Growth, Nitrogen Uptake, and Nutritional Value of a Diverse Panel of Shrub Willow (Salix spp.) Genotypes in Response to Nitrogen Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Growth and Physiological Measurements
2.3. Statistical Analysis
3. Results
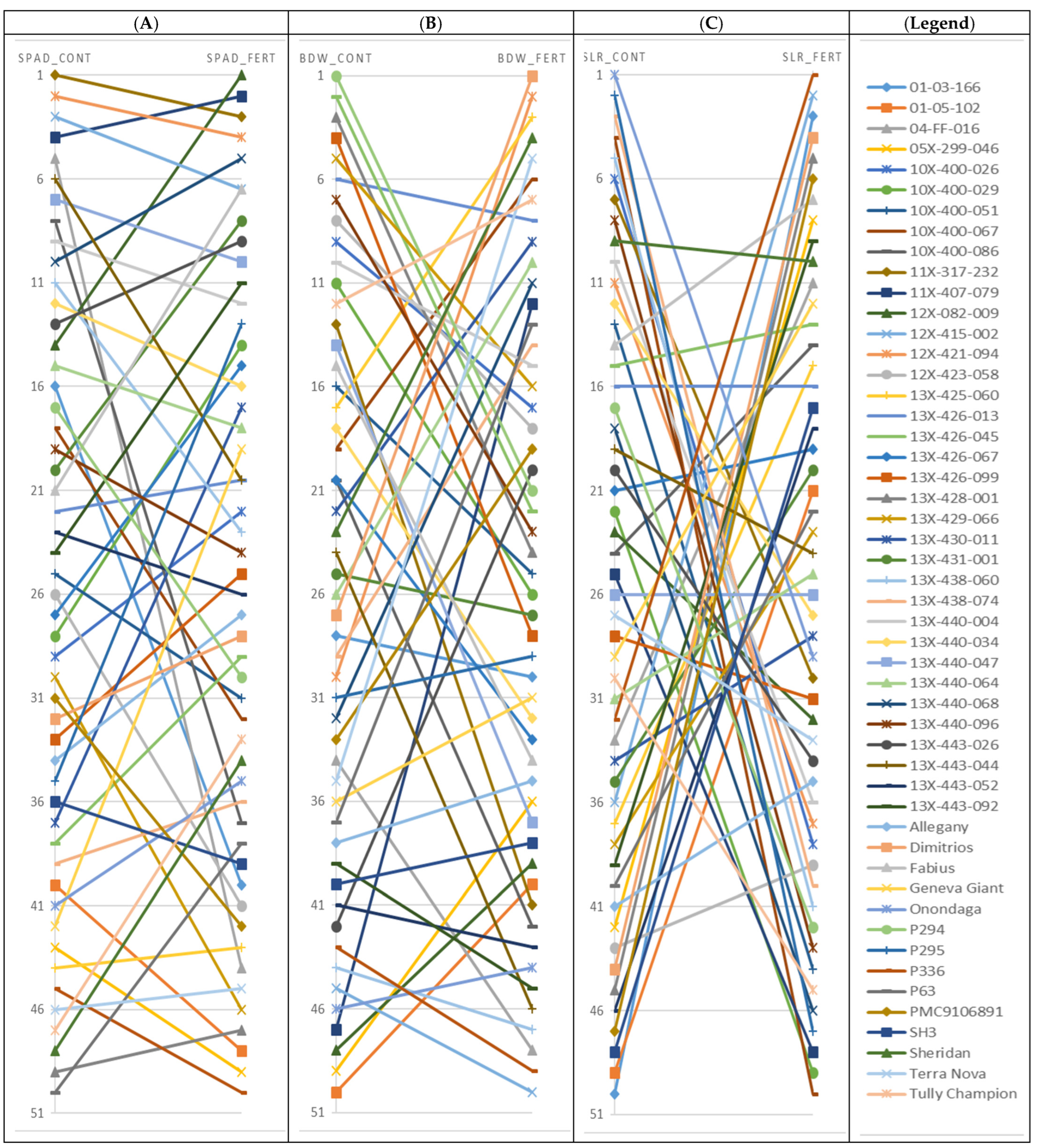
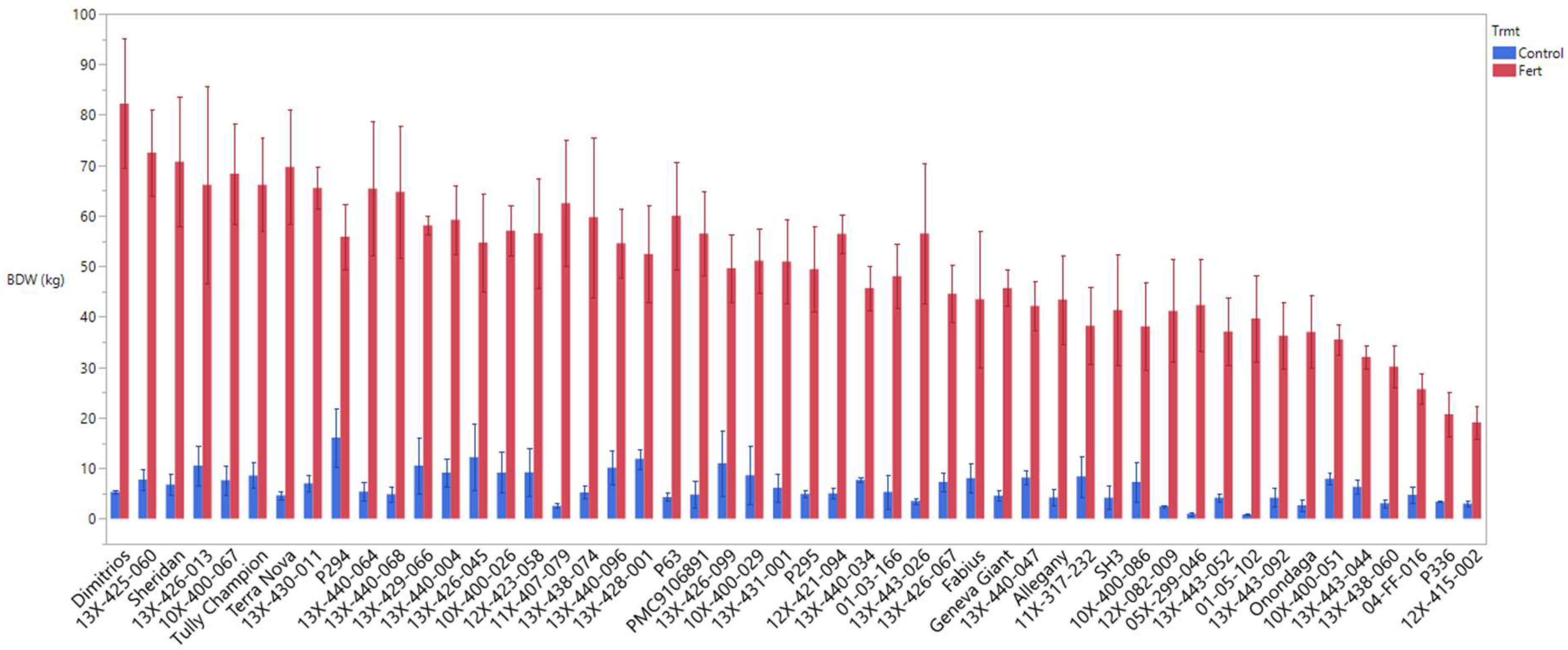
3.1. Biomass Productivity and Growth Development
3.2. Morphological Characteristics
3.3. Nutritional Value
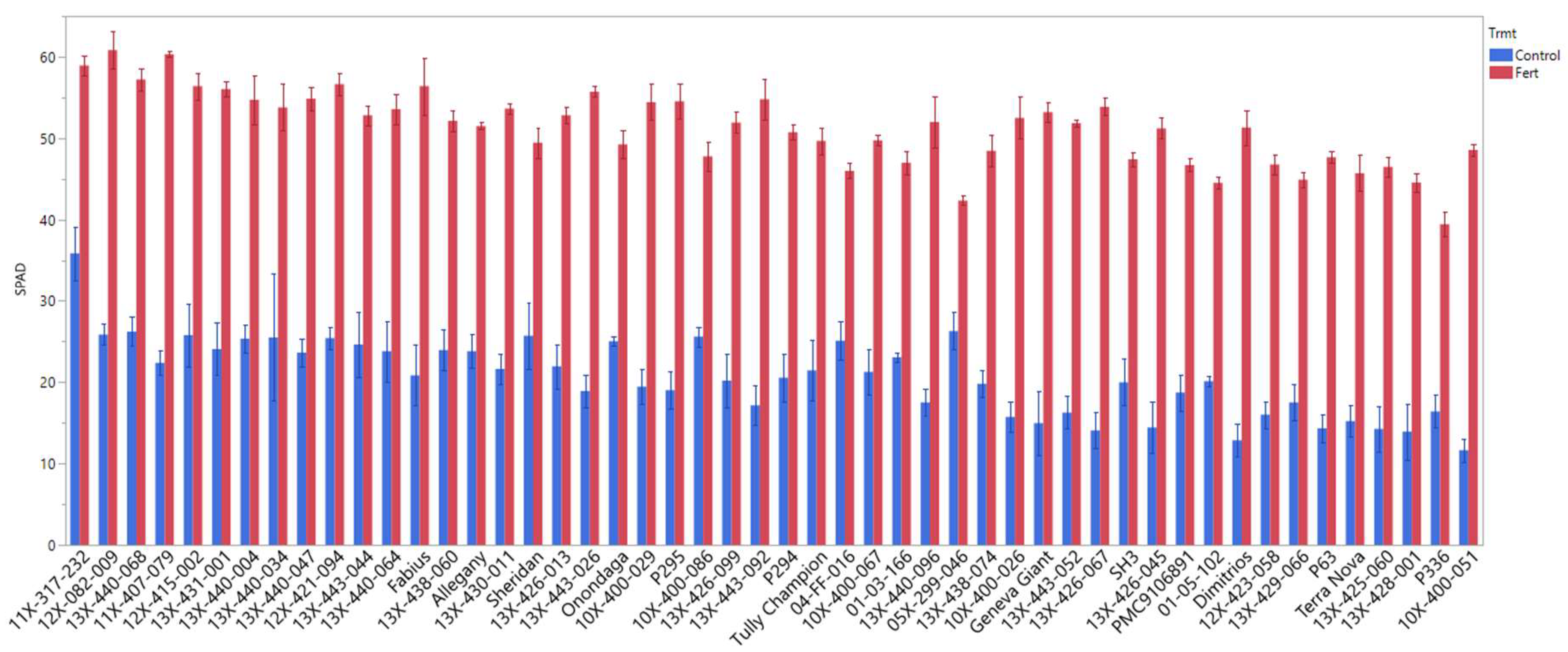
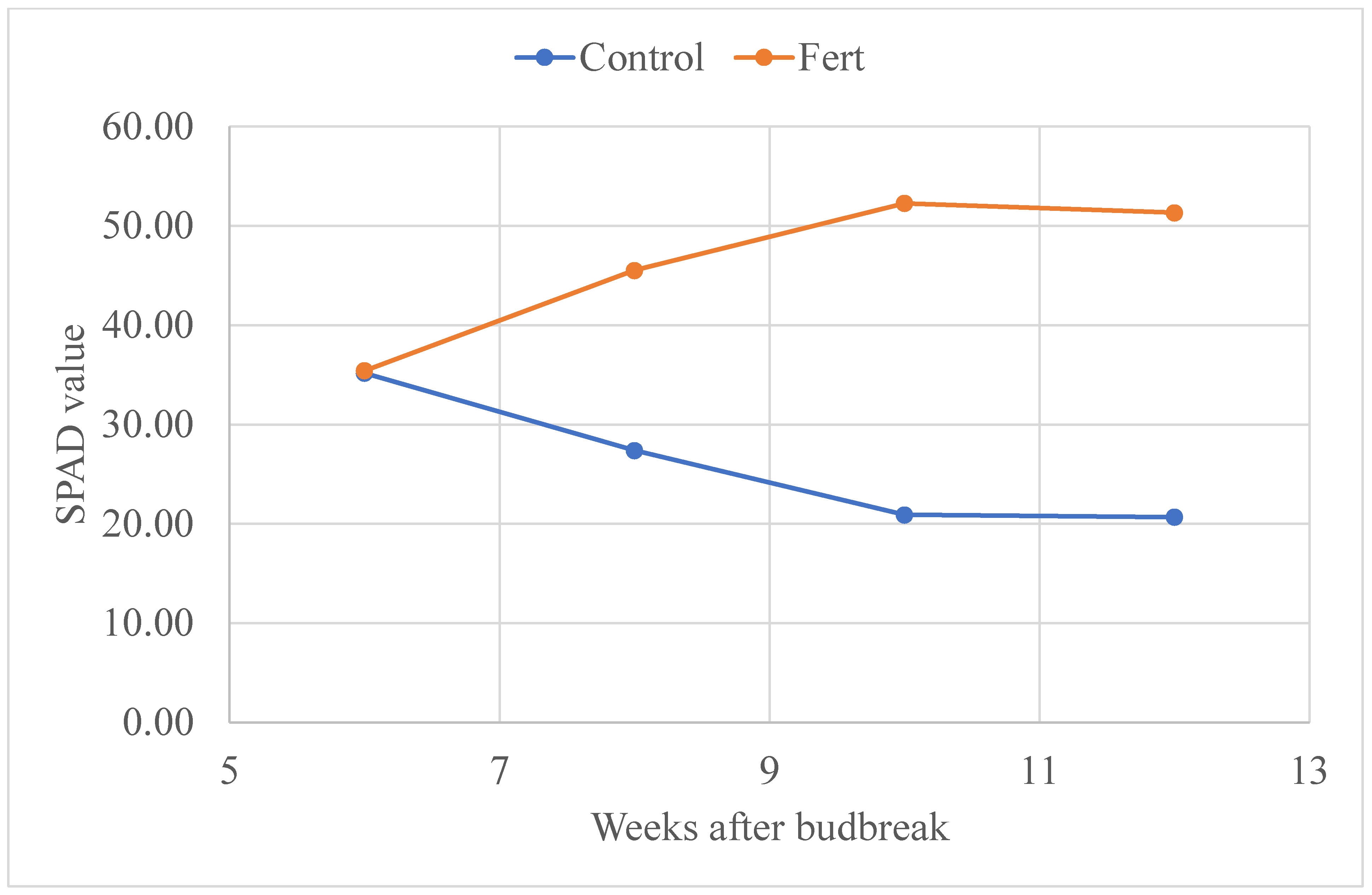
3.4. SPAD Value
3.5. Correlation between Traits
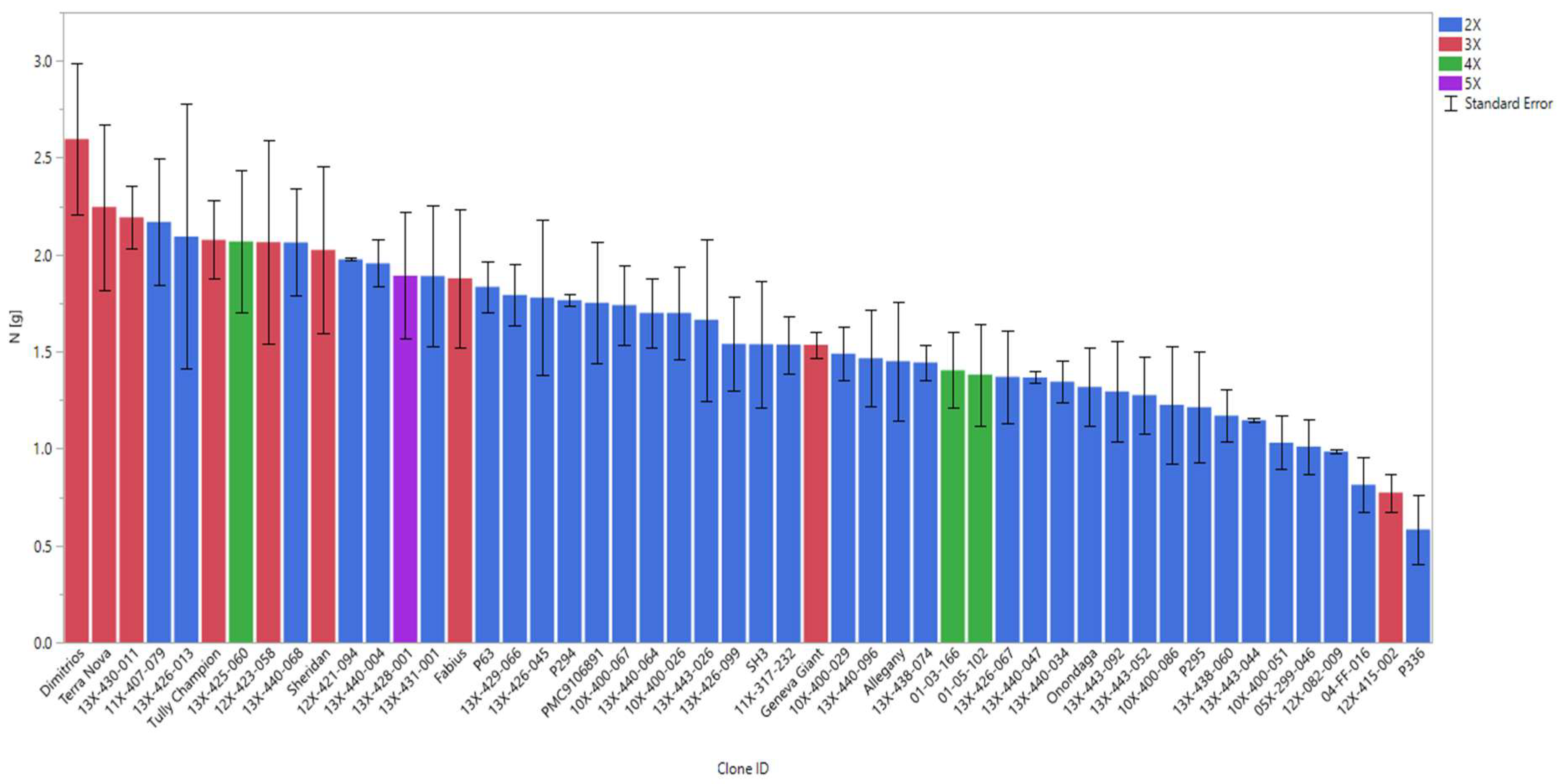
3.6. Performance of Genotypes under High Fertilizer and Their Response to Low Fertilizer (Control)
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Clone ID | Epithet | Pedigree | Sex | Ploidy |
| 01-03-166 | Salix cinerea | F | 4X | |
| 01X-266-016 | Sheridan | S. viminalis × (S. viminalis × S. miyabeana) | F | 3X |
| 05X-281-068 | Geneva Giant | (S. koriyanagi × S. purpurea) × S. miyabeana | F | 3X |
| 05X-299-046 | S. eriocephala | F | 2X | |
| 99202-004 | Fabius | S. viminalis × S. miyabeana | F | 3X |
| 99202-011 | Tully Champion | S. viminalis × S. miyabeana | F | 3X |
| 99239-015 | Allegany | S. koriyanagi × S. purpurea | F | 2X |
| P294 | S. suchowensis | F | 2X | |
| P295 | S. suchowensis | F | 2X | |
| P336 | S. integra | F | 2X | |
| SH3 | S. koriyanagi | F | 2X | |
| - | Terra Nova | (S. viminalis × S. triandra) × S. miyabeana | F | 3X |
| PMC9106891 | S. sericea | F? | 2X | |
| 01-05-102 | S. cinerea | M | 4X | |
| 04-FF-016 | S. koriyanagi | M | 2X | |
| 99113-012 | Onondaga | S. koriyanagi × S. purpurea | M | 2X |
| - | Dimitrios | (S. schwerinii × S. viminalis) × S. aeygyptiaca | M | 3X |
| P63 | S. suchowensis | M | 2X | |
| 10X-400-026 | S. purpurea × S. suchowensis | M | 2X | |
| 10X-400-029 | S. purpurea × S. suchowensis | F | 2X | |
| 10X-400-051 | S. purpurea × S. suchowensis | M | 2X | |
| 10X-400-067 | S. purpurea × S. suchowensis | F | 2X | |
| 10X-400-086 | S. purpurea × S. suchowensis | M | 2X | |
| 11X-317-232 | S. purpurea | M | 2X | |
| 11X-407-079 | S. purpurea × S. viminalis | F | 2X | |
| 12X-082-009 | S. purpurea | F | 2X | |
| 12X-415-002 | S. purpurea × S. miyabeana | F | 3X | |
| 12X-421-094 | S. viminalis × S. purpurea | F | 2X | |
| 12X-423-058 | S. viminalis × S. miyabeana | F | 3X | |
| 13X-425-060 | S. miyabeana | F | 4X | |
| 13X-426-013 | S. integra × S. purpurea | F | 2X | |
| 13X-426-045 | S. integra × S. purpurea | F | 2X | |
| 13X-426-067 | S. integra × S. purpurea | F | 2X | |
| 13X-426-099 | S. integra × S. purpurea | F | 2X | |
| 13X-428-001 | S. miyabeana × S. dasyclados | NA | 5X | |
| 13X-429-066 | S. koriyanagi × S. suchowensis | NA | 2X | |
| 13X-430-011 | S. miyabeana × S. viminalis | F | 3X | |
| 13X-431-001 | (S. koriyanagi × S. purpurea) × S. suchowensis | NA | 2X | |
| 13X-438-060 | S. purpurea × S. koriyanagi | M | 2X | |
| 13X-438-074 | S. purpurea × S. koriyanagi | F | 2X | |
| 13X-440-004 | S. suchowensis × S. purpurea | M | 2X | |
| 13X-440-034 | S. suchowensis × S. purpurea | F | 2X | |
| 13X-440-047 | S. suchowensis × S. purpurea | M | 2X | |
| 13X-440-064 | S. suchowensis × S. purpurea | M | 2X | |
| 13X-440-068 | S. suchowensis × S. purpurea | F | 2X | |
| 13X-440-096 | S. suchowensis × S. purpurea | M | 2X | |
| 13X-443-026 | S. suchowensis× S. purpurea | M | 2X | |
| 13X-443-044 | S. suchowensis× S. purpurea | M | 2X | |
| 13X-443-052 | S. suchowensis× S. purpurea | F | 2X | |
| 13X-443-092 | S. suchowensis× S. purpurea | F | 2X |
| DF | BDW | SLR | DMC | MSL | SSL | MSD | SSD | |
| Fixed effects | ||||||||
| Treatment | 1 | 1241.8 *** | 81.7 *** | 1548.5 *** | 1024.3 *** | 681.2 *** | 1161.9 *** | 616.1 *** |
| Genotype | 49 | 2.9 *** | 11.9 *** | 2.8 *** | 5 *** | 4.9 *** | 5.9 *** | 4.5 *** |
| Interaction | 49 | 2.3 *** | 3.5 *** | 1.9 ** | 2.6 *** | 2.1 ** | 2.1 *** | 1.9 ** |
| Random effects, | ||||||||
| Block 𝜎2 (%) | 22.1 | 15.9 | 0.4 | 16.6 | 16.2 | 12.8 | 11.5 | |
| Error 𝜎2 (%) | 77.9 | 84.1 | 99.6 | 83.4 | 83.8 | 87.2 | 88.5 |
| DF | LA | SLA | SN | SA | PSB | SPAD | REC | |
| Fixed effects | ||||||||
| Treatment | 1 | 1257.1 *** | 849.7 *** | 17.4 *** | 899.8 *** | 915.5 *** | 2674 *** | 2904 *** |
| Genotype | 49 | 28.6 *** | 4.8 *** | 5.9 *** | 5.5 *** | 4.3 *** | 10.8 *** | 2.2 *** |
| Interaction | 49 | 9.9 *** | 2.1 *** | 0.1 ns | 2.5 *** | 4.3 *** | 1.8 * | 1.8 *** |
| Random effects, | ||||||||
| Block 𝜎2 (%) | 1.9 | 0.8 | 2.1 | 3.9 | 0.2 | 8.9 | 14.6 | |
| Error 𝜎2 (%) | 98.1 | 99.2 | 97.9 | 96.1 | 99.8 | 91.1 | 85.4 |
| DF | CP | ADF | aNDF | TDN | NEL | NEM | NEG | RFV | DE | NFC | N (g) | |
| Fixed effects | ||||||||||||
| Treatment | 1 | 516.6 *** | 0.1 ns | 3.7 ns | 0.4 ns | 1.8 ns | 0.8 ns | 0.9 ns | 1.7 ns | 27.5 *** | 224.6 *** | 117.9 *** |
| Genotype | 49 | 2.9 *** | 3.1 *** | 3.7 *** | 3.4 *** | 3.8 *** | 3.5 *** | 3.5 *** | 3.5 *** | 3.9 *** | 3.7 *** | 3.1 *** |
| Interaction | ||||||||||||
| Random effects, | ||||||||||||
| Block 𝜎2 (%) | 40.9 | 18.3 | 23.2 | 24.3 | 22.2 | 23.7 | 22.3 | 23.2 | 21.8 | 24.6 | 22.3 | |
| Error 𝜎2 (%) | 59.1 | 81.7 | 76.8 | 75.7 | 77.8 | 76.3 | 77.7 | 76.8 | 78.2 | 75.4 | 77.7 |
References
- Alva, A.K.; Paramasivam, S.; Fares, A.; Delgado, J.A.; Mattos, D., Jr.; Sajwan, K. Nitrogen and irrigation management practices to improve nitrogen uptake efficiency and minimize leaching losses. J. Crop Improv. 2006, 15, 369–420. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Zhou, X.; Helmers, M.J.; Asbjornsen, H.; Kolka, R.; Tomer, M.D.; Cruse, R.M. Nutrient removal by prairie filter strips in agricultural landscapes. J. Soil Water Conserv. 2014, 69, 54–64. [Google Scholar] [CrossRef]
- Ssegane, H.; Negri, M.C.; Quinn, J.; Urgun-Demirtas, M. Multifunctional landscapes: Site characterization and field-scale design to incorporate biomass production into an agricultural system. Biomass Bioenergy 2015, 80, 179–190. [Google Scholar] [CrossRef]
- Ssegane, H.; Zumpf, C.; Cristina Negri, M.; Campbell, P.; Heavey, J.P.; Volk, T.A. The economics of growing shrub willow as a bioenergy buffer on agricultural fields: A case study in the Midwest Corn Belt. Biofuels Bioprod. Biorefining 2016, 10, 776–789. [Google Scholar] [CrossRef]
- Stettler, R.F. Cottonwood and the River of Time; University of Washington Press: Seattle, DC, USA, 2009. [Google Scholar]
- Rytter, R.; Hansson, A. Seasonal amount, growth and depth distribution of fine roots in an irrigated and fertilized Salix viminalis L. plantation. Biomass Bioenergy 1996, 11, 129–137. [Google Scholar] [CrossRef]
- Crow, P.; Houston, T. The influence of soil and coppice cycle on the rooting habit of short rotation poplar and willow coppice. Biomass Bioenergy 2004, 26, 497–505. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Muir, J.P.; Naumann, H.D.; Norris, A.B.; Ramírez-Restrepo, C.A.; Mertens-Talcott, S.U. Nutritional Aspects of Ecologically Relevant Phytochemicals in Ruminant Production. Front. Vet. Sci. 2021, 8, 155. [Google Scholar] [CrossRef]
- Villalba, J.J.; Provenza, F.D.; Gibson, N.; López-Ortíz, S. Veterinary medicine: The value of plant secondary compounds and diversity in balancing consumer and ecological health. In Sustainable Food Production Includes Human and Environmental Health; Springer: Dordrecht, The Netherlands, 2014; pp. 165–190. [Google Scholar] [CrossRef]
- Muklada, H.; Klein, J.D.; Glasser, T.A.; Dvash, L.; Azaizeh, H.; Halabi, N.; Davidovich-Rikanati, R.; Lewinsohn, E.; Landau, S.Y. Initial evaluation of willow (Salix acmophylla) irrigated with treated wastewater as a fodder crop for dairy goats. Small Rumin. Res. 2018, 163, 76–83. [Google Scholar] [CrossRef]
- Muklada, H.; Voet, H.; Deutch, T.; Zachut, M.; Kra, G.; Blum, S.E.; Krifuks, O.; Glasser, T.A.; Klein, J.D.; Davidovich-Rikanati, R.; et al. The effect of willow fodder feeding on immune cell populations in the blood and milk of late-lactating dairy goats. Animal 2020, 14, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Kemp, P.D.; Barry, T.N.; Douglas, G.B. Edible forage yield and nutritive value of poplar and willow. NZGA Res. Pract. Ser. 2003, 10, 53–63. [Google Scholar] [CrossRef]
- Douglas, G.B.; Bulloch, B.T.; Foote, A.G. Cutting management of willows (Salix spp.) and leguminous shrubs for forage during summer. N. Z. J. Agric. Res. 1996, 39, 175–184. [Google Scholar] [CrossRef]
- Moore, K.M.; Barry, T.N.; Cameron, P.N.; Lopez-Villalobos, N.; Cameron, D.J. Willow (Salix spp.) as a supplement for grazing cattle under drought conditions. Anim. Feed Sci. Technol. 2003, 104, 1–11. [Google Scholar] [CrossRef]
- McWilliam, E.L.; Barry, T.N.; Lopez-Villalobos, N.; Cameron, P.N.; Kemp, P.D. Effects of willow (Salix) versus poplar (Populus) supplementation on the reproductive performance of ewes grazing low quality drought pasture during mating. Anim. Feed Sci. Technol. 2005, 119, 69–86. [Google Scholar] [CrossRef]
- Boeckler, G.A.; Gershenzon, J.; Unsicker, S.B. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochemistry 2011, 72, 1497–1509. [Google Scholar] [CrossRef]
- Orians, C.M.; Griffiths, M.E.; Roche, B.M.; Fritz, R.S. Phenolic glycosides and condensed tannins in Salix sericea, S. eriocephala and their F1 hybrids: Not all hybrids are created equal. Biochem. Syst. Ecol. 2000, 28, 619–632. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, C.A.; Barry, T.N.; Marriner, A.; López-Villalobos, N.; McWilliam, E.L.; Lassey, K.R.; Clark, H. Effects of grazing willow fodder blocks upon methane production and blood composition in young sheep. Anim. Feed Sci. Technol. 2010, 155, 33–43. [Google Scholar] [CrossRef]
- McCabe, S.M.; Barry, T.N. Nutritive value of willow (Salix spp.) for sheep, goats and deer. J. Agric. Sci. 1988, 111, 1–9. [Google Scholar] [CrossRef]
- Carlson, C.H.; Smart, L.B. Electrical capacitance as a predictor of root dry weight in shrub willow (Salix; Salicaceae) parents and progeny. Appl. Plant Sci. 2016, 4, 1600031. [Google Scholar] [CrossRef]
- SAS Institute Inc. The SAS System for Windows; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Fabio, E.S.; Smart, L.B. Effects of nitrogen fertilization in shrub willow short rotation coppice production—A quantitative review. GCB Bioenergy 2013, 10, 548–564. [Google Scholar] [CrossRef]
- Serapiglia, M.J.; Gouker, F.E.; Smart, L.B. Early selection of novel triploid hybrids of shrub willow with improved biomass yield relative to diploids. BMC Plant Biol. 2014, 14, 74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mamashita, T.; Larocque, G.R.; DesRochers, A.; Beaulieu, J.; Thomas, B.R.; Mosseler, A.; Major, J.; Sidders, D. Short-term growth and morphological responses to nitrogen availability and plant density in hybrid poplars and willows. Biomass Bioenergy 2015, 81, 88–97. [Google Scholar] [CrossRef]
- Weih, M.; Nordh, N.E. Characterising willows for biomass and phytoremediation: Growth, nitrogen and water use of 14 willow clones under different irrigation and fertilisation regimes. Biomass Bioenergy 2002, 23, 397–413. [Google Scholar] [CrossRef]
- Bonosi, L.; Ghelardini, L.; Weih, M. Growth responses of 15 Salix genotypes to temporary water stress are different from the responses to permanent water shortage. Trees 2010, 24, 843–854. [Google Scholar] [CrossRef]
- Fabio, E.S.; Leary, C.J.; Smart, L.B. Tolerance of novel inter-specific shrub willow hybrids to water stress. Trees 2019, 33, 1015–1026. [Google Scholar] [CrossRef]
- Weih, M.; Rönnberg-Wästjung, A.C. Shoot biomass growth is related to the vertical leaf nitrogen gradient in Salix canopies. Tree Physiol. 2007, 27, 1551–1559. [Google Scholar] [CrossRef]
- Kemp, P.D.; Mackay, A.D.; Matheson, L.A.; Timmins, M.E. The forage value of poplars and willows. Proc. N. Z. Grassl. Assoc. 2001, 63, 115–119. [Google Scholar] [CrossRef]
- Hoffman, P.; Shaver, R. A Quick Guide to Understanding Forage Test Results. Available online: https://fyi.extension.wisc.edu/forage/a-quick-guide-to-understanding-forage-test-results/ (accessed on 3 August 2022).
- Ramírez-Restrepo, C.A.; Barry, T.N. Alternative temperate forages containing secondary compounds for improving sustainable productivity in grazing ruminants. Anim. Feed Sci. Technol. 2005, 120, 179–201. [Google Scholar] [CrossRef]
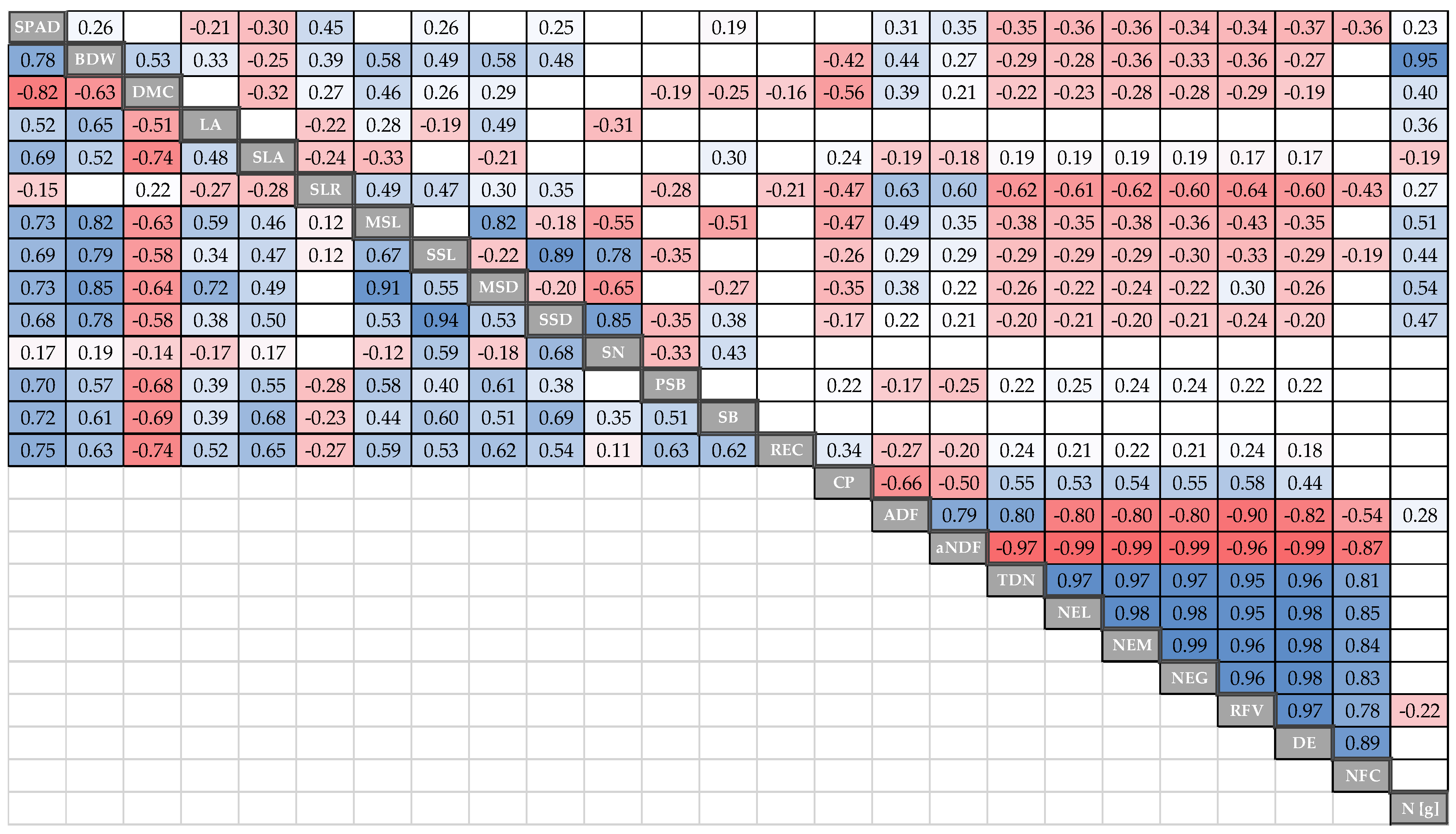


| Trait Abbreviation | Description | Units | Measured [Weeks] | No. of Observations |
|---|---|---|---|---|
| Biomass productivity | ||||
| SPAD | SPAD | SPAD units | 6, 8, 10, 12 | 1600 |
| BDW | Aboveground biomass dry weight | g plant−1 | 12 | 400 |
| SSL | Sum stem length | cm | 12 | 931 |
| MSL | Mean stem length | cm | 12 | 931 |
| PSB | Proportional sylleptic branch | 12 | 931 | |
| SA | Stem angle | degree | 12 | 931 |
| SN | Stem number | - | 12 | 400 |
| MSD | Mean stem diameter | mm | 12 | 931 |
| SSD | Sum stem diameter | mm | 12 | 931 |
| LA | Leaf area | cm2 | 12 | 1200 |
| SLA | Specific leaf area | cm2 g−1 | 12 | 1200 |
| SLR | Stem: leaf ratio | g g−1 | 12 | 400 |
| DMC | Dry matter content | g g−1 | 12 | 400 |
| Belowground | ||||
| REC | Root electrical capacitance | nF | 12 | 400 |
| Trait | Control | Fertilizer | |
|---|---|---|---|
| Biomass productivity | Mean + SE n = 200 | Mean + SE n = 200 | |
| BDW | 6.43 ± 0.41 | *** | 51.15 ± 1.57 |
| DMC | 0.39 ± 0.01 | *** | 0.26 ± 0.001 |
| SLR | 1.06 ± 0.02 | *** | 0.92 ± 0.02 |
| SML | 52.57 ± 1.91 | *** | 121.39 ± 2.2 |
| SSL | 110.59 ± 4.51 | *** | 285.05 ± 7.56 |
| MSD | 3.99 ± 0.08 | *** | 7.68 ± 0.13 |
| SSD | 8.4 ± 0.22 | *** | 17.97 ± 0.44 |
| Morphological characteristics | n = 200 | n = 200 | |
| LA | 6.3 ± 0.29 | *** | 20.64 ± 0.91 |
| SLA | 118.55 ± 1.79 | *** | 180.09 ± 1.99 |
| SN | 2.18 ± 0.06 | *** | 2.49 ± 0.07 |
| SA | 3.69 ± 0.68 | *** | 34.46 ± 1.18 |
| PSB | 0.01 ± 0.01 | *** | 0.31 ± 0.01 |
| SPAD 5/20 | 20.67 ± 0.47 | *** | 51.31 ± 0.38 |
| SPAD | 27.82 ± 0.38 | *** | 44.45 ± 0.32 |
| REC | 8.68 ± 0.23 | *** | 56.29 ± 1.66 |
| Nutritional value | n = 10 | n = 150 | |
| CP | 4.54 ± 0.18 | *** | 19.46 ± 0.19 |
| ADF | 40.33 ± 1.12 | 38.75 ± 0.36 | |
| NDF | 47.01 ± 1.11 | 47.07 ± 0.39 | |
| TDN | 60.4 ± 0.4 | 60.9 ± 0.12 | |
| NEL | 0.61 ± 0.01 | 0.61 ± 0.002 | |
| NEM | 0.57 ± 0.01 | 0.58 ± 0.002 | |
| NEG | 0.32 ± 0.01 | 0.32 ± 0.002 | |
| RFV | 114.4 ± 4.31 | 117.75 ± 1.44 | |
| DE | 1.12 ± 0.01 | *** | 1.07 ± 0.005 |
| NFC | 38.43 ± 1.16 | *** | 23.47 ± 0.34 |
| N | 17.84 ± 1.35 | *** | 161.39 ± 4.85 |
| Effect | DF | F Value | p Value |
|---|---|---|---|
| Clone | 49 | 11.79 | <0.0001 |
| Trmt | 1 | 5153.15 | <0.0001 |
| Time | 1 | 5.81 | 0.0201 |
| Time × Trmt | 1 | 3561.32 | <0.0001 |
| Time × Clone | 49 | 2.88 | <0.0001 |
| Clone × Trmt | 49 | 2.33 | <0.0001 |
| Time × Time | 1 | 24.08 | <0.0001 |
| Time × Time × Trmt | 1 | 663.25 | <0.0001 |
| Time × Time × Clone | 49 | 1.51 | 0.0223 |
| Time × Time × Clone × Trmt | 49 | 2.61 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muklada, H.; Fabio, E.S.; Smart, L.B. Growth, Nitrogen Uptake, and Nutritional Value of a Diverse Panel of Shrub Willow (Salix spp.) Genotypes in Response to Nitrogen Fertilization. Agronomy 2022, 12, 2678. https://doi.org/10.3390/agronomy12112678
Muklada H, Fabio ES, Smart LB. Growth, Nitrogen Uptake, and Nutritional Value of a Diverse Panel of Shrub Willow (Salix spp.) Genotypes in Response to Nitrogen Fertilization. Agronomy. 2022; 12(11):2678. https://doi.org/10.3390/agronomy12112678
Chicago/Turabian StyleMuklada, Hussein, Eric S. Fabio, and Lawrence B. Smart. 2022. "Growth, Nitrogen Uptake, and Nutritional Value of a Diverse Panel of Shrub Willow (Salix spp.) Genotypes in Response to Nitrogen Fertilization" Agronomy 12, no. 11: 2678. https://doi.org/10.3390/agronomy12112678
APA StyleMuklada, H., Fabio, E. S., & Smart, L. B. (2022). Growth, Nitrogen Uptake, and Nutritional Value of a Diverse Panel of Shrub Willow (Salix spp.) Genotypes in Response to Nitrogen Fertilization. Agronomy, 12(11), 2678. https://doi.org/10.3390/agronomy12112678






