Effects of Strip-Till and Simultaneous Fertilization at Three Soil Depths on Soil Biochemical and Biological Properties
Abstract
1. Introduction
- I.
- Soil organic matter (SOM) and exogenous organic matter (EOM) degradation rates will be primarily most enhanced in topsoil, although long-term, enhanced activity in topsoil is likely to reduce enzyme activities compared to mid-depth (10–15 cm) soil, due to the consumption of easily utilizable sources and leaching of nutrients from the surface to deeper soil layers.
- II.
- Respiratory carbon mineralization will be highest in topsoil during the whole growing season due to the best aeration and the highest biomass of aerobic microbes.
- III.
- Due to the strip-till application of digestate to the entire soil profile, increased microbial activities will extend to the greatest depth at the highest digestate dose.
- IV.
- Nitrogen mineralization will be lower compared to carbon mineralization at the greatest depth, putatively due to the limited content of organic nitrogen in the digestate and the oxygen-dependency of nitrification processes.
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil Sampling and Measurements
2.3. Statistical Analysis
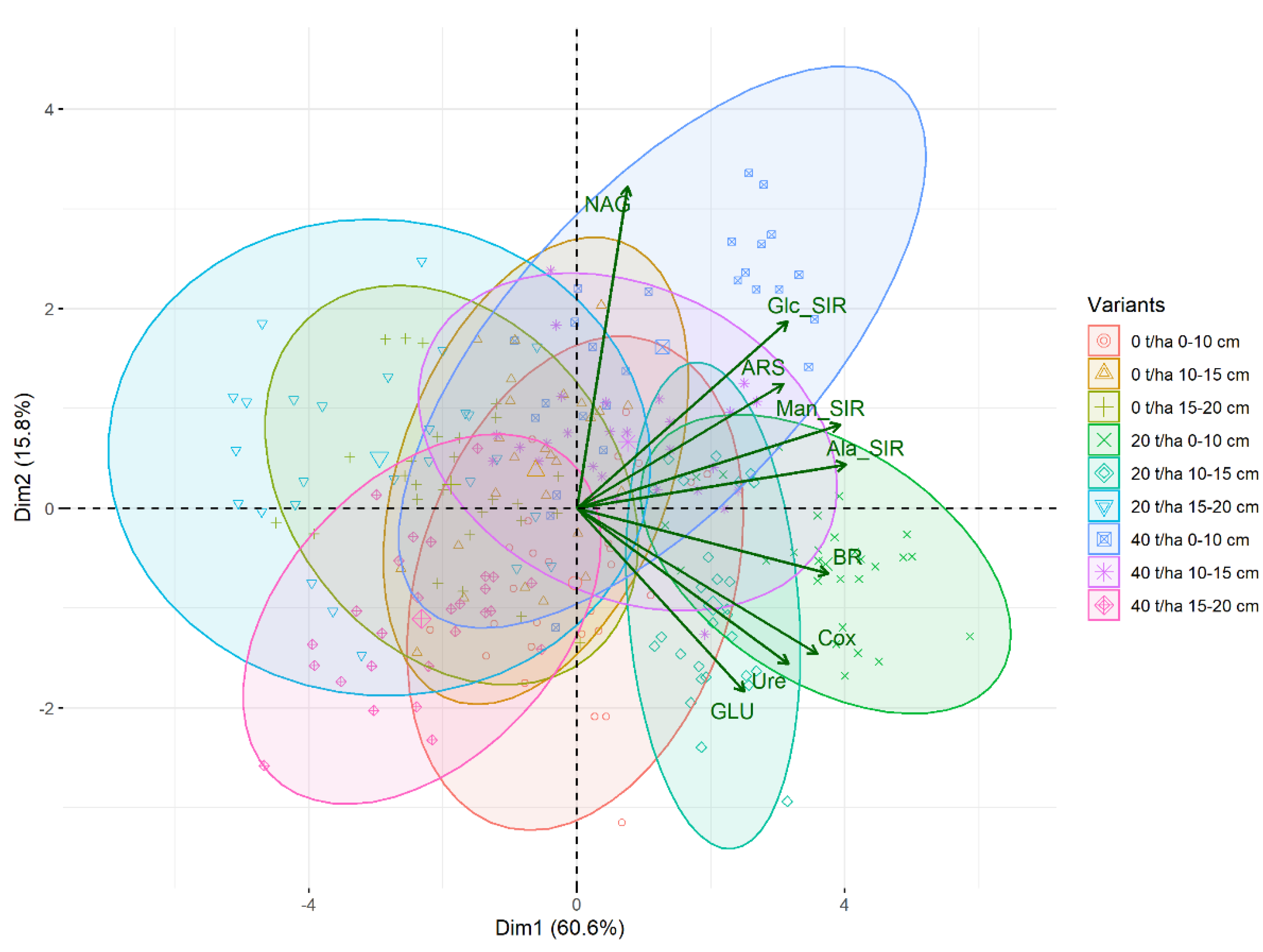
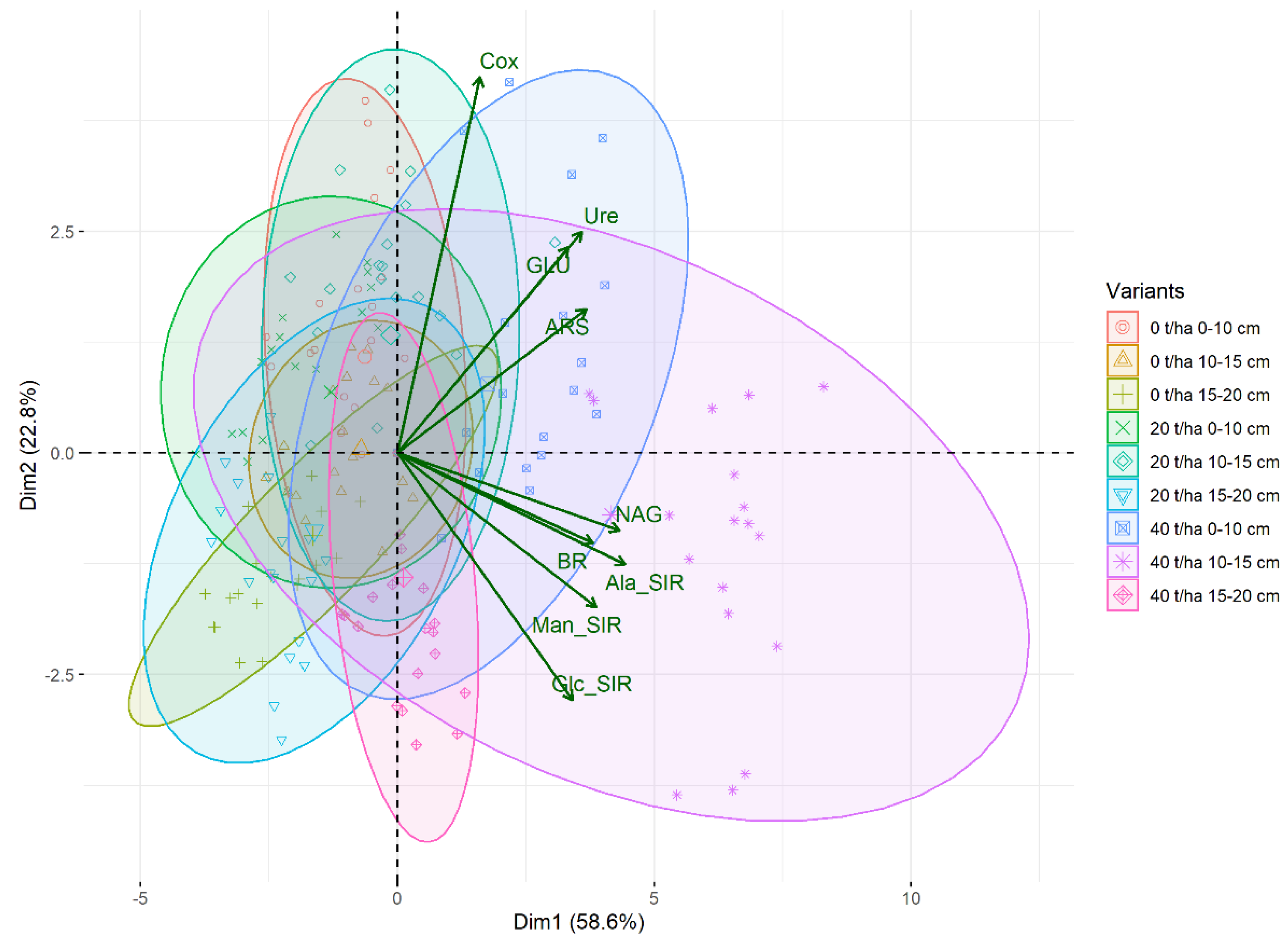
3. Results
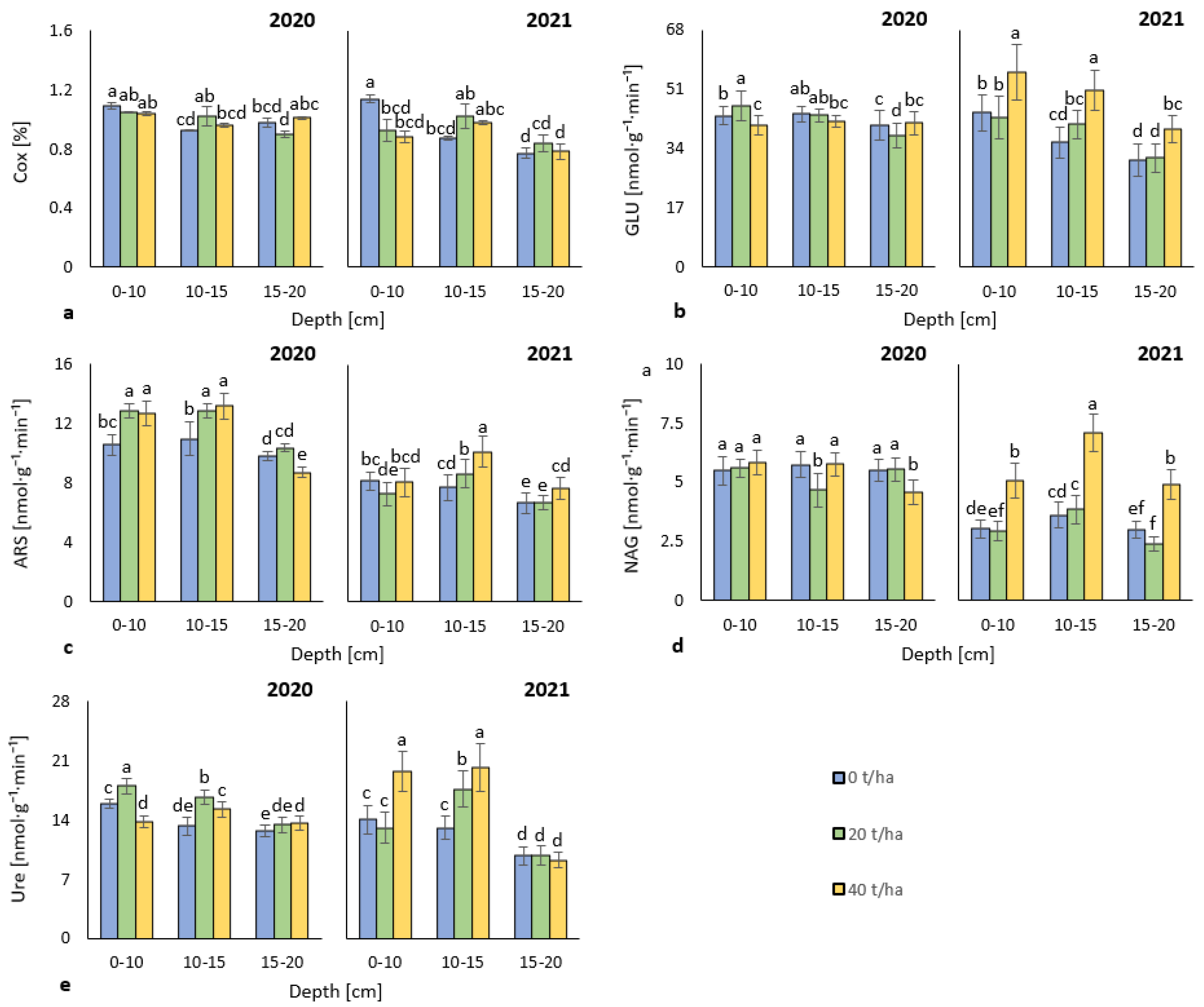
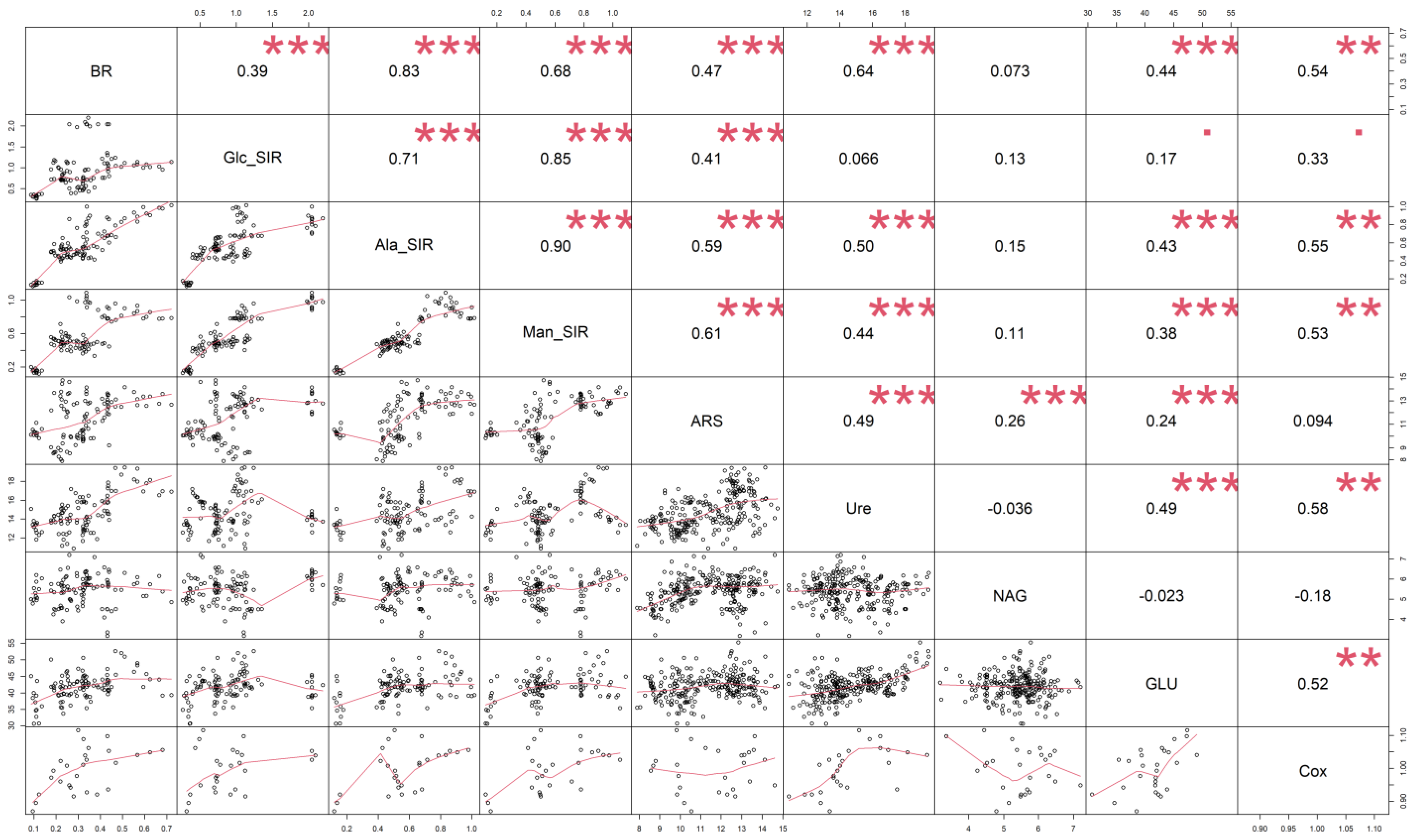
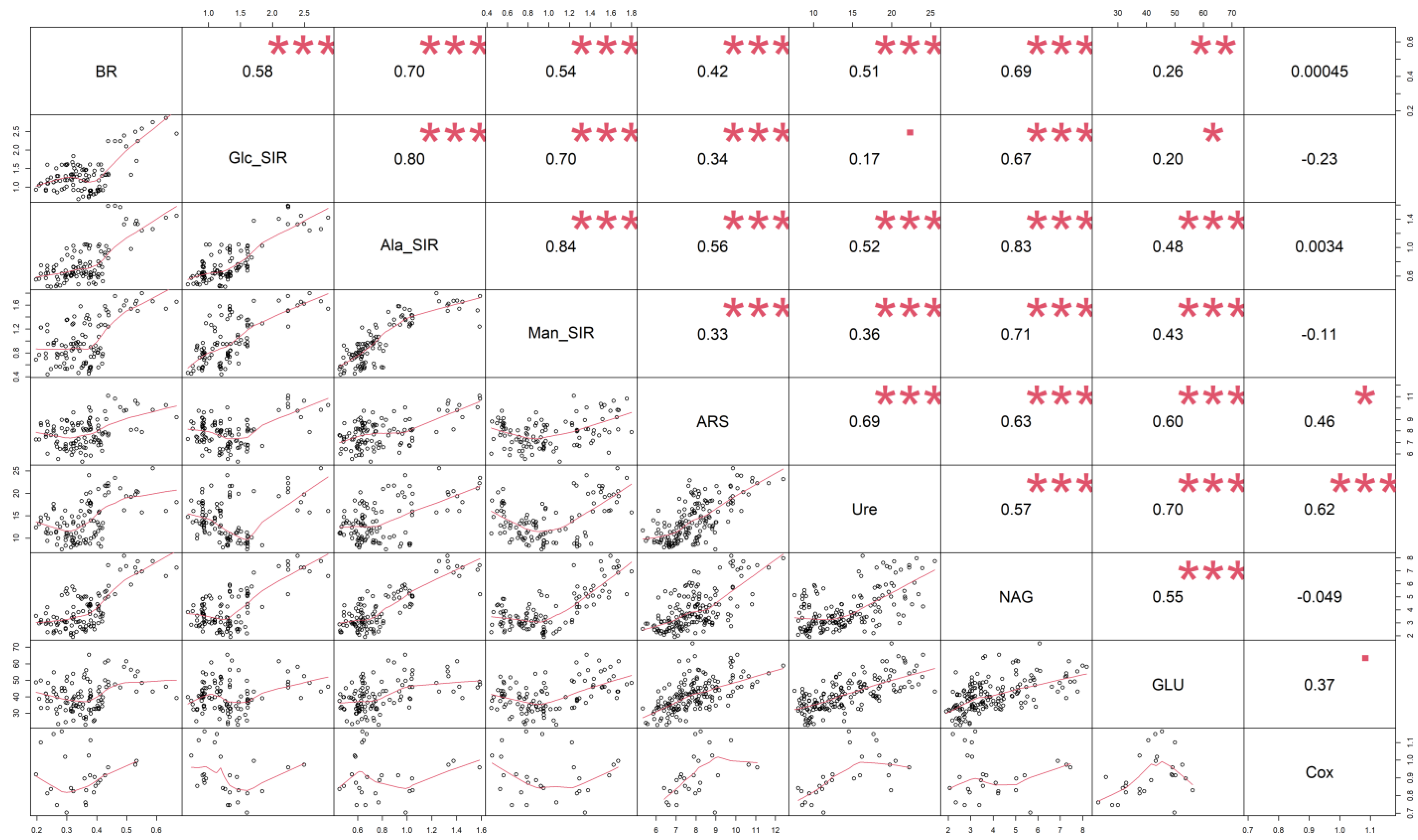
3.1. Soil Oxidizable Carbon and Enzyme Activities
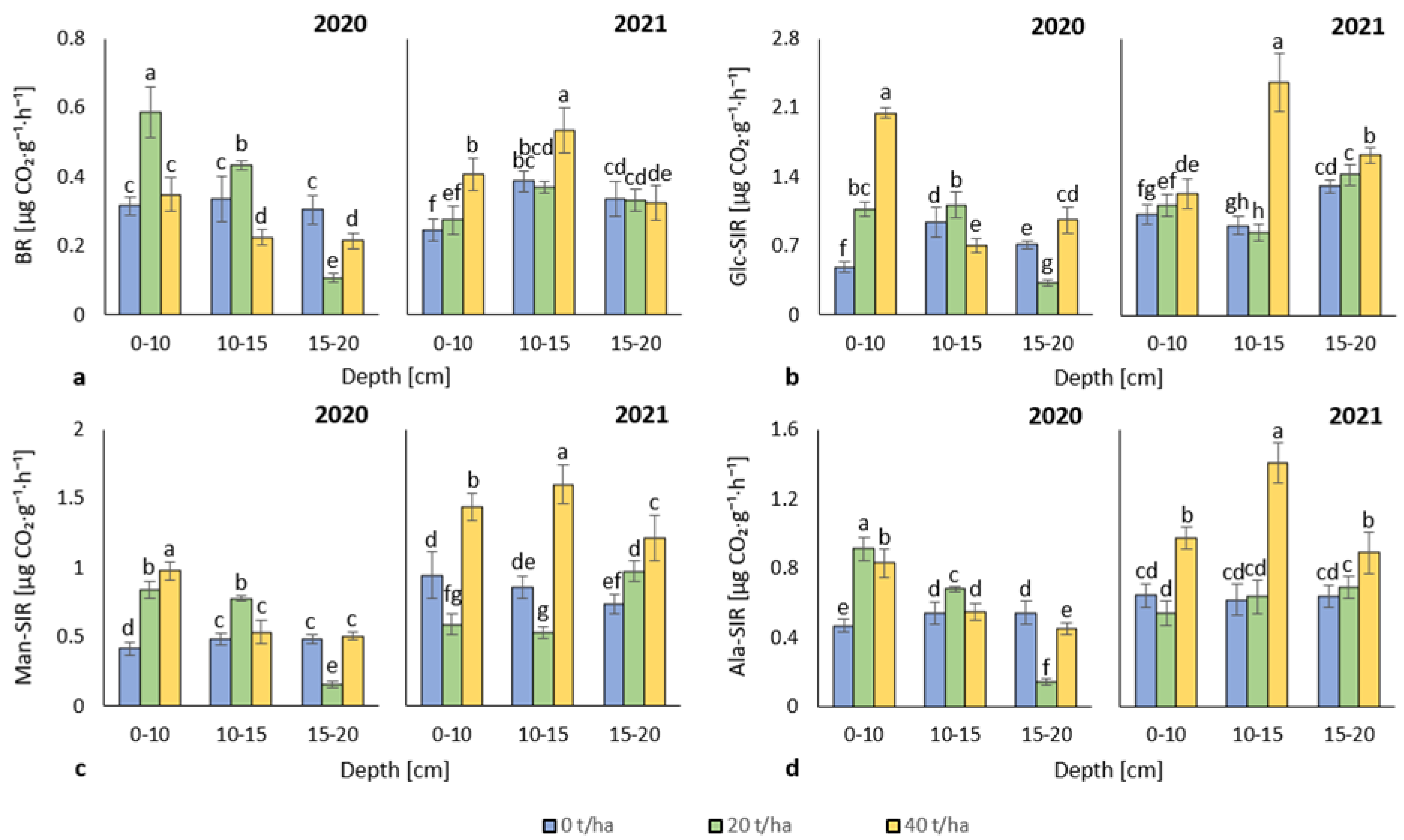
3.2. Soil Respiration
4. Discussion
4.1. Soil Oxidizable Carbon and Enzyme Activities
4.2. Soil Respiration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, D.; Huang, Y.; An, S.; Sun, H.; Bhople, P.; Chen, Z. Soil physicochemical and microbial characteristics of contrasting land-use types along soil depth gradients. Catena 2018, 162, 345–353. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Velmourougane, K.; Bhattacharyya, T.; Sarkar, D.; Pal, D.K.; Prasad, J.; Sidhu, G.S.; Nair, K.M.; Sahoo, A.K.; Das, T.H.; et al. Impacts of agro-climates and land use systems on culturable microbial population in soils of the Indo-Gangetic Plains, India. Curr. Sci. 2014, 107, 1464–1469. [Google Scholar]
- Shrestha, R.K.; Lal, R. Land use impacts on physical properties of 28 years old reclaimed mine soils in Ohio. Plant Soil 2008, 306, 249–260. [Google Scholar] [CrossRef]
- Reza, S.K.; Baruah, U.; Nayak, D.C.; Dutta, D.; Singh, S.K. Effects of Land-use on Soil Physical, Chemical and Microbial Properties in Humid Subtropical Northeastern India. Natl. Acad. Sci. Lett. 2018, 41, 141–145. [Google Scholar] [CrossRef]
- Celik, I.; Barut, Z.B.; Ortas, I.; Gok, M.; Demirbas, A.; Tulun, Y.; Akpinar, C. Impacts of different tillage practices on some soil microbiological properties and crop yield under semi-arid Mediterranean conditions. Int. J. Plant Prod. 2011, 5, 237–254. [Google Scholar]
- Miura, F.; Nakamoto, T.; Kaneda, S.; Okano, S.; Nakajima, M.; Murakami, T. Dynamics of soil biota at different depths under two contrasting tillage practices. Soil Biol. Biochem. 2008, 40, 406–414. [Google Scholar] [CrossRef]
- Sun, B.; Jia, S.; Zhang, S.; McLaughlin, N.B.; Zhang, X.; Liang, A.; Chen, X.; Wei, S.; Liu, S. Tillage, seasonal and depths effects on soil microbial properties in black soil of Northeast China. Soil Tillage Res. 2016, 155, 421–428. [Google Scholar] [CrossRef]
- Yadav, D.; Rani, K.; Wati, L. Impact of tillage practices on physico-chemical and microbiological properties of soil in wheat-pearl-millet cropping system. Range Manag. Agrofor. 2020, 41, 276–283. [Google Scholar]
- Lavrenko, S.O.; Lavrenko, N.M.; Maksymov, D.O.; Maksymov, M.V.; Didenko, N.O.; Islam, K.R. Variable tillage depth and chemical fertilization impact on irrigated common beans and soil physical properties. Soil Tillage Res. 2021, 212, 105024. [Google Scholar] [CrossRef]
- Vrtilek, P.; Smutny, V.; Neudert, L. Evaluation of the Impact of Different Soil Tillage on Physical Soil Properties. In Proceedings of the 24th International PhD Students Conference for Undergraduate and Postgraduate Students (MendelNet), Mendel Univ Brno, Fac AgriSciences, Brno, Czech Republic, 8–9 November 2017; pp. 164–168. [Google Scholar]
- Álvaro-Fuentes, J.; Morell, F.J.; Madejón, E.; Lampurlanés, J.; Arrúe, J.L.; Cantero-Martínez, C. Soil biochemical properties in a semiarid Mediterranean agroecosystem as affected by long-term tillage and N fertilization. Soil Tillage Res. 2013, 129, 69–74. [Google Scholar] [CrossRef]
- Mallory, J.J.; Mohtar, R.H.; Heathman, G.C.; Schulze, D.G.; Braudeau, E. Evaluating the effect of tillage on soil structural properties using the pedostructure concept. Geoderma 2011, 163, 141–149. [Google Scholar] [CrossRef]
- Helgason, B.L.; Walley, F.L.; Germida, J.J. Fungal and Bacterial Abundance in Long-Term No-Till and Intensive-Till Soils of the Northern Great Plains. Soil Sci. Soc. Am. J. 2009, 73, 120–127. [Google Scholar] [CrossRef]
- van Groenigen, K.-J.; Bloem, J.; Bååth, E.; Boeckx, P.; Rousk, J.; Bodé, S.; Forristal, D.; Jones, M.B. Abundance, production and stabilization of microbial biomass under conventional and reduced tillage. Soil Biol. Biochem. 2010, 42, 48–55. [Google Scholar] [CrossRef]
- Godsey, C.; Kochenower, R.; Taylor, R. Strip-till Considerations in Oklahoma. Okla. Coop. Ext. Serv. 2017, 2, PSS-2134. [Google Scholar]
- Bakhitova, A.R.; Mamontov, V.G.; Enakiev, Y.I.; Stefanov, K.G. Variability of Soil Nutritional Parameters in Small-plot Experiment with Mineral Fertilizers Application in Different Depth. In Proceedings of the 7th International Conference on Energy Efficiency and Agricultural Engineering (EE and AE), Ruse, Bulgaria, 12–14 November 2020.
- Ozdemir, N.; Yakupoglu, T.; Dengiz, O. The effects of bio-solid and tea waste application into different levels of eroded soil on N, P and K concentrations. Environ. Monit. Assess. 2008, 156, 109. [Google Scholar] [CrossRef]
- USDA. Conservation practice definitions and their corresponding potential to adversely impact cultural resources. Nat. Resour. Conserv. Serv. 2013, 11. [Google Scholar]
- Koszel, M.; Lorencowicz, E. Agricultural Use of Biogas Digestate as a Replacement Fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 119–124. [Google Scholar] [CrossRef]
- Slepetiene, A.; Volungevicius, J.; Jurgutis, L.; Liaudanskiene, I.; Amaleviciute-Volunge, K.; Slepetys, J.; Ceseviciene, J. The potential of digestate as a biofertilizer in eroded soils of Lithuania. Waste Manag. 2020, 102, 441–451. [Google Scholar] [CrossRef]
- ISO_14235:1998; Soil quality—Determination of organic carbon by sulfochromic oxidation. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO_20130:2018; Soil quality—Measurement of enzyme activity patterns in soil samples using colorimetric substrates in micro-well plates. International Organization for Standardization: Geneva, Switzerland, 2018.
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Duffkova, R.; Macurova, H. Soil biological quantity and quality parameters of grasslands in various landscape zones. Plant Soil Environ. 2011, 57, 577–582. [Google Scholar] [CrossRef]
- Diederich, K.M.; Ruark, M.D.; Krishnan, K.; Arriaga, F.J.; Silva, E.M. Increasing Labile Soil Carbon and Nitrogen Fractions Require a Change in System, Rather Than Practice. Soil Sci. Soc. Am. J. 2019, 83, 1733–1745. [Google Scholar] [CrossRef]
- Ma, X.Z.; Chen, L.J.; Chen, Z.H.; Wu, Z.J.; Zhang, L.L.; Zhang, Y.L. Soil glycosidase activities and water soluble organic carbon under different land use types. Rev. Cienc. Suelo Y Nutr. Veg. 2010, 10, 93–101. [Google Scholar] [CrossRef][Green Version]
- Pereira, A.A.; Borges, A.C.; Matos, M.P.; Diniz, I.C.C.; Matos, A.T. Degradation rate of anaerobically digested sewage sludge in soil. J. Water Sanit. Hyg. Dev. 2018, 8, 17–26. [Google Scholar] [CrossRef]
- Musatti, A.; Ficara, E.; Mapelli, C.; Sambusiti, C.; Rollini, M. Use of solid digestate for lignocellulolytic enzymes production through submerged fungal fermentation. J. Environ. Manag. 2017, 199, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Plaza, C.; Hernández, D.; García-Gil, J.C.; Polo, A. Microbial activity in pig slurry-amended soils under semiarid conditions. Soil Biol. Biochem. 2004, 36, 1577–1585. [Google Scholar] [CrossRef]
- Meyer, A.H.; Wooldridge, J.; Dames, J.F. Variation in urease and β-glucosidase activities with soil depth and root density in a ‘Cripp’s Pink’/M7 apple orchard under conventional and organic management. S. Afr. J. Plant Soil 2015, 32, 227–234. [Google Scholar] [CrossRef]
- Tabatabai, M.; Bremner, J. Factors affecting soil arylsulfatase activity. Soil Sci. Soc. Am. J. 1970, 34, 427–429. [Google Scholar] [CrossRef]
- Deng, S.P.; Tabatabai, M.A. Effect of tillage and residue management on enzyme activities in soils: III. Phosphatases and arylsulfatase. Biol. Fertil. Soils 1997, 24, 141–146. [Google Scholar] [CrossRef]
- Balota, E.L.; Machineski, O.; Truber, P.V. Soil enzyme activities under pig slurry addition and different tillage systems. Acta Scientiarum. Agron. 2011, 33, 729–737. [Google Scholar] [CrossRef]
- Lalande, R.; Gagnon, B.; Simard, R.R.; Côté, D. Soil microbial biomass and enzyme activity following liquid hog manure application in a long-term field trial. Can. J. Soil Sci. 2000, 80, 263–269. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Siles, J.A.; Cajthaml, T.; García-Romera, I.; Tlustoš, P.; Száková, J. Effect of digestate and fly ash applications on soil functional properties and microbial communities. Eur. J. Soil Biol. 2015, 71, 1–12. [Google Scholar] [CrossRef]
- Li, N.; Xu, Y.-Z.; Han, X.-Z.; He, H.-B.; Zhang, X.-d.; Zhang, B. Fungi contribute more than bacteria to soil organic matter through necromass accumulation under different agricultural practices during the early pedogenesis of a Mollisol. Eur. J. Soil Biol. 2015, 67, 51–58. [Google Scholar] [CrossRef]
- Wang, L.L.; Li, W.Z.; Wang, Z.J.; Wang, Z.W.; Sui, C.; Li, Y. Effects of digestate application depth on soil nitrogen volatilization and vertical distribution. Int. J. Agric. Biol. Eng. 2016, 9, 101–107. [Google Scholar] [CrossRef]
- Hu, X.; Liu, H.; Xu, C.; Huang, X.; Jiang, M.; Zhuang, H.; Huang, L. Effect of Digestate and Straw Combined Application on Maintaining Rice Production and Paddy Environment. Int. J. Environ. Res. Public Health 2021, 18, 5714. [Google Scholar] [CrossRef] [PubMed]
- Elbl, J.; Simeckova, J.; Skarpa, P.; Kintl, A.; Brtnicky, M.; Vaverkova, M.D. Comparison of the Agricultural Use of Products from Organic Waste Processing with Conventional Mineral Fertilizer: Potential Effects on Mineral Nitrogen Leaching and Soil Quality. Agronomy 2020, 10, 226. [Google Scholar] [CrossRef]
- Habova, M.; Vlcek, V.; Simeckova, J.; Hybler, V.; Pospisilova, L.; Jandak, J. Response of microbial associations to fertilizers application. In Proceedings of the 23rd International PhD Students Conference (MendelNet), Mendel Univ Brno, Fac AgriSciences, Brno, Czech Republic, 9–10 November 2016; pp. 411–416. [Google Scholar]
- Fang, C.; Moncrieff, J.B. The variation of soil microbial respiration with depth in relation to soil carbon composition. Plant Soil 2005, 268, 243–253. [Google Scholar] [CrossRef]
- Spohn, M.; Chodak, M. Microbial respiration per unit biomass increases with carbon-to-nutrient ratios in forest soils. Soil Biol. Biochem. 2015, 81, 128–133. [Google Scholar] [CrossRef]
- Horta, C.; Carneiro, J.P. Use of Digestate as Organic Amendment and Source of Nitrogen to Vegetable Crops. Appl. Sci. 2021, 12, 248. [Google Scholar] [CrossRef]
- Greenberg, I.; Kaiser, M.; Gunina, A.; Ledesma, P.; Polifka, S.; Wiedner, K.; Mueller, C.W.; Glaser, B.; Ludwig, B. Substitution of mineral fertilizers with biogas digestate plus biochar increases physically stabilized soil carbon but not crop biomass in a field trial. Sci Total Environ. 2019, 680, 181–189. [Google Scholar] [CrossRef]
| Year | pH (-) | Cox (%) | Ntot (%) | |
|---|---|---|---|---|
| 2020 | Mean | 6.87 | 1.09 | 0.16 |
| SD | 0.09 | 0.02 | 0.00 | |
| 2021 | Mean | 7.14 | 1.14 | 0.14 |
| SD | 0.02 | 0.03 | 0.00 |
| Year | DM (%) | N/NH4 in FM (g∙kg−1) | N/NO3 in FM (g∙kg−1) | Nmin in FM (g∙kg−1) | P in FM (g∙kg−1) | K in FM (g∙kg−1) | |
|---|---|---|---|---|---|---|---|
| 2020 | Mean | 3.943 | 1.514 | 0.491 | 2.005 | 0.159 | 3.738 |
| SD | 0.080 | 0.057 | 0.017 | 0.074 | 0.010 | 0.217 | |
| 2021 | Mean | 7.100 | 3.390 | 0.305 | 3.695 | 0.408 | 2.953 |
| SD | 0.377 | 0.036 | 0.005 | 0.033 | 0.024 | 0.151 | |
| 2021 vs. 2020 | Relative values | 1.8 | 2.2 | 0.6 | 1.8 | 2.6 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holatko, J.; Hammerschmiedt, T.; Kintl, A.; Kucerik, J.; Malicek, O.; Latal, O.; Baltazar, T.; Brtnicky, M. Effects of Strip-Till and Simultaneous Fertilization at Three Soil Depths on Soil Biochemical and Biological Properties. Agronomy 2022, 12, 2597. https://doi.org/10.3390/agronomy12112597
Holatko J, Hammerschmiedt T, Kintl A, Kucerik J, Malicek O, Latal O, Baltazar T, Brtnicky M. Effects of Strip-Till and Simultaneous Fertilization at Three Soil Depths on Soil Biochemical and Biological Properties. Agronomy. 2022; 12(11):2597. https://doi.org/10.3390/agronomy12112597
Chicago/Turabian StyleHolatko, Jiri, Tereza Hammerschmiedt, Antonin Kintl, Jiri Kucerik, Ondrej Malicek, Oldrich Latal, Tivadar Baltazar, and Martin Brtnicky. 2022. "Effects of Strip-Till and Simultaneous Fertilization at Three Soil Depths on Soil Biochemical and Biological Properties" Agronomy 12, no. 11: 2597. https://doi.org/10.3390/agronomy12112597
APA StyleHolatko, J., Hammerschmiedt, T., Kintl, A., Kucerik, J., Malicek, O., Latal, O., Baltazar, T., & Brtnicky, M. (2022). Effects of Strip-Till and Simultaneous Fertilization at Three Soil Depths on Soil Biochemical and Biological Properties. Agronomy, 12(11), 2597. https://doi.org/10.3390/agronomy12112597








