were introduced, the zinc content in the very light and heavy soils amounted to 1.628 to 2.933 mg Zn kg−1 d.m. and 3.73 to 4.95 mg Zn kg−1 d.m., respectively (Table 3).

Table 4.
Zinc content in very light and heavy soils throughout the incubation experiment (mg Zn kg−1 d.m. ± SD).
After 120 days of incubation, the zinc content in the treatments of very light and heavy soils with the addition of waste sulfur and its mixtures with organic materials was, as a rule, comparable to or significantly higher than that in the control treatment (without additives). Among the treatments with the applied materials in very light soil, the treatment with the addition of waste sulfur at the SI sulfur dose and biochar and the treatment with the addition of waste sulfur at the SII sulfur dose and digestate were characterized by significantly higher zinc content, while treatments with the lowest zinc were waste sulfur at the SI and SII sulfur doses and the mixture of waste sulfur at the SII sulfur dose with manure. Regarding the zinc concentration in heavy soil treatments at the end of the experimental period, treatments with the addition of waste sulfur at the SII sulfur dose and the mixture of waste sulfur at the SI sulfur dose with digestate had the highest zinc content. Statistically significant differences among other treatments with the addition of the tested materials were relatively small and related only to some objects.
At the end of the experiment, a slight effect of the sulfur dose on the zinc concentration of both soils tested (regardless of organic material addition) was observed. Throughout the incubation period, the content of the discussed element in treatments with the addition of waste sulfur at the SII sulfur dose and its mixtures with organic materials was comparable to or significantly higher than the content of this element determined in the treatments with the addition of waste sulfur at the SI sulfur dose and its mixtures with organic materials.
In relation to treatments with the addition of only waste sulfur, a beneficial effect of the introduced organic materials (manure, biochar and digestate) on zinc concentration in both tested soils was observed (especially in the treatments of very light soil).
On the day of the waste sulfur and organic material application, the manganese content in very light soil ranged from 2.99 to 4.86 mg Mn kg−1 d.m., and in heavy soil, it varied from 5.00 to 6.92 mg Mn kg−1 d.m. (Table 4). During incubation, the manganese content in both soils increased. One hundred and twenty days after the materials were introduced, the manganese content in the very light and heavy soils amounted to 4.93 to 6.38 mg Mn kg−1 d.m. and 13.18 to 14.26 mg Mn kg−1 d.m., respectively (Table 5).

Table 5.
Manganese content in very light and heavy soils throughout the incubation experiment (mg Mn kg−1 d.m. ± SD).
After 120 days of incubation, the manganese content in the treatments of very light and heavy soils with the addition of waste sulfur and its mixtures with organic materials was comparable to or significantly higher than that in the control treatment (without additions). Among the treatments with the applied materials in very light soil, the treatment with the addition of waste sulfur at the SI sulfur dose and biochar and the treatment with the addition of waste sulfur at the SII sulfur dose and digestate were characterized by the highest manganese content. Statistically significant differences among other treatments with the addition of the tested materials were relatively minor and related only to some objects. Regarding the manganese concentration in heavy soil treatments after 120 days of the experiment, statistically significant differences among treatments with the addition of the tested materials were relatively small and related only to some objects. However, all determined values of the studied element content were significantly higher than those determined in the control treatment.
At the end of the experiment, a minor effect of the sulfur dose on the manganese concentration of both tested soils (regardless of organic material addition) was observed. Throughout the incubation period, the content of the discussed element in treatments with the addition of waste sulfur at the SII sulfur dose and its mixtures with organic materials was, as a rule, comparable to or significantly higher than the content of this element determined in the treatments with the addition of waste sulfur at the SI sulfur dose and its mixtures with organic materials.
In relation to the treatments with the addition of only waste sulfur, a beneficial effect of the introduced organic materials (manure, biochar and digestate) on the manganese concentration in both tested soils was observed.
The presented findings indicate that the application of waste sulfur and its mixtures with organic materials affected the zinc and manganese contents of both tested soils. After 120 days from the introduction of the tested materials, in comparison to the control treatment, the contents of discussed elements in the very light and heavy soils increased. Similar results have been previously reported; e.g., Soaud et al. [46], after the application of elemental sulfur at a dose of 444 mg kg−1 of soil, found that the concentrations of Mn and Zn increased (in comparison to the control treatment) in all three tested soils during a 128-day incubation experiment. At the end of the incubation period, the zinc concentration after amending the soil with elemental sulfur ranged from 0.21 to 1.93 mg kg−1, while in the control treatment, the determined element content amounted to 0.34 to 1.77 mg kg−1. As a rule, the zinc concentration tended to decrease during the incubation period. After 128 days of the experiment, treatments with elemental sulfur were characterized by manganese contents ranging from 2.89 to 4.47 mg kg−1, while control treatments were characterized by this element content ranging from 0.23 to 1.00 mg kg−1. Cifuentes and Lindemann [47] found that after the introduction of elemental sulfur (at a dose of 5 g S0 kg−1 soil), the available Mn content had increased significantly by the end of the 270-day field experiment, while this treatment had no effect on other nutrients (P, Fe and Zn). The addition of organic matter (fresh manure, composted manure and the remains of bermudagrass) increased the contents of P and Zn. The authors highlighted that the increase in elemental sulfur oxidation resulting from the organic matter added to S0-treated soils significantly increased Mn availability only (and had no effect on P, Fe or Zn) in comparison to treatments with the addition of only S0 or organic matter. The lack of an increase in the availability of P, Fe or Zn after the application of only S0 could be the result of an insufficient sulfur dose and its slow and incomplete oxidation or a high soil buffering capacity, which resists soil acidification. Furthermore, elements could have been immobilized by soil microorganisms, chemically reprecipitated in less mobile forms or not affected at all [47]. Skwierawska et al. [48] found that after the application of S0 at annual doses of 40 kg ha−1, 80 kg ha−1 and 120 kg ha−1, the Zn and Mn concentrations in the soil after a three-year field experiment amounted to 3.69, 3.30 and 3.99 mg Zn kg−1 soil, respectively (2.92 mg Zn kg−1 soil in the control treatment), and 93.19, 90,16 and 91.80 mg Mn kg−1 soil, respectively (85.75 mg Mn kg−1 soil in the control treatment). The depletion of these element resources could have resulted from their enhanced solubility in the soil solution and their utilization by plants. The opposite results were presented by Cui and Wang [49], who found a decrease in the available Zn concentration after soil fertilization with elemental sulfur, while Tabak et al. [50] stated that significant variations in the concentrations of available forms of heavy metals (Fe, Mn, Zn, Cu, Cr, Ni, Pb and Cd) as a result of elemental sulfur application were found only for some of the determined elements and used reagents (0.01 mol L−1 CaCl2, Mehlich 3, 1 mol L−1 HCl). This can be explained by the slight impact of sulfur introduction on soil pH and the low contents of elements (constituting impurities) other than sulfur in the applied waste.
Introduced elemental sulfur undergoes biological transformation into ions (SO42−), constituting an available form of this element [51]. Together with sulfur oxidation in soil, sulfate ions are produced, and hence, soil acidification is induced [52,53]. There are many factors that affect the solubility and bioavailability of nutrients and trace elements; however, the most crucial is the value of soil pH. Any factor affecting the pH value affects other soil features by shaping its reaction. As a rule, the contents of the available forms of macroelements decrease with decreasing pH values. This is especially visible in the case of phosphorus, the availability of which can be reduced by up to 90% in an acidic environment [54]. On the other hand, as the pH value decreases, the solubility of trace elements increases. At lower pH values, the mobility of cations is enhanced, while the activity of anions is reduced. As the pH value increases, the electromagnetic charge of soil colloids (organic matter, iron and aluminum oxides, and 1:1 and 2:1 clay minerals) also increases, as a result of which cation immobilization and anion mobility are higher. Furthermore, together with the increasing soil reaction, metal precipitation into carbonates, chlorides, hydroxides, phosphates and sulfates also increases.
Both manganese and zinc are essential nutrients involved in physiological processes occurring during plant growth and development [47,55,56,57]. Manganese participates in water photolysis in chloroplasts, the regulation of enzyme activities and protection from oxidative membrane damage, while zinc constitutes an element of some enzymes, maintains membrane integrity, and regulates auxin synthesis and pollen production [58] based on various sources. Although the availability of these elements constitutes an important factor in increasing crop yield quality and quantity, their toxic nature could be revealed under acidic soil conditions [55,59,60].
3.2. Regression Models to Predict Changes in Manganese and Zinc Availability over Time
The results of the regression analysis of the Mn availability changes over time in very light soil for particular experimental treatments are presented in Figure 1. However, out of nine treatments, only two regression models turned out to be statistically significant: SI + B (soil with the addition of waste sulfur (sulfur dose: I) and biochar) and SII + D (soil with the addition of waste sulfur (sulfur dose: II) and digestate). The coefficients of determination (R2) for the developed regression equations ranged from 0.9139 (SI + B) to 0.8073 (SII + D), and the adjusted coefficients of determination (R2adj.) reached values from 0.8852 to 0.7430, respectively. The coefficient of determination for other treatments ranged from 0.0974 to 0.7434.
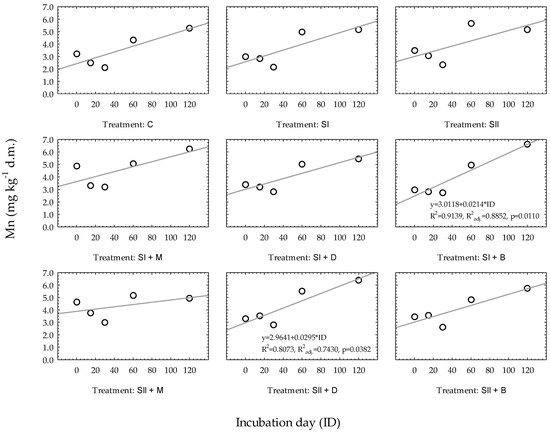
Figure 1.
Linear regression models of Mn changes over time based on experimental treatments in very light soil. Regression equations are presented only when p ≤ 0.05. * constitute a multiplication mark.
For all regression models developed for Zn in very light soil in all experimental treatments, the p values were below 0.05 (Figure 2). The results showed that in the control treatment, the prediction of Zn availability in very light soil was the most precise (R2 = 0.9842, R2adj. = 0.9790). In other treatments, the regression equation developed for the combination of waste sulfur (sulfur dose: I) and biochar (SI + B) could also predict Zn with very high accuracy (R2 = 0.9706, R2adj. = 0.9608). In other cases, R2 ranged from 0.7962 (SII + M) to 0.9611 (SII + D).
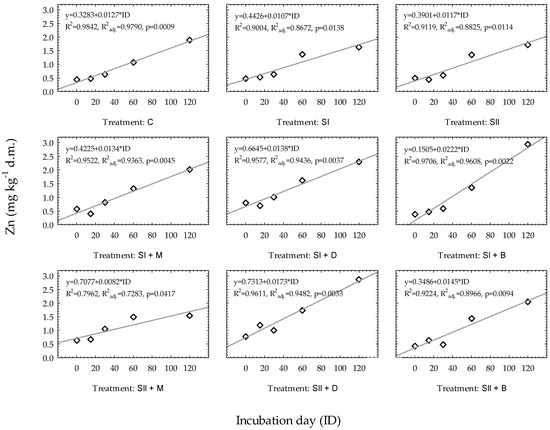
Figure 2.
Linear regression models of Zn changes over time based on experimental treatments in very light soil. Regression equations are presented only when p ≤ 0.05. * constitute a multiplication mark.
As shown in Figure 3, only two simple regression models of Mn changes in heavy soil were statistically significant, and both of them were amended with waste sulfur at dose I and dose II (SI and SII, respectively). The coefficients of determination (R2) for the presented regression equations ranged from 0.8012 (SI) to 0.7804 (SII), and the adjusted coefficients of determination (R2adj.) reached values from 0.7350 to 0.7071, respectively. Other linear models were less accurate: R2 ranged from 0.5934 to 0.7287, and p values were below 0.05.
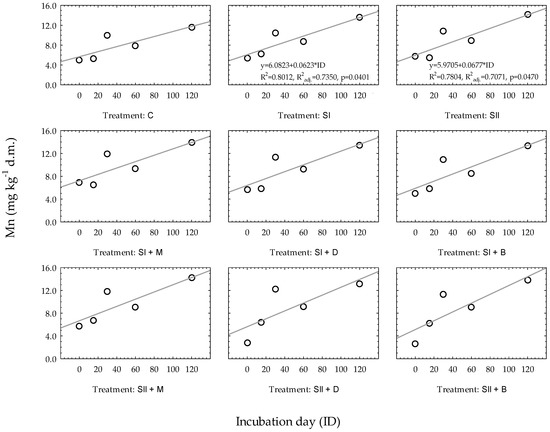
Figure 3.
Linear regression models of Mn changes over time based on experimental treatments in heavy soil. Regression equations are presented only when p ≤ 0.05. * constitute a multiplication mark.
Contrary to the observed statistical significance of the models of Zn in all treatments for very light soil, for heavy soil, only four regression models had p values lower than 0.05 (Figure 4). In heavy soil, Zn changes over time were predicted the most accurately in the following treatments: SII (soil with the addition of waste sulfur (sulfur dose: II); R2 = 0.9020, R2adj. = 0.8693), SI + M (waste sulfur (sulfur dose: I) and manure; R2 = 0.8229, R2adj. = 0.7639), SI + D (waste sulfur (sulfur dose: I) and digestate; R2 = 0.8250, R2adj. = 0.7667) and SI + B (waste sulfur (sulfur dose: I) and biochar; R2 = 0.8235, R2adj. = 0.7647). In other cases, the models were not significant (p > 0.05), and R2 ranged from 0.4681 to 0.8777. An additional statistical analysis allowed the authors to develop significant polynomial regression models for SI (soil with the addition of waste sulfur (sulfur dose: I); R2 = 0.9850, R2adj. = 0.9699; ) and for SII + M (waste sulfur (sulfur dose: II) and manure; R2 = 0.9683, R2adj. = 0.9365; ). Linear models were not statistically significant for these experimental treatments.
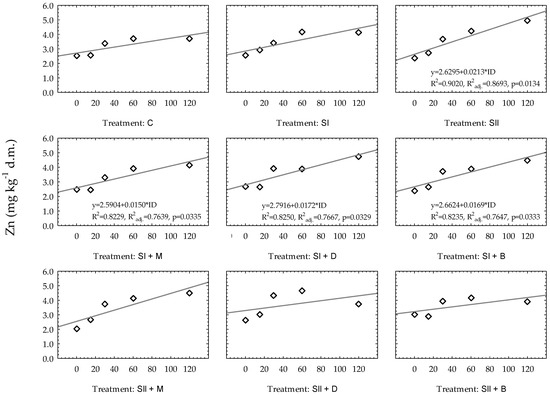
Figure 4.
Linear regression models of Zn changes over time based on experimental treatments in heavy soil. Regression equations are presented only when p ≤ 0.05. * constitute a multiplication mark.
Figure 5 shows the observed Mn and Zn contents plotted against the fitted values calculated on the basis of the obtained regression equations, indicating how well the models describe the changes in the availability of these elements in the soil in a particular experimental treatment (SI + B: soil with the addition of waste sulfur (sulfur dose: I) and biochar). Despite the high coefficients of determination for both presented models, a clearly better fit of the data can be observed for the Zn model.
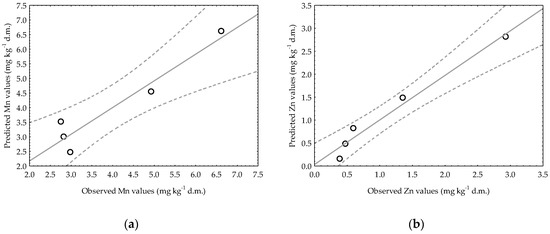
Figure 5.
Observed versus predicted plots of linear regression models for Mn (a) and Zn (b) content changes over time in very light soil for SI + B treatment; these experimental combinations were chosen because they had the highest R2 values (R2 = 0.9139 for Mn and R2 = 0.9706 for Zn).
A similar procedure for plotting observed versus predicted data was used for the chosen time models developed for heavy soil (Figure 6). Treatment SI for Mn and treatment SII for Zn were selected according to their statistical significance. In addition, the highest possible value of the coefficient of determination R2 was chosen, which was 0.8012 for Mn (SI) and 0.9020 for Zn (SII). For heavy soil data, the data points for the Zn model are much closer to the projected regression line, and it can be concluded that the regression model fits the data reasonably well and much better than that of Mn.
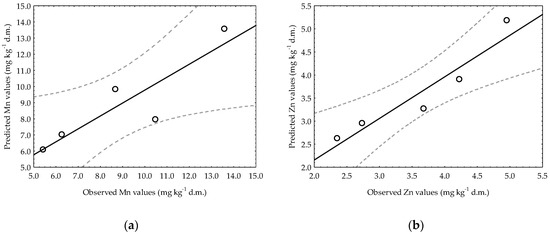
Figure 6.
Observed versus predicted plots of linear regression models of Mn (a) and Zn (b) content changes over time in heavy soil for SI treatment (Mn) and SII treatment (Zn); these experimental combinations were chosen because they had the highest R2 values (R2 = 0.8012 for Mn and R2 = 0.9020 for Zn).
3.3. Application of Multiple Regression Analysis for Predicting Manganese and Zinc Availability in Soils
Multiple linear regression analysis was used to develop models that describe how the y variable (Mn and Zn) relates to a number of explanatory variables (xn: soil pH value, S-SO4 and dehydrogenase activity). The general structure of the base regression equation for particular elements used for the analysis was: , where b0 is the intercept; b1 … bn are regression coefficients (not standardized); and x1, x2 and x3 are the soil pH value, S-SO4 content and dehydrogenase (D) activity, respectively. The models were developed on the basis of data from five incubation periods (days 0, 15, 30, 60 and 120). Statistical analyses were performed separately for very light and heavy soils. After stepwise regression analysis with backward elimination, the final and simplified models are presented in Table 6.

Table 6.
Estimated equation parameters and statistics for stepwise (backward elimination) multiple regression of tested elements.
In the regression equations after the backward elimination procedure, the explanatory variables were often not the same. Of the four regression models, there was a variable related to soil pH in the three equations: for Zn content in both very light and heavy soils and for Mn content in heavy soil; in the case of the regression equation for Zn content in heavy soil, the sulfate sulfur content turned out to be an additional variable remaining after the analysis (Table 6). Dehydrogenase activity only remained in one regression equation, which was developed for Mn content in very light soil. The coefficient of determination was the highest (R2 = 0.8409), and Se was the lowest (0.3164) for the model describing Zn content in heavy soil.
It can be seen that in Figure 7a,b, the observed data and their adjustment to the regression line are rather dispersed. As mentioned before, the only case when the variable indicating the activity of dehydrogenase remained in the regression equation was the model describing the availability of Mn in very light soil. Despite the fact that the statistical analysis proved the significance of the model, the coefficient of determination was very low (R2 = 0.1934), indicating the poor fit and precision of the model. For a given model, there was higher variability around the regression line (Figure 7a), which is the reason for its lower R2 value. In very light soil, the regression equation for Zn consisted of pH, but the accuracy of the model was much lower compared to that obtained for Zn in heavy soil, as evidenced in Table 5 by R2 (0.4257) and Se (0.5072), together with the visual presentation in Figure 7b. The equation for Mn in heavy soil (Figure 7c), which contains an explanatory variable denoting soil pH, is characterized by a high coefficient of determination (R2 = 0.6060), although the Se value should be considered quite high (2.0888). The regression line approximates the real data points quite well for Zn in heavy soil, which can be observed in Figure 7d, and the model can explain around 84% of the Zn content variability in the soil. Judging by the Se value, when predicting the value of the dependent variable (Zn), the authors made an error of 0.3164 mg kg−1 d.m.
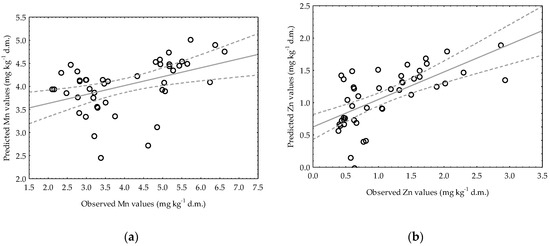
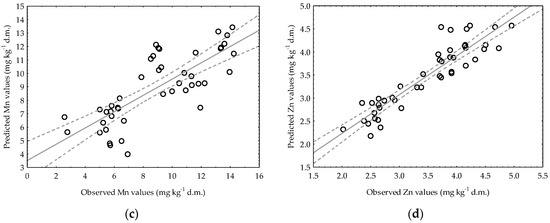
Figure 7.
Observed Mn and Zn contents in very light (a,b) and heavy soils (c,d) versus predicted data generated by the developed simplified regression models.
Multiple regression analysis resulted in a significant relationship between Mn and Zn availability in very light and heavy soils and some of the explanatory variables (soil pH, S-SO4 content and dehydrogenase activity). The results are also presented using a Pareto chart of t-values for the regression coefficients (Figure 8). The Pareto chart shows the size of the effect that each variable involved in the regression model has on the dependent variable (Mn and Zn) in decreasing order, and the line going across the columns indicates how large an effect has to be (i.e., how long a column must be) to be statistically significant. The columns of the plot are in proportion to the calculated values of the t-statistics of each effect. As observed, soil pH was a predictive variable for Zn availability in light and heavy soils as well as for Mn content in heavy soil. The sulfate sulfur content itself seemed to also be an important predictor for Zn content in heavy soil, but less significant than the pH value. In this case, higher values of the t-score for pH in comparison to the S-SO4 content indicate that a large difference exists between these two variable inputs (approximately 3-fold). The availability of Mn in very light soil significantly depended mostly on dehydrogenase activity.
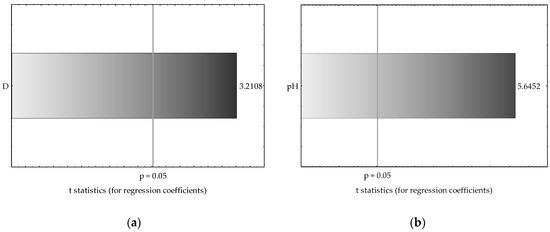
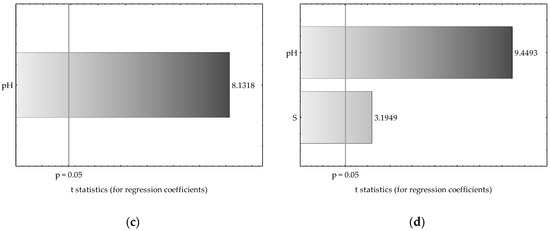
Figure 8.
Pareto chart of t-values for the most significant regression coefficients in the developed regression models for Mn and Zn contents in very light (a,b) and heavy soils (c,d). Pareto chart contrasting t-values of regression coefficients accounting for the regression model with the significant p-value cut-off (vertical gray line).
3.4. Heatmaps of Selected Soil Parameters as a Function of Different Treatments
Figure 9 reports the heatmaps obtained analyzing the four determined soil parameters (i.e., soil pH, dehydrogenase activity, S-SO4, and Mn and Zn contents) that differed among treatments. Two heatmaps, referring to very light soil (Figure 9a) and heavy soil (Figure 9b), were created. The color pattern ranges from dark green for the lowest values and increases gradually until becoming dark red for the highest values. Particular patterns in the heatmaps differed between the very light and heavy soils (values were higher for heavy soil, as is shown in the figure legend). In addition, the arrangement of the treatments shown on the left side of both heatmaps’ panels is different between very light and heavy soils. Interesting groups of dependencies can be observed; for example, the lowest pH was found in samples of heavy soil, especially after 60 and 120 incubation days, together with lower dehydrogenase activity and higher Mn content (for all treatments after 120 incubation days), and the Zn content, in most cases, also increased after a longer incubation time. A high content of available sulfur was found on the 60th day of incubation when a double dose of sulfur was applied at the beginning of the experiment. Light soil samples usually had higher pH at the beginning of the experiment and then fell, but to a lesser extent than heavy soil, in the following measurement days, being lower after 120 days of incubation. Greater amounts of Mn and Zn were observed on day 120 of incubation, especially for treatments including biochar and digestate for SI and SII and manure for SI. In the initial period of the experiment, the content of S-SO4 in the very light soil samples was low; only on the 15th day (SII + D), the 30th day (SII + D and SII + M) and especially on the 60th day of incubation (SII + D and SII + M) did the values turn out to be the highest. Conversely, the higher activity of dehydrogenase, regardless of the treatment, was observed in the first days of the experiment, up to the 30th day of incubation.
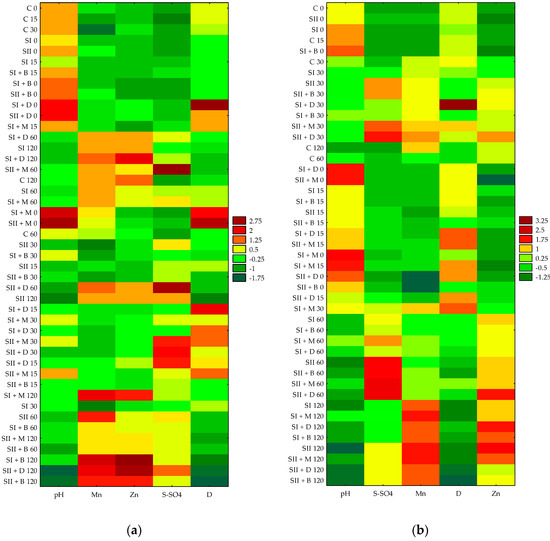
Figure 9.
Heatmaps of selected soil parameters (pH, D activity, S-SO4, and Mn and Zn contents) in very light (a) and heavy soils (b).
3.5. Principal Component Analysis (PCA) Biplots to Show the Distribution of Experimental Treatments
Principal component analysis (PCA) was used to display patterns in the data set containing observations described by the quantitative parameters of soil samples (Figure 10). The PC1 and PC2 components for parameters obtained for very light soil had eigenvalues equal to 3.23 and 0.80, while for heavy soil, these eigenvalues were 3.62 and 0.69, respectively. PC1 and PC2 accounted for 64.51% and 16.07% of the observed variation for very light soil. The amount of variance retained by the principal components (PC1 and PC2) for heavy soil was 72.37% and 13.85%, respectively. For both soil types, the separation of the treatments was shown, but data clustering was more related to incubation than to the experimental treatments themselves.
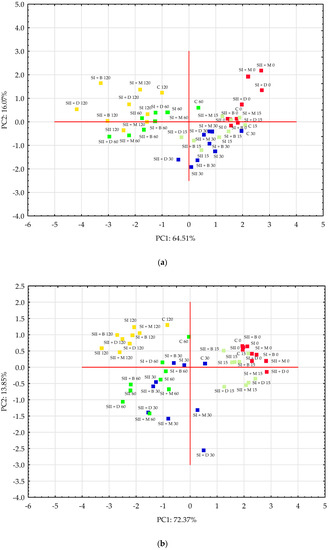
Figure 10.
Ordination diagram of principal component analysis (PCA) showing similarities among experimental treatments in very light (a) and heavy soils (b). Different colors of the squares indicate incubation days: 0 (■); 15 (■); 30 (■); 60 (■); 120 (■).
In the score plot, the data obtained on incubation days 0, 15 and 30 for very light soil were clustered on the positive side of the PC1 axis, with the exception of the SII + D treatment on the 15th and 30th incubation days (negative sides of both PC1 and PC2 axes) (Figure 10a). Most data obtained on the 30th incubation day were clustered in the lower right quadrant (negative PC2 values), while for incubation day 0, the data were clustered in the upper right quadrant (positive PC2 values), with the exception of SI and SI + B. The negative side of PC1 comprised data measured for treatments on the 60th and 120th incubation days (an exception is C 60). Most of the treatment data collected on the 120th day were grouped in the upper left quadrant of the score plot, especially for the SI and C combination. The highest values for horizontal coordinates were recorded for treatments: SII + M 0 and SI + D 0 (positive) and SII + D 120 (negative). Extreme values on the vertical axis were recorded for treatments: SI + M 0 (positive) and SII 30 (negative). The parameters clustering on the positive side of PC1 were pH and dehydrogenase activity (loadings: 0.86 and 0.76, respectively), and those clustering on the negative side were Zn, Mn and S-SO4 (loadings: −0.90, −0.79 and −0.70, respectively). PC2 had high positive loadings of Mn (0.58), pH (0.39), Zn (0.35) and dehydrogenase activity (0.26), and the only negative contributor to PC2 was S-SO4 (−0.35). The first principal component mainly represents variables such as Zn availability in samples of very light soil and soil pH, while the second principal component mainly represents Mn availability.
The data for heavy soil measured on incubation days 0 and 15 were clustered on the positive side of PC1, while data on the 60th and 120th incubation days were located on the negative side of PC1 (Figure 10b). The data on the 30th day were distributed in all plot quadrants. Almost all points for 0-day treatments were located in the upper right quadrant, with the exception of SII + D 0, which had negative coordinates for PC2. Negative PC1 but positive PC2 coordinates were observed for the parameters measured on the 120th incubation day for all treatments. Parameters measured on the 15th day of treatments with manure (M) and digestate (D) for both sulfur doses were grouped in the bottom right quadrant of the plot. On the other hand, treatments with the double sulfur dose, based on data from the 30th and 60th days, were clustered in the bottom left quadrant. Extreme values on the horizontal axis were recorded for treatments: SII + M 0 and SII + D 0 (positive) and SII 120 (negative). Extreme values on the vertical axis were recorded for treatments: C 120 and SI 120 (positive) and SI + D 30 (negative). The parameters clustering on the positive side of PC1 were soil pH and dehydrogenase activity (loadings: 0.95 and 0.72, respectively), whereas the loadings of Zn, Mn and S-SO4 were negative (−0.95, −0.75 and −0.74). A positive loading for PC2 was observed only for Mn, but it was very low, below 0.01. Other soil parameters (dehydrogenase activity, S-SO4, Zn and soil pH) clustered on the negative side of PC2 with loadings of −0.60, −0.56, −0.12 and −0.08, respectively. The first principal component mainly represents variables such as Zn availability in the samples of heavy soil and soil pH, while the second principal component mainly represents dehydrogenase activity and S-SO4 content.
3.6. Comments on the Results of Advanced Statistical Analyses
The presented findings indicate significant relationships between the soil pH value, S-SO4, Zn and Mn contents, dehydrogenase activity and experimental duration. The results of the regression analysis revealed that the applied materials had a more significant influence on the zinc content than on the manganese content during the incubation period. The present study used multiple linear regression analysis to identify which soil parameters (pH value, S-SO4 content and dehydrogenase activity) most significantly affect Mn and Zn contents. This technique is widely used in soil research [61,62,63,64]. Most of the regression equations after the backward elimination procedure regarded soil pH as a key variable affecting the availability of Zn and Mn contents in both light and heavy soils. This was also confirmed using a Pareto chart of t-values for the most significant regression coefficients in the developed regression models. As presented in the heatmaps, with the prolonging of the incubation period, increasing contents of sulfate sulfur and decreasing values of pH and dehydrogenase activity, the Zn and Mn contents in both tested soils increased. Data clustering in the principal component analysis was more related to incubation duration than the experimental treatments.
The presented findings are in line with our previous research [65]. From the mentioned experiment, after the preparation of the PCA ordination including soil parameters and conditions for conducting the experiment, it was concluded that the S0 dose and incubation duration could decrease the soil enzyme activity (dehydrogenase and catalase) and pH value and also increase the content of sulfate sulfur. Furthermore, the liming treatment reduced the relationship strength between the discussed parameters. However, in the case of the elemental sulfur dose and sulfate sulfur content, the lime introduction increased the correlation between these variables. The addition of calcium compounds such as calcium carbonate to low-pH soil increases its oxidative capacity, hence the oxidation of elemental sulfur [66]. Dall’Orsoletta et al. [67] reported that the application of elemental sulfur at increasing doses decreases soil pH. As Kulczycki [68] stated, after studying the effect of the discussed amendment in different doses and types of soils, decreasing soil pH impairs the oxidation rate of the introduced sulfur. Similar results were presented by Zhao et al. [69], who highlighted a positive correlation between these parameters. A close relationship between the sulfate sulfur content and pH results from the fact that the soil reaction shapes both the abundance and activity of sulfur oxidizers [49,70]. These species transform unavailable elemental sulfur form into mobile ions, and their efficiency decreases together with decreasing soil pH values [70,71]. A reduction in dehydrogenase activity over the incubation study period was presented by Mierzwa-Hersztek et al., as well [72]. In addition to decreasing the soil pH value, lowering the value of the discussed parameter could be a result of the scarcity of easily degradable carbon substrates, as well as nutrient resources [73,74,75]. As mentioned in the above section, lower soil pH benefits the mobility of trace elements and reduces the mobility of macroelements. This is a result of the consequences induced by the increased activity of hydrogen ions (H+). Various relationships among soil parameters have been presented across research reports. Wang et al. [76], after testing the effect of six different pH values on element concentrations in two soils, reported that, together with the decreasing value of soil pH, the concentrations of plant-available Al, Cd, Mn and Zn increased, while plant-available Ca and Mg contents decreased. According to this, lowering the pH value significantly influenced plant Zn and Cd uptake. In addition, Soaud et al. [46] highlighted the relationship between an increase in the nutrient concentration and a decrease in the pH value of the tested soils after conducting a 128-day incubation experiment and amending the soil with elemental sulfur. In contrast, Matos Castañon et al. [77], after conducting a pot experiment, concluded that increasing doses of elemental sulfur did not affect the soil abundance of available P, K and Mg, in spite of lowering the pH of the examined soil. Hanousek et al. [78], after testing three study sites, found a significant negative correlation between soil pH and the sulfate sulfur content, as well as a significant positive correlation between soil pH and the Al content. Mattiello et al. [53] found that sulfur oxidation was not correlated with available Zn in the acid soil, contrary to high-pH soil, where it was. Additionally, among the two tested soils, acidic soil was characterized by a higher concentration of this element compared to slightly alkaline soil.
4. Conclusions
Sustainable agriculture is based on practices that take into account the need to protect the environment and natural resources while meeting increasing production goals by leveraging the opportunities introduced by technological development. The aim is to limit the use of chemical plant protection products and mineral fertilizers in favor of organic fertilizers and to minimize the use of fossil fuels.
As observed, soil pH was a predictive variable of Zn availability in light and heavy soils, as well as for Mn content in heavy soil. The sulfate sulfur content itself seemed to also be an important predictor of Zn content in heavy soil but was less significant than the pH value. In this case, higher values of the t-score for pH in comparison to the S-SO4 content indicate that a large difference exists between these two variable inputs (approximately 3-fold). The availability of Mn in very light soil significantly depended mostly on dehydrogenase activity.
The use of sulfur waste in the plant production process is consistent with the concept of a circular economy. It allows the reintegration of sulfur into the trophic chain and reduces the consumption of non-renewable resources necessary for the production of traditional mineral fertilizers. The various methods of statistical data analysis used by the authors could help researchers and practitioners in advancing their interpretation of results to provide a fuller picture of the changes caused by experimental procedures.
Efficient energy management (heat and electricity) is becoming a priority in the context of the international competitiveness of the fertilizer production industry. More and more stringent regulations enforce the use of solutions to limit emissions to the atmosphere.
The production of conventional sulfur fertilizers is an energy-intensive process that requires the input of non-renewable resources. The agricultural use of sulfur waste allows plants to recover nutrients and reduce the consumption of mineral fertilizers. This could result in reducing energy expenditure and the environmental burden associated with the production of conventional fertilizers.
The presented results constitute a foundation for future theoretical and practical studies. Since research on the use of waste sulfur and its mixtures with organic materials in plant fertilization is limited, the presented results are a valuable tool for assessing the potential of activities to re-use the waste in question. As the presented results refer to preliminary tests, it is advisable to continue and replicate them in real growing conditions. This will allow extending the scope of research to include the assessment of the impact of waste sulfur on the properties of soil and plant material under variable environmental factors.
Author Contributions
Conceptualization, A.L., B.F.-M. and A.K. (Andrzej Kalisz); methodology, A.L., B.F.-M. and A.K. (Andrzej Kalisz); software, A.L., B.F.-M., A.K. (Andrzej Kalisz), Z.G.-S. and A.K. (Agnieszka Kowalczyk); validation, A.L., B.F.-M. and A.K. (Andrzej Kalisz); formal analysis, A.L., B.F.-M., A.K. (Andrzej Kalisz) and Z.G.-S.; investigation, A.L., B.F.-M. and A.K. (Andrzej Kalisz); resources, A.L., B.F.-M., A.K. (Andrzej Kalisz), Z.G.-S. and A.K. (Agnieszka Kowalczyk); data curation, A.L., B.F.-M. and A.K. (Andrzej Kalisz); writing—original draft preparation, A.L., B.F.-M., A.K. (Andrzej Kalisz) and Z.G.-S.; writing—review and editing, A.L., B.F.-M., A.K. (Andrzej Kalisz) and Z.G.-S.; visualization, A.L., B.F.-M. and A.K. (Andrzej Kalisz); supervision, A.L., B.F.-M. and A.K. (Andrzej Kalisz); project administration, A.L., B.F.-M., A.K. (Andrzej Kalisz), Z.G.-S. and A.K. (Agnieszka Kowalczyk); funding acquisition, A.L., B.F.-M., A.K. (Andrzej Kalisz), Z.G.-S. and A.K. (Agnieszka Kowalczyk). All authors have read and agreed to the published version of the manuscript.
Funding
This publication was financed by a subsidy granted to the Cracow University of Economics (057/ZZE/2022/POT). This publication was financed by a subsidy granted to the University of Agriculture in Krakow by the Ministry of Science and Education of the Republic of Poland and the Institute of Technology and Life Sciences, National Research Institute.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gródek-Szostak, Z.; Luc, M.; Szeląg-Sikora, A.; Sikora, J.; Niemiec, M.; Ochoa Siguencia, L.; Velinov, E. Promotion of RES in a Technology Transfer Network. Case Study of the Enterprise Europe Network. Energies 2020, 13, 3445. [Google Scholar] [CrossRef]
- Gródek-Szostak, Z.; Adamczyk, J.; Luc, M.; Suder, M.; Tora, J.; Kotulewicz-Wisińska, K.; Zysk, W.; Szeląg-Sikora, A. Hard Cash in Hard Times—The Effect of Institutional Support for Businesses Shaken by COVID-19. Sustainability 2022, 14, 4399. [Google Scholar] [CrossRef]
- Wojnarowska, M.; Sołtysik, M.; Prusak, A. Impact of eco-labelling on the implementation of sustainable production and consumption. Environ. Impact Assess. Rev. 2021, 86, 106505. [Google Scholar] [CrossRef]
- Nilsson, M.; Griggs, D.; Visbeck, M. Map the interactions between sustainable development goals. Nature 2016, 534, 320–322. [Google Scholar] [CrossRef] [PubMed]
- The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas); VINNOVA. Swedish Research and Innovation Strategy for a Bio-Based Economy. 2012. Available online: https://www.formas.se/download/18.462d60ec167c69393b91e60f/1549956092919/Strategy_Biobased_Ekonomy_hela.pdf (accessed on 25 August 2022).
- McCormick, K.; Kautto, N. The bioeconomy in Europe: An overview. Sustainability 2013, 5, 2589–2608. [Google Scholar] [CrossRef]
- Donner, M.; de Vries, H. How to innovate business models for a circular bio-economy? Bus. Strategy Environ. 2021, 30, 1932–1947. [Google Scholar] [CrossRef]
- European Commission. Closing the Loop—An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015.
- European Commission. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy society and the environment Updated Bioeconomy Strategy; European Commission: Brussels, Belgium, 2018.
- OECD. The Bioeconomy to 2030 Designing a Policy Agenda-Main Findings and Policy Conclusions Organisation for Economic Co-Operation and Development (OECD); OECD: Paris, France, 2009. [Google Scholar]
- UN General Assembly. Transforming our World: The 2030 Agenda for Sustainable Development—Resolution A/RES/70/1 Adopted by the General Sssembly on 25 September 2015. Available online: https://www.un.org/en/development/desa/population/migration/generalassembly/docs/globalcompact/A_RES_70_1_E.pdf (accessed on 25 August 2022).
- Geng, Y.; Sarkis, J.; Bleischwitz, R. How to globalize the circular economy. Nature 2019, 565, 153–155. [Google Scholar] [CrossRef]
- Schroeder, P.; Anggraeni, K.; Weber, U. The relevance of circular economy practices to the sustainable development goals. J. Ind. Ecol. 2018, 23, 77–95. [Google Scholar] [CrossRef]
- European Commission. EU Biodiversity Strategy for 2030–Bringing Nature Back Into Our Lives. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1590574123338&uri=CELEX:52020DC0380 (accessed on 20 August 2022).
- Jones, A.; Panagos, P.; Barcelo, S.; Bouraoui, F.; Bosco, C.; Dewitte, O.; Gardi, C.; Erhard, M.; Hervas, D.F.; Hiederer, R.; et al. The State of Soil in Europe: A Contribution of the JRC to the European Environment Agency’s Environment State and Outlook Report—SOER 2010; Publications office of the European Union: Luxembourg, 2012; p. 80. [CrossRef]
- FAO; ITPS. Status of the World’s Soil Resources (SWSR)—Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy, 2015; p. 607. Available online: http://www.fao.org/3/i5199e/i5199e.pdf (accessed on 20 August 2022).
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Oberč, B.P.; Schnell, A.A. Approaches to Sustainable Agriculture. Exploring the Pathways towards the Future of Farming; Iucn, E., Ed.; IUCN EURO: Brussels, Belgium, 2020. [Google Scholar]
- Kandil, A.H.T.; Cheira, M.F.; Gado, H.S.; Soliman, M.H.; Akl, H.M. Ammonium sulfate preparation from phosphogypsum waste. J. Radiat. Res. Appl. Sci. 2017, 10, 24–33. [Google Scholar] [CrossRef]
- Törnwall, E.; Pettersson, H.; Thorin, E.; Schwede, S. Post-treatment of biogas digestate—An evaluation of ammonium recovery, energy use and sanitation. Energy Procedia 2017, 142, 957–963. [Google Scholar] [CrossRef]
- Lamastra, L.; Suciu, N.A.; Trevisan, M. Sewage sludge for sustainable agriculture: Contaminants’ contents and potential use as fertilizer. Chem. Biol. Technol. Ag. 2018, 5, 10. [Google Scholar] [CrossRef]
- Szymańska, M.; Sosulski, T.; Szara, E.; Wąs, A.; Sulewski, P.; van Pruissen, G.W.P.; Cornelissen, R.L. Ammonium sulphate from a bio-refinery system as a fertilizer-agronomic and economic effectiveness on the farm scale. Energies 2019, 12, 4721. [Google Scholar] [CrossRef]
- Lisowska, A.; Filipek-Mazur, B.; Komorowska, M.; Niemiec, M.; Bar-Michalczyk, D.; Kuboń, M.; Tabor, S.; Gródek-Szostak, Z.; Szeląg-Sikora, A.; Sikora, J.; et al. Environmental and production aspects of using fertilizers based on waste elemental sulfur and organic materials. Materials 2022, 15, 3387. [Google Scholar] [CrossRef] [PubMed]
- Blake-Kalff, M.M.A.; Zhao, F.J.; Hawkesford, M.J.; Mcgrath, S.P. Using plant analysis to predict yield losses caused by sulphur deficiency. Ann. Appl. Biol. 2001, 138, 123–127. [Google Scholar] [CrossRef]
- Scherer, H.W. Sulphur in crop production—Invited paper. Eur. J. Agron. 2001, 14, 81–111. [Google Scholar] [CrossRef]
- Mukwevho, E.; Ferreira, Z.; Ayeleso, A. Potential role of sulfur-containing antioxidant systems in highly oxidative environments. Molecules 2014, 19, 19376–19389. [Google Scholar] [CrossRef] [PubMed]
- Engardt, M.; Simpson, D.; Schwikowski, M.; Granat, L. Deposition of sulphur and nitrogen in Europe 1900–2050. Model calculations and comparison to historical observations. Tellus B Chem. Phys. Meteorol. 2017, 69, 1328945. [Google Scholar] [CrossRef]
- Siebielec, G.; Smreczak, B.; Klimkowicz-Pawlas, A.; Kowalik, M.; Kaczyński, R.; Koza, P.; Ukalska-Jaruga, A.; Łysiak, M.; Wójtowicz, U.; Poręba, L.; et al. Report from the third stage of the contract implementation. In Monitoring of Chemistry of Arable Soils in Poland in 2015–2017; IUNG-PIB Puławy: Puławy, Poland, 2017; pp. 1–170. [Google Scholar]
- Giovanelli, J.; Mudd, S.H.; Datko, A.H. Sulphur amino acids in plants. In The Biochemistry of Plants; Miflin, B.J., Lea, P.J., Eds.; Academic Press: New York, NY, USA, 1980; Volume 5, pp. 453–506. [Google Scholar]
- Haq, K.; Ali, M. Biologically active sulphur compounds of plant origin. In Sulphur in Plants; Abrol, Y.P.R., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 375–386. [Google Scholar] [CrossRef]
- Droux, M. Sulfur assimilation and the role of sulfur in plant metabolism: A survey. Photosynth. Res. 2004, 79, 33–48. [Google Scholar] [CrossRef]
- Durenkamp, M.; De Kok, L.J. Impact of pedospheric, atmospheric sulphur nutrition on sulphur metabolism of Allium cepa L. a species with a potential sink capacity for secondary sulphur compounds. J. Exp. Bot. 2004, 55, 1821–1830. [Google Scholar] [CrossRef]
- Kertesz, M.A.; Fellows, E.; Schmalenberger, A. Rhizobacteria and plant sulfur supply. Adv. Appl. Microbiol. 2007, 62, 235–268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Tausz, M.; De Kok, L.J. Role of sulfur for plant production in agricultural and natural ecosystems. In Sulfur Metabolism in Phototrophic Organisms; Hell, R., Dahl, C., Knaff, D.B., Leustek, T., Eds.; Springer: New York, NY, USA, 2008; Volume 27, pp. 417–435. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Castellarín, J.M.; Miralles, D.J.; Pedrol, H.M. Sulfur fertilization improves nitrogen use efficiency in wheat by increasing nitrogen uptake. Field Crops Res. 2009, 113, 170–177. [Google Scholar] [CrossRef]
- Katerova, Z.; Miteva, L.P.E. Glutathione and herbicide resistance in plants. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Anjum, N.A., Chan, M.T., Umar, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 191–207. [Google Scholar] [CrossRef]
- Rendig, V.V.; Oputa, C.; McComb, E.A. Effects of sulfur deficiency on non-protein nitrogen, soluble sugars, and N/S ratios in young corn (Zea mays L.) plants. Plant Soil. 1976, 44, 423–437. [Google Scholar] [CrossRef]
- Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; GarcíaEnciso, E.L.; González-Morales, S.; Juárez-Maldonado, A.; Rincón-Sánchez, F.; Benavides-Mendoza, A. From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef]
- Marschner, H. Mineral nutrition of higher plants, 2nd ed.; Academic Press: London, UK, 1995; pp. 1–889. [Google Scholar]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- PTG. Particle size distribution and textural classes of soils and mineral materials–classification of Polish Society of Soil Science 2008. Rocz. Glebozn.-Soil Sci. Annu. 2009, 60, 5–16. Available online: http://ssa.ptg.sggw.pl/files/artykuly/2009_60/2009_tom_60_2/tom_60_2_005-016.pdf (accessed on 25 August 2022).
- Analiza chemiczno-rolnicza gleby-Pobieranie próbek. pracowana na podstawie PN-R-04031:1997. Available online: http://oschr-bydgoszcz.pl/Dokumenty/Instrukcja%20pobierania%20probek%20glebowych.pdf (accessed on 20 August 2022).
- Kabata-Pendias, A.; Piotrowska, M.; Motowicka-Terelak, T.; Maliszewska-Kordybach, B.; Filipiak, K.; Krakowiak, A.; Pietruch, C. Podstawy oceny chemicznego zanieczyszczenia gleb. Metale ciężkie, siarka i WWA. Biblioteka Monitoringu Środowiska; PIOŚ, IUNG: Warszawa, Poland, 1995; p. 41. [Google Scholar]
- Thalmann, A. Methods of dehydrogenase activity determination with triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Warzyński, H.; Sosnowska, A.; Harasimiuk, A. Effect of variable content of organic matter and carbonates on results of determination of granulometric composition by means of Casagrande’s areometric method in modification by Prószyński. Soil Sci. Annu. 2018, 69, 39–48. [Google Scholar] [CrossRef]
- Soaud, A.A.; Al Darwish, F.H.; Saleh, M.E.; El-Tarabily, K.A.; Sofian-Azirun, M.; Rahman, M.M. Effects of elemental sulfur, phosphorus, micronutrients and Paracoccus versutus on nutrient availability of calcareous soils. Aust. J. Crop Sci. 2011, 5, 554–561. [Google Scholar]
- Cifuentes, F.R.; Lindemann, W.C. Organic matter stimulation of elemental sulfur oxidation in a calcareous soil. Soil Sci. Soc. Am. J. 1993, 57, 727–731. [Google Scholar] [CrossRef]
- Skwierawska, M.; Zawartka, L.; Zawadzki, B. The effect of different rates and forms of sulphur applied on changes of soil agrochemical properties. Plant Soil Environ. 2008, 54, 171–177. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q. Interaction effect of zinc and elemental sulfur on their uptake by spring wheat. J. Plant Nutr. 2005, 28, 639–649. [Google Scholar] [CrossRef]
- Tabak, M.; Lisowska, A.; Filipek-Mazur, B.; Antonkiewicz, J. The effect of amending soil with waste elemental sulfur on the availability of selected macroelements and heavy metals. Processes 2020, 8, 1245. [Google Scholar] [CrossRef]
- Baikhamurova, M.O.; Sainova, G.A.; Akbasova, A.D.; Anarbekova, G.D.; Ozler, M.A. The influence of the mixture of vermicompost and sulphur-perlite-containing waste on the yield and the quality of crops. J. Water Land Dev. 2021, 49, 213–218. [Google Scholar] [CrossRef]
- Zhao, C.; Degryse, F.; Gupta, V.; McLaughlin, M.J. Elemental sulfur oxidation in Australian cropping soils. Soil Sci. Soc. Am. J. 2015, 79, 89–96. [Google Scholar] [CrossRef]
- Mattiello, E.M.; da Silva, R.C.; Degryse, F.; Baird, R.; Gupta, V.V.S.R.; McLaughlin, M.J. Sulfur and zinc availability from co-granulated Zn-enriched elemental sulfur fertilizers. J. Agric. Food Chem. 2017, 65, 1108–1115. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Ameliorating soil acidity of tropical oxisols by liming for sustainable crop production. Adv. Agron. 2008, 65, 345–399. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 905–927. [Google Scholar] [CrossRef]
- Alloway, B.J. Micronutrient Deficiencies in Global Crop Production; Springer: Heidelberg, Germany, 2008; p. 370. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–a review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 5794869. [Google Scholar] [CrossRef]
- Harisuseno, D.; Cahya, E.N. Determination of soil infiltration rate equation based on soil properties using multiple linear regression. J. Water Land Dev. 2020, 47, 77–88. [Google Scholar] [CrossRef]
- Pentoś, K.; Mbah, J.T.; Pieczarka, K.; Niedbała, G.; Wojciechowski, T. Evaluation of multiple linear regression and machine learning approaches to predict soil compaction and shear stress based on electrical parameters. Appl. Sci. 2022, 12, 8791. [Google Scholar] [CrossRef]
- Rajan, N.M.; Neelamegam, P.; Thatheyus, A.J. Multiple linear and non-linear regression analyses of various soil and terrain indices with regard to their efficiency in the determination of temporal changes in LST values within Trichy district of Tamil Nadu, India. Environ. Monit. Assess. 2022, 194, 138. [Google Scholar] [CrossRef]
- Brubaker, S.C.; Jones, A.J.; Frank, K.; Lewis, D.T. Regression models for estimating soil properties by landscape position. Soil Sci. Soc. Am. J. 1994, 58, 1763–1767. [Google Scholar] [CrossRef]
- Tabak, M.; Lisowska, A.; Filipek-Mazur, B. Bioavailability of sulfur from waste obtained during biogas desulfurization and the effect of sulfur on soil acidity and biological activity. Processes 2020, 8, 863. [Google Scholar] [CrossRef]
- Attoe, O.J.; Olson, R.A. Factors affecting rate of oxidation in soils of elemental sulfur and that added in rock phosphate-sulfur fusions. Soil Sci. 1966, 101, 317–325. [Google Scholar] [CrossRef]
- Dall’Orsoletta, D.J.; Mumbach, G.L.; Brignoli, F.M.; Gatiboni, L.C. Elemental sulfur recommendation for pH reduction in soils from Southern Brazil. Rev. Bras. Eng. Agricola Ambient. 2022, 26, 212–218. [Google Scholar] [CrossRef]
- Kulczycki, G. The effect of elemental sulfur fertilization on plant yields and soil properties. Adv. Agron. 2021, 167, 105–181. [Google Scholar] [CrossRef]
- Zhao, C.; Gupta, V.V.S.R.; Degryse, F.; McLaughlin, M.J. Abundance and diversity of S-oxidising bacteria and their role in elemental sulphur oxidation in Australian cropping soils. Biol. Fert. Soils 2016, 53, 159–169. [Google Scholar] [CrossRef]
- Harahuc, L.; Lizama, H.M.; Suzuki, I. Selective inhibition of the oxidation of ferrous iron or sulfur in Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 2000, 66, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Balík, J.; Kulhánek, M.; Černý, J.; Száková, J.; Pavlíková, D.; Čermák, P. Differences in soil sulfur fractions due to limitation of atmospheric deposition. Plant. Soil Environ. 2009, 55, 344–352. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Wolny-Koładka, K.; Gondek, K.; Gałązka, A.; Gawryjołek, K. Effect of coapplication of biochar and nutrients on microbiocenotic composition, dehydrogenase activity index and chemical properties of sandy soil. Waste Biomass Valor. 2020, 11, 3911–3923. [Google Scholar] [CrossRef]
- Scherer, H.W.; Metker, D.J.; Welp, G. Effect of long-term organic amendments on chemical and microbial properties of a luvisol. Plant Soil Environ. 2011, 57, 513–518. [Google Scholar] [CrossRef]
- Cenini, V.; Fornara, D.; Mcmullan, G.; Ternan, N.; Carolan, R.; Crawley, M.J.; Clement, J.C.; Lavorel, S. Linkages between extracellular enzyme activities and the carbon and nitrogen content of grassland soils. Soil Biol. Biochem. 2016, 96, 198–206. [Google Scholar] [CrossRef]
- Siczek, A.; Frąc, M.; Gryta, A.; Kalembasa, S.; Kalembasa, D. Variation in soil microbial population and enzyme activities under faba bean as affected by pentachlorophenol. Appl. Soil Ecol. 2020, 150, 103466. [Google Scholar] [CrossRef]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; Reeves, R.D. Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 2006, 281, 325–337. [Google Scholar] [CrossRef]
- Matos Castañon, T.H.F.; de Aquino, B.F.; Bonfim-Silva, E.M.; Almeida Lima, I.M.; Barreto Damasce, A.P.A. Management of sulphur fertilizer in forage sorghum crop cultivated in eutrophic cambisol with alkaline pH. Aust. J. Crop Sci. 2019, 13, 1258–1266. [Google Scholar] [CrossRef]
- Hanousek, O.; Prohaska, T.; Kulhanek, M.; Balik, J.; Tejnecky, V.; Berger, T.W. Fractionation of sulfur (S) in beech (Fagus sylvatica) forest soils in relation to distance from the stem base as useful tool for modeling S biogeochemistry. Model. Earth Syst. Environ. 2017, 3, 1065–1079. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).