Phytotoxic Effects of Three Origanum Species Extracts and Essential Oil on Seed Germinations and Seedling Growths of Four Weed Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Isolation of Essential Oils
2.2. GC–MS Analysis
2.3. Seed Germination and Seedling Growth Experiments In Vivo and In Vitro Conditions
2.4. In Greenhouse Conditions
2.5. Statistical Analysis
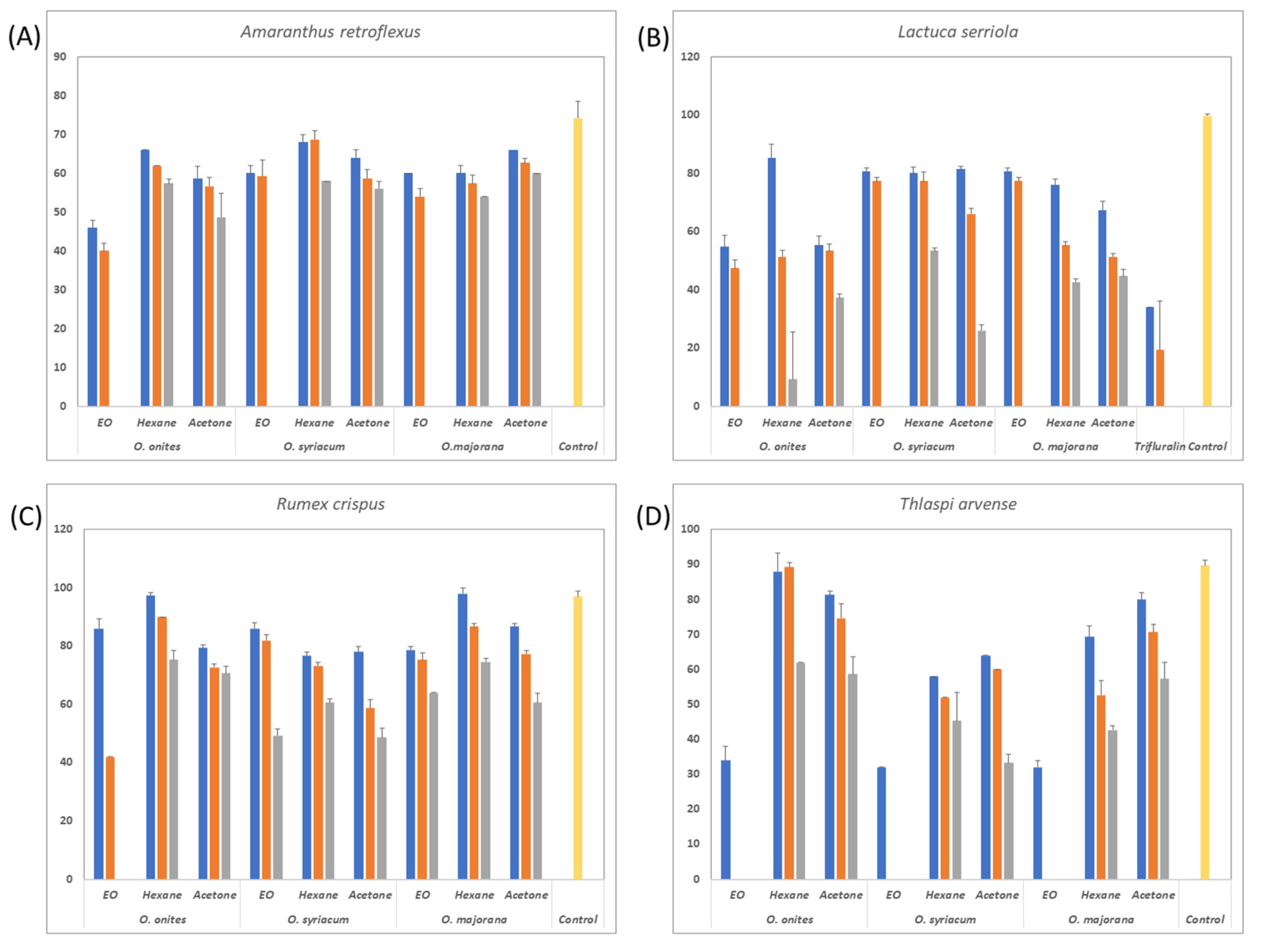
3. Results
3.1. Chemical Composition of the Essential Oils
3.2. Herbicidal Effects of the Oil and Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chauhan, B.S. Grand challenges in weed management. Front. Agron. 2020, 1, 1–4. [Google Scholar] [CrossRef]
- Majrashi, A.A. Preliminary assessment of weed population in vegetable and fruit farms of Taif, Saudi Arabia. Braz. J. Biol. 2022, 82, 1–9. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development strategies and prospects of nano-based smart pesticide formulation. J. Agric. Food Chem. 2018, 66, 6504–6512. [Google Scholar] [CrossRef]
- Liang, J.; Yu, M.; Guo, L.; Cui, B.; Zhao, X.; Sun, C.; Wang, Y.; Liu, G.; Cui, H.; Zeng, Z. Bioinspired development of P(St–MAA)–avermectin nanoparticles with high affinity for foliage to enhance folia retention. J. Agric. Food Chem. 2018, 66, 6578–6584. [Google Scholar] [CrossRef]
- Sarkar, M.R.; Rashid, M.H.O.; Rahman, A.; Kafi, M.A.; Hosen, M.I.; Rahman, M.S.; Khan, M.N. Recent advances in nanomaterials based sustainable agriculture: An overview. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100687. [Google Scholar]
- Islam, F.; Wang, J.; Farooq, M.A.; Khan, M.S.; Xu, L.; Zhu, J.; Zhao, M.; Stéphane, M. Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ. Int. 2018, 111, 332–351. [Google Scholar] [CrossRef]
- Böcker, T.; Möhring, N.; Finger, R. Herbicide free agriculture? A bio-economic modelling application to Swiss wheat production. Agric. Syst. 2019, 173, 378–392. [Google Scholar] [CrossRef]
- Tandon, S.; Pant, R. Kinetics of diuron under aerobic condition and residue analysis in sugarcane under subtropical field conditions. Environ. Technol. 2019, 40, 86–93. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Jena, H.M. A systemic assessment of the environmental impacts and remediation strategies for chloroacetanilide herbicides. J. Water Process Eng. 2019, 31, 100860. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Romagni, J.G.; Rimando, A.M. Natural Products as Sources of Herbicides: Current Status and Future Trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Chhokar, R.S.; Sharma, R.K.; Chauhan, D.S.; Mongia, A.D. Evaluation of herbicides against Phalaris minor in wheat in north-western. Indian plains. Weed Res. 2006, 46, 40–49. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Sutay, S. Inhibitory Effects of Monoterpenes on Seed Germination and Seedling Growth. Z. Naturforsch. C 2007, 62, 207–214. [Google Scholar] [CrossRef]
- Salamci, E.; Kordali, S.; Kotan, R.; Cakir, A.; Kaya, Y. Chemical Composition, Antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem. Syste. Ecol. 2007, 35, 569–581. [Google Scholar] [CrossRef]
- Kordali, S.; Usanmaz, A.; Cakir, A.; Komaki, A.; Ercisli, S. Antifungal and herbicidal effects of fruit essential oils of four Myrtus communis genotypes. Chem. Biodivers. 2016, 13, 77–84. [Google Scholar] [CrossRef]
- Hazrati, H.; Saharkhiz, M.J.; Niakousari, M.; Moein, M. Natural herbicide activity of Satureja hortensis L. essential oil nanoemulsion on the seed germination and morphophysiological features of two important weed species. Ecotoxicol. Environ. Saf. 2017, 142, 423–430. [Google Scholar] [CrossRef]
- Kaab, S.B.; Lins, L.; Hanafi, M.; Rebey, I.B.; Deleu, M.; Fauconnier, M.L.; Ksouri, R.; Jijacli, M.H.; De Clerck, C. Cynara cardunculus crude extract as a powerful natural herbicide and insight into the mode of action of its bioactive molecules. Biomolecules 2020, 10, 1–17. [Google Scholar]
- Bozhuyuk, A.U. Herbicidal Activity and Chemical Composition of Two Essential Oils on Seed Germinations and Seedling Growths of Three Weed Species. J. Essent. Oil Bear. Plants 2020, 23, 821–831. [Google Scholar] [CrossRef]
- De Mastro, G.; El Mahdi, J.; Ruta, C. Bioherbicidal potential of the essential oils from mediterranean lamiaceae for weed control in organic farming. Plants 2021, 10, 818. [Google Scholar] [CrossRef]
- Taban, A.; Somayeh Rastegar, S.; Nasirzadeh, M.; Saharkhiz, M.J. Essential oil composition and comparative phytotoxic activity of fennel, summer savory, Mexican marigold and feverfew: A potential bioherbicide. Vegetos 2022, 35, 502–510. [Google Scholar] [CrossRef]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Barton, A.F.M.; Dell, B.; Knight, A.R. Herbicidal activity of cineole derivatives. J. Agric. Food Chem. 2010, 58, 10147–10155. [Google Scholar] [CrossRef]
- Jouini, A.; Verdeguer, M.; Pinton, S.; Araniti, F.; Palazzolo, E.; Badalucco, L.; Laudicina, V.A. Potential effects of essential oils extracted from mediterranean aromatic plants on target weeds and soil microorganisms. Plants 2020, 9, 1289. [Google Scholar] [CrossRef]
- Gokturk, T.; Chachkhiani-Anasashvili, N.; Kordali, S.; Dumbadze, G.; Bozhuyuk, A.U. Insecticidal effects of some essential oils against box tree moth (Cydalima perspectalis Walker (Lepidoptera: Crambidae)). Int. J. Trop. Insect Sci. 2020, 40, 637–643. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sanchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A Review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Dirmenci, T.; Yazici, T.; Özcan, T.; Çelenk, S.; Martin, E. A New Species and a New Natural Hybrid of Origanum L. (Lamiaceae) from the West of Turkey. Turk. J. Bot. 2018, 42, 73–90. [Google Scholar] [CrossRef]
- Dirmenci, T.; Özcan, T.; Yazıcı, T.; Arabacı, T.; Martin, E. Morphological, cytological, palynological and molecular evidence on two new hybrids: An example of homoploid hybridization in Origanum (Lamiaceae). Phytotaxa 2018, 371, 145–167. [Google Scholar] [CrossRef]
- Dirmenci, T.; Özcan, T.; Acar, M.; Arabacı, T.; Yazıcı, T.; Martin, E. A rearranged homoploid hybrid species of Origanum (Lamiaceae): O. × munzurense Kit Tan & Sorger. Bot. Lett. 2019, 166, 153–162. [Google Scholar]
- Arabaci, T.; Dirmenci, T.; Yıldız, B. A New Hybrid of the Genus Origanum L. (Lamiaceae): Origanum × malatyanum. Bagbahce Bilim Derg. 2020, 7, 10–15. [Google Scholar]
- Baytop, T. Türkiye’de Bitkiler ile Tedavi, Geçmişte ve Bugün; Nobel Tıp Kitabevleri: İstanbul, Türkiye, 1999. [Google Scholar]
- Bozdemir, Ç. Economic Importance and Usage Fields of Oregano Species Growing in Turkey. Yüzüncü Yil Üniv. J. Agr. Sci. 2019, 29, 583–594. [Google Scholar]
- TÜİK 2019. Available online: https://www.tuik.gov.tr (accessed on 6 February 2020).
- Marrelli, M.; Statti, G.A.; Conforti, F. Origanum spp.: An update of their chemical and biological profiles. Phytochem. Rev. 2018, 17, 873–888. [Google Scholar] [CrossRef]
- Naquvi, K.J.; Ahamad, J.; Salma, A.; Ansari, S.H.; Najmi, A.K. A critical review on traditional uses, phytochemistry and pharmacological uses of Origanum vulgare Linn. Int. Res. J. Pharm. 2019, 10, 7–11. [Google Scholar] [CrossRef]
- Gong, H.Y.; Liu, W.H.; LV, G.Y.; Zhou, X. Analysis of Essential Oils of Origanum vulgare from Six Production Areas of China and Pakistan. Rev. Bras. Farmacogn. 2014, 24, 25–32. [Google Scholar] [CrossRef]
- Sokmen, M.; Serkedjleva, J.; Dalerera, D.; Gulluce, M.; Pollsslou, M.; Tape, B.; Akpulat, H.A.; Sahin, F.; Sökmen, A. In vitro antioxidant, antimicrobial and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum acutidens. J. Agric. Food Chem. 2004, 52, 3309–3312. [Google Scholar] [CrossRef]
- Figueredo, G.; Özcan, M.M.; Chalchat, J.C.; Bağcı, Y.; Chalard, P. Chemical Composition of Essential Oil of Hyssopus officinalis L. and Origanum acutidens. J. Essent. Oil Bear. Plants 2012, 15, 300–306. [Google Scholar] [CrossRef]
- Kizil, S.; Hasimi, N.; Tolan, V. Biological Activities of Origanum, Satureja, Thymbra and Thymus Species Grown in Turkey. J. Essent. Oil Bear. Plants 2014, 17, 460–468. [Google Scholar] [CrossRef]
- Aseyd Nezhad, S.; Es-haghi, A.; Tabrizi, M.H. Green synthesis of cerium oxide nanoparticle using Origanum majorana L. leaf extract, its characterization and biological activities. Appl. Organomet. Chem. 2019, 34, 1–10. [Google Scholar] [CrossRef]
- Shehadeh, M.; Jaradat, N.; Al-Masri, M.; Naser Zaid, A.; Hussein, F.; Khasati, A.; Darwish, R. Rapid, cost-effective and organic solvent-free production of biologically active essential oil from Mediterranean wild Origanum Syr. Saudi Pharm. J. 2019, 27, 612–618. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakcı, R.; Kesdek, M.; Mete, E. Antifungal, Phytotoxic, and Insecticidal Properties of Essential Oil Isolated from Turkish Origanum acutidens and Three Components, cavacrol, thymol and p-Cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Tozlu, E.; Cakir, A.; Kordali, S.; Tozlu, G.; Ozer, H.; Aytas Akcin, T. Chemical compositions and insecticidal effects of essential oils isolated from Achillea gypsicola, Satureja hortensis, Origanum acutidens and Hypericum scabrum against broadbean weevil (Bruchus dentipes). Sci. Hortic. 2011, 130, 9–17. [Google Scholar] [CrossRef]
- Baj, T.; Baryluk, A.; Sieniawska, E. Application of mixture design for optimum antioxidant activity of mixtures of essential oils from Ocimum basilicum L., Origanum majorana L. and Rosmarinus officinalis L. Indus. Crops Prod. 2018, 115, 52–61. [Google Scholar] [CrossRef]
- Dhaouadi, S.; Rouissi, W.; Mougou-Hamdane, A.; Hannachi, I.; Nasraoui, B. Antifungal activity of essential oils of Origanum majorana and Lavender angustifolia against Fusarium wilt and root rot disease of melon plants. Tunis. J. Plant Prot. 2018, 13, 39–55. [Google Scholar]
- Erenler, R.; Demirtas, I.; Karan, T.; Gul, F.; Kayir, O.; Karakoc, O.C. Chemical constituents, quantitative analysis and insecticidal activities of plant extract and essential oil from Origanum onites L. Trends Phytochem. Res. 2018, 2, 91–96. [Google Scholar]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.S.; Caputo, L. Antimicrobial and Phytotoxic Activity of Origanum heracleoticum and O. majorana Essential Oils Growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef]
- Karan, T.; Belguzar, S.; Selvi, B. Antibacterial Activity of Essential Oils of Origanum bilgeri, Origanum onites, Satureja spicigera Leaves Against Agricultural Plant Pathogenic Bacteria. J. Essent. Oil Bear. Plants 2021, 24, 1159–1168. [Google Scholar] [CrossRef]
- Becer, E.; Altundag, E.M.; Başer, K.H.C.; Vatansever, H.S. Cytotoxic activity and antioxidant effects of Origanum onites essential oil and its two major contents, carvacrol and p-Cymene on human colorectal (HCT116) and hepatocelluler carcinoma (HepG2) cell lines. J. Essent. Oil Res. 2022, 34, 1–10. [Google Scholar] [CrossRef]
- Kaskatepe, B.; Aslan Erdem, S.; Ozturk, S.; Safi Oz, Z.; Subasi, E.; Koyuncu, M.; Vlainic, J.; Kosalec, I. Antifungal and Anti-Virulent Activity of Origanum majorana L. Essential Oil on Candida albicans and In Vivo Toxicity in the Galleria mellonella Larval Model. Molecules 2022, 27, 663. [Google Scholar] [CrossRef]
- Kordali, S.; Tazegül, A.; Cakir, A. Phytotoxic Effects of Nepeta meyeri Benth. Extracts and Essential Oil on Seed Germinations and Seedling Growths of Four Weed Species. Rec. Nat. Prod. 2015, 9, 404–418. [Google Scholar]
- Özer, Z.; Gören, A.C.; Kılıc, T.; Öncü, M.; Çarıkçı, S.; Dirmenci, T. The phenolic contents, antioxidant and anticholinesterase activity of section Amaracus (Gled.) Vogel and Anatolican Ietsw. of Origanum L. species. Arab. J. Chem. 2020, 13, 5027–5039. [Google Scholar] [CrossRef]
- Gokturk, T.S.; Kordali, S.; Usanmaz Bozhuyuk, A. Insecticidal effects of essential oils against nymphal and adult stage of Ricania simulans (Hemiptera: Ricanidae). Nat. Prod. Commun. 2017, 12, 973–976. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Akcin, T.A.; Mete, E.; Aydin, T.; Kilic, H. Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan. (Asteraceae). Ind. Crop. Prod. 2009, 29, 562–570. [Google Scholar] [CrossRef]
- Ramluckan, K.; Moodley, K.G.; Bux, F. An evaluation of the efficacy of using selected solvents for the extraction of lipids from algal biomass by the soxhlet extraction method. Fuel 2014, 116, 103–108. [Google Scholar] [CrossRef]
- Yildirim, B.A.; Kordali, S.; Yildirim, S.; Yildirim, F.; Ercisli, S. Antidiabetic and Antioxidant Effects of Vitis Vinifera L. Cv. ‘Kara Erik‘ Seed Extract in Strept Ozotocin Diabetic Rats. Oxid. Commun. 2017, 40, 209–219. [Google Scholar]
- Sarikurkcu, C.; Ceylan, O.; Zeljković, S.Ć. Micromeria myrtifolia: Essential Oil Composition and Biological Activity. Nat. Prod. Commun. 2019, 14, 1934578X19851687. [Google Scholar] [CrossRef]
- Cetin, B.; Cakmakci, S.; Cakmakci, R. The investigation of antimicrobial activity of thyme and oregano essential oils. Turk. J. Agric. For. 2011, 35, 145–154. [Google Scholar]
- Çakır, A.; Özer, H.; Aydın, T.; Kordali, Ş.; Çavuşoğlu, A.; Akçin, T.; Mete, E.; Akçin, A. Phytotoxic and Insecticidal Properties of Essential Oils andExtracts of Four Achillea Species. Rec. Nat. Prod. 2016, 10, 154–167. [Google Scholar]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Üstüner, T.; Kordali, S.; Usanmaz Bozhüyük, A. Herbicidal and Fungicidal Effects of Cuminum cyminum, Mentha longifolia and Allium sativum Essential Oils on Some Weeds and Fungi. Rec. Nat. Prod. 2018, 56, 619–629. [Google Scholar] [CrossRef]
- Fradi, A.J.; Al-Araji, A.M.Y. Effect of Eucalyptus camaldulensis terpenes, alkaloids and phenols against Fusarium oxysporum. Iraqi J. Sci. 2015, 56, 2807–2810. [Google Scholar]
- Harčárová, M.; Čonková, E.; Proškovcová, M.; Váczi, P.; Marcinčáková, D.; Bujňák, L. Comparison of antifungal activity of selected essential oils against Fusarium graminearum in vitro. Ann Agric Environ. Med. 2021, 28, 414–418. [Google Scholar] [CrossRef]
- Dudai, N.; Chaimovitsh, D.; Larkov, O.; Fischer, R.; Blaicher, Y.; Mayer, A. Allelochemicals released by leaf residues of Micromeria fruticosa in soils, their uptake and metabolism by inhibited wheat seed. Plant Soil 2009, 314, 311–317. [Google Scholar] [CrossRef]
- Gendy, A.N.E.; Leonardi, M.; Mugnaini, L.; Bertelloni, F.; Ebani, V.V.; Nardoni, S.; Mancianti, F.; Hendawy, S.; Omer, E.; Pistelli, L. Chemical composition and antimicrobial activity of essential oil of wild and cultivated Origanum syriacum plants grown in Sinai, Egypt. Ind. Crops Prod. 2015, 67, 201–207. [Google Scholar] [CrossRef]
- Al Hafi, M.; El Beyrouthy, M.; Ouaini, N.; Stien, D.; Rutledge, D.; Chaillou, S. Chemical Composition and Antimicrobial Activity of Origanum libanoticum, Origanum ehrenbergii, and Origanum syriacum Growing Wild in Lebanon. Chem. Biodivers 2016, 13, 555–560. [Google Scholar] [CrossRef]
- Karan, T.; Simsek, S.; Yildiz, I.; Erenler, R. Chemical Composition and Insecticidal Activity of Origanum syriacum L. Essential Oil Against Sitophilus oryzae and Rhyzopertha dominica. Int. J. Second. Metab. 2018, 5, 87–93. [Google Scholar]
- El Khoury, R.; Michael Jubeli, R.; El Beyrouthy, M.; Baillet Guffroy, A.; Rizk, T.; Tfayli, A.; Lteif, R. Phytochemical screening and antityrosinase activity of carvacrol, thymoquinone, and four essential oils of Lebanese plants. J. Cosmet. Derm. 2019, 18, 944–952. [Google Scholar] [CrossRef]
- Badawy, A.A.; El-mohandes, M.A.; Algharib, A.M.; Hatab, B.E.; Omer, E.A. The essential oil and its main constituents of Origanum syriacum ssp. sinaicum grown wild in Saint Katherine Protectorate, South Sinai, Egypt. Al-Azhar J. Agric. Res. 2020, 45, 116–131. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalli, N.; Skourti, A.; Karagianni, E.S.; Nika, E.P.; Kontodimasd, D.C.; Cappellaccie, L.; Petrellie, R.; Cianfaglionef, K.; et al. Effectiveness of eight essential oils against two key stored-product beetles, Prostephanus truncatus (Horn) and Trogoderma granarium Everts. Food Chem. Toxicol. 2020, 139, 1–13. [Google Scholar] [CrossRef]
- Alonazi, M.A.; Jemel, I.; Moubayed, N.; Alwhibi, M.; El-Sayed, N.N.E.; Ben Bacha, A. Evaluation of the in vitro anti-inflammatory and cytotoxic potential of ethanolic and aqueous extracts of Origanum syriacum and Salvia lanigera leaves. Environ. Sci. Pollut Res. Int. 2021, 28, 19890–19900. [Google Scholar] [CrossRef]
- Hassan, Y.A.; Khedr, A.I.M.; Alkabli, J.; Elshaarawy, R.F.M.; Nasr, A.M. Co-delivery of imidazolium Zn(II)salen and Origanum syriacum essential oil by shrimp chitosan nanoparticles for antimicrobial applications. Carbohyd. Polym. 2021, 260, 117834. [Google Scholar] [CrossRef]
- El-Alam, I.; Zgheib, R.; Iriti, M.; El Beyrouthy, M.; Hattouny, P.; Verdin, A.; Fontaine, J.; Chahine, R.; Hadj Sahraoui, A.L.; Makhlouf, H. Origanum syriacum Essential Oil Chemical Polymorphism According to Soil Type. Foods 2019, 8, 90. [Google Scholar] [CrossRef]
- Zgheib, R.; Chaillou, S.; Ouaini, N.; Kassouf, A.; Rutledge, D.; El Azzi, D.; El Beyrouthy, M. Chemometric Tools to Highlight the Variability of the Chemical Composition and Yield of Lebanese Origanum syriacum L. Essent. Oil. Chem. Biodivers. 2016, 13, 1326–1347. [Google Scholar] [CrossRef]
- AL-Mariri, A.; Odeh, A.; Alobeid, B.; Boukai, H. In vitro antibacterial activity of Origanum syriacum L. essential oils against gram-negative bacteria. Avicenna J. Clin. Microbiol. Infect 2019, 6, 26–30. [Google Scholar] [CrossRef]
- Jan, S.; Mir, J.I.; Shafi, W.; Faktoo, S.Z.; Singh, D.B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Divergence in tissue-specific expression patterns of genes associated with the terpeniod biosynthesis in two oregano species Origanum vulgare L., and Origanum majorana. Ind. Crops Prod. 2018, 123, 546–555. [Google Scholar] [CrossRef]
- Abbasi-Maleki, S.; Kadkhoda, Z.; Taghizad-Farid, R. The antidepressant-like effects of Origanum majorana essential oil on mice through monoaminergic modulation using the forced swimming test. J. Tradit Complement Med. 2020, 10, 327–335. [Google Scholar] [CrossRef]
- Demirel, N.; Erdoğan, C. Insecticidal effects of essential oils from Labiatae and Lauraceae families against cowpea weevil, Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in stored pea seeds. Entomol. Appl. Sci. Lett. 2017, 4, 13–19. [Google Scholar]
- Waller, S.B.; Madrid, I.M.; Ferraz, V.; Picoli, T.; Cleff, M.B.; de Faria, R.O.; Meireles, M.C.A.; de Mello, J.R.B. Cytotox and anti-Sporothrix brasiliensis activity of the Origanum majorana Linn. oil. Braz. J. Microbiol. 2016, 47, 896–901. [Google Scholar] [CrossRef]
- Radaelli, M.; da Silvaa, B.P.; Weidlicha, L.; Hoehne, L.; Flach, A.; da Costac, L.A.M.; Ethur, E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef]
- Elmhalli, F.; Garboui, S.S.; Karlson, A.K.B.; Mozūraitis, R.; Baldauf, S.L.; Grandi, G. Acaricidal activity against Ixodes ricinus nymphs of essential oils from the Libyan plants Artemisia herba alba, Origanum majorana and Juniperus phoenicea. Vet. Parasitol: Reg. Stud. Rep. 2021, 24, 100575. [Google Scholar] [CrossRef]
- Waller, S.B.; Rıpoll, M.K.; Sılva, A.L.; Serra, E.F.; Dıas, T.P.; Neves, V.B.D.; Melo, L.P.D.; Lindemann, P.; De Almeida, M.O.; Gomes, A.; et al. Activities and mechanisms of oregano, marjoram and rosemary essential oils against Malassezia pachydermatitis isolates from canine and feline otitis. Turk. J. Vet. Anim. Sci. 2022, 46, 549–558. [Google Scholar]
- Dantas, A.; dos, S.; Klein-Júnior, L.C.; Machado, M.S.; Guecheva, T.N.; Santos dos, L.D.; Zanette, R.A.; de Mello, F.B.; Henriques, J.A.P.; de Mello, J.R.B. Origanum majorana Essential Oil Lacks Mutagenic Activity in the Salmonella/Microsome and Micronucleus Assays. Sci. World J. 2016, 2016, 1–7. [Google Scholar]
- Ouedrhiri, W.; Balouiri, M.; Bouhdid, S.; Moja, S.; Chahdi, F.O.; Taleb, M.; Greche, H. Mixture design of Origanum compactum, Origanum majorana and Thymus serpyllum essential oils: Optimization of their antibacterial effect. Ind. Crops Prod. 2019, 89, 1–9. [Google Scholar] [CrossRef]
- Ghazal, T.S.A.; Schelz, Z.; Vidács, L.; Szemerédi, N.; Veres, K.; Spengler, G.; Hohmann, J. Antimicrobial, Multidrug Resistance Reversal and Biofilm Formation Inhibitory Effect of Origanum majorana Extracts, Essential Oil and Monoterpenes. Plants 2022, 11, 1432. [Google Scholar] [CrossRef]
- Mossa, A.T.; Nawwar, G. Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Hum. Exp. Toxicol. 2011, 30, 1501–1513. [Google Scholar] [CrossRef]
- Kimera, F.; Sewilam, H.; Fouad, W.M.; Suloma, A. Efficient utilization of aquaculture effluents to maximize plant growth, yield, and essential oils composition of Origanum majorana cultivation. Ann. Agric. Sci. 2021, 66, 1–7. [Google Scholar] [CrossRef]
- Amor, G.; Caputo, L.; La Storia, A.; De Feo, V.; Mauriello, G.; and Fechtali, T. Chemical Composition and Antimicrobial Activity of Artemisia herba-alba and Origanum majorana Essential Oils from Morocco. Molecules 2019, 24, 4021. [Google Scholar] [CrossRef]
- Mady, H.Y.; Ahmed, M.M.; El Namaky, A.H. Efficiency of Origanum majorana essential oil as insecticidal agent against Rhynchophorus ferrugineus the red palm weevil (Olivier) (Coleoptera: Curculionidae). J. Biopest 2021, 14, 32–40. [Google Scholar]
- Stefanaki, A.; Cook, C.M.; Lanaras, T.; Kokkini, S. The Oregano plants of Chios Island (Greece): Essential oils of Origanum onites L. growing wild in different habitats. Ind. Crops Prod. 2016, 82, 107–113. [Google Scholar] [CrossRef]
- Ozdemir, R.C.; Taştan, Y.; Guney, K. Prevention of Saprolegniasis in rainbow trout (Oncorhynchus mykiss) eggs using oregano (Origanum onites) and laurel (Laurus nobilis) essential oils. J. Fish Dis. 2022, 45, 51–58. [Google Scholar] [CrossRef]
- Yigit, N.O.; Kocaayan, H. Efficiency of thyme (Origanum onites) and coriander (Coriandrum sativum) essential oils on anesthesia and histopathology of rainbow trout (Oncorhynchus mykiss). Aquaculture 2023, 52, 738813. [Google Scholar] [CrossRef]
- Yilar, M.; Bayar, Y.; Onaran, A. Chemical composition and allelopathic effect of Origanum onites L. essential oil. Plant Prot. Bull. 2019, 59, 71–78. [Google Scholar]
- Spyridopoulou, K.; Fitsiou, E.; Bouloukosta, E.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Oreopoulou, A.; Papavassilopoulou, E.; Pappa, A.; Chlichlia, K. Extraction, Chemical Composition, and Anticancer Potential of Origanum onites L. Essential Oil. Molecules 2019, 24, 2612. [Google Scholar] [CrossRef]
- Sarıkaya, A.G. Leaf and Flower Volatile Oil Components of Two Thyme Taxa Origanum onites L. and Thymbra spicata var. spicata L. in Turkey. Eur. J. Sci. Technol. 2019, 17, 346–350. [Google Scholar] [CrossRef]
- Ozdemir, N.; Ozgen, Y.; Kiralan, M.; Bayrak, A.; Arslan, N.; Ramadan, M.F. Effect of different drying methods on the essential oil yield, composition and antioxidant activity of Origanum vulgare L. and Origanum onites L. J. Food Meas. Charact. 2018, 12, 820–825. [Google Scholar] [CrossRef]
- Senatore, F.; De Fusco, R.; De Feo, V. Essential oils from Salvia spp. (Lamiaceae). I. chemical composition of the essential oils from Salvia glutinosa L. growing wild in Southern Italy. J. Essent. Oil Res. 1997, 9, 151–157. [Google Scholar] [CrossRef]
- Karimian, P.; Kavoosi, G.H.; Amirghofran, Z. Anti-oxidative and anti-infammatory efects of Tagetes minuta essential oil in activated macrophages. Asian Pac. J. Trop. Biomed. 2014, 4, 219–227. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M.; Changizi, S.; Vosoughi, M. Essential oil composition of Salvia virgata Jacq. from Iran. J. Essent. Oil-Bear. Plants 2005, 8, 330–333. [Google Scholar] [CrossRef]
- Alizadeh, A. Essential Oil Constituents, Antioxidant and Antimicrobial Activities of Salvia virgata Jacq. from Iran. J. Essent. Oil-Bear. Plants 2013, 16, 172–182. [Google Scholar] [CrossRef]
- Moadeli, S.N.; Rowshan, V.; Abotalebi, A. Comparison of the essential oil components in wild and cultivate population of Salvia virgata. Int. Res. J. Appl. Basic Sci. 2013, 4, 337–340. [Google Scholar]
- Raut, J.S.; Karuppayil, S.M.; Raut, J.S.; Karuppayil, S.M.A. Status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Yilar, M.; Kadioglu, İ.; Telci, İ. Determination of Essential Oil Compositions of Some Salvia Species Naturally Growing in Tokat Province. Turk. J. Agric. Nat. Sci. 2015, 2, 313–319. [Google Scholar]
- Saharkhiz, J.M.; Smaeili, S.; Merikhi, M. Essential oil analysis and phytotoxic activity of two ecotypes of Zataria multiflora Boiss. growing in Iran. Nat. Prod. Res. 2010, 24, 1598–1609. [Google Scholar] [CrossRef]
- Ghasemi, G.; Alirezalu, A.; Ghosta, Y.; Jarrahi, A.; Safavi, S.A.; Mohammadi, M.A.; Barba, F.J.; Munekata, P.E.S.; Domínguez, R.; Lorenzo, J.M. Composition, Antifungal, Phytotoxic, and Insecticidal Activities of Thymus kotschyanus Essential Oil. Molecules 2020, 25, 1152. [Google Scholar] [CrossRef]
- Gharibvandia, A.; Karimmojenia, H.; Ehsanzadeha, P.; Rahimmaleka, M.; Mastinu, A. Weed management by allelopathic activity of Foeniculum vulgare essential oil. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2022, 156, 1–9. [Google Scholar] [CrossRef]
- Tursun, N.; Işıkber, A.A.; Alma, M.H.; Bozhüyük, A.U. Inhibitory Effect of Oregano and Laurel Essential Oils and Their Main Components on Seed Germination of Some Weed and Crop Species. Selcuk J. Agric. Food Sci. (SJAFS) 2022, 36, 275–281. [Google Scholar]
- Yasar, A.; Karaman, Y.; Gokbulut, I.; Tursun, A.O.; Tursun, N.; Uremis, I.; Arslan, M. Chemical Composition and Herbicidal Activities of Essential Oil from Aerial Parts of Origanum Hybrids Grown in Different Global Climate Scenarios on Seed Germination of Amaranthus palmeri. J. Essent. Oil Bear. Plants 2021, 24, 603–616. [Google Scholar] [CrossRef]
- Said, A.; Aoun, T.; Elhaji, N.; Marin, P.D.; Giweli, A. Allelopathic Effects on Seeds Germination of Lactuca sativa L. Seeds and Antibacterial Activity of Thymus capitatus Essential Oil from Zintan-Libya flora. Am. Sci. Res. J. Eng. Technol. Sci. (ASRJETS) 2016, 17, 121–131. [Google Scholar]
- Flores-Macías, A.; Reyes-Zarate, G.G.; da Camara, C.A.G.; López-Ordaz, R.; C.Guillén, J.; Ramos-López, M.Á. Chemical composition and phytotoxic potential of Eucalyptus globulus essential oil against Lactuca sativa and two herbicide-resistant weeds: Avena fatua and Amaranthus hybridus. TIP Rev. Espec. En Cienc. Quím.-Biol. 2021, 24, 1–8. [Google Scholar] [CrossRef]
- Ruiz-Vásquez, L.; Ruiz Mesia, L.; Caballero Ceferino, H.D.; Ruiz Mesia, W.; Andrés, M.F.; Díaz, C.E.; Gonzalez-Coloma, A. Antifungal and Herbicidal Potential of Piper Essential Oils from the Peruvian Amazonia. Plants 2022, 11, 1793. [Google Scholar] [CrossRef]
- Azizan, K.A.; Zamani, A.I.; Nor Muhammad, N.A.; Khairudin, K.; Yusoff, N.; Nawawi, M.F. Dose-Dependent Effect of Wedelia trilobata Essential Oil (EO) on Lettuce (Lactuca sativa L.) with Multivariate Analysis. Chem. Biodivers. 2022, 19, e202100833. [Google Scholar] [CrossRef]
- Nikolova, M.; Traykova, B.; Yankova-Tsvetkova, E.; Stefanova, T.; Dzhurmanski, A.; Aneva, I.; Berkov, S. Herbicide Potential of Selected Essential Oils From Plants of Lamiaceae and Asteraceae Families. Acta Agrobot. 2021, 74, 1–7. [Google Scholar] [CrossRef]
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.; Putievsky, E.; Lerner, H. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Varela, R.M.; Simonet, A.M.; Carrera, C.; Molinillo, J.M. Allelopathy as a new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23. [Google Scholar] [CrossRef]
- Synowiec, A.; Lenart–Boron, A.; Kalemba, D. Effect of soil application of microencapsulated caraway oil on weed infestation and maize yield. Int. J. Pest Manag. 2018, 64, 315–323. [Google Scholar] [CrossRef]
- Verdeguer, M.; Castañeda, L.G.; Torres–Pagan, N.; Llorens–Molina, J.A.; Carrubba, A. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus Essential Oils. Molecules 2020, 25, 562. [Google Scholar] [CrossRef]
- Hamrouni, L.; Hanana, M.; Amri, I.; Romane, A.E.; Gargouri, S.; Jamoussi, B. Allelopathic effects of essential oils of Pinus halepensis Miller: Chemical composition and study of their antifungal and herbicidal activities. Arch. Phytopathol. Pflanzenschutz 2015, 48, 145–158. [Google Scholar] [CrossRef]
- Ibanez, M.D.; Blazquez, M.A. Phytotoxicity of essential oils on selected weeds: Potential hazard on food crops. Plants 2018, 7, 79. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Calmasur, O.; Kordali, S.; Kaya, O.; Aslan, I. Toxicity of essential oil vapours obtained from Achillea spp. to Sitophilus granarius (L.) and Tribolium confusum (Jacquelin du Val). J. Plant Dis. Prot. 2006, 113, 37–41. [Google Scholar]
- Semerdjieva, I.; Atanasova, D.; Maneva, V.; Zheljazkov, V.; Radoukova, T.; Astatkie, T.; Dincheva, I. Allelopathic effects of Juniper essential oils on seed germination and seedling growth of some weed seeds. Ind. Crops Prod. 2022, 180, 114768. [Google Scholar] [CrossRef]
- Synowiec, A.; Kalemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic potential of essential oils from temperate climate plants against the germination of selected weeds and crops. J. Pest Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]

| Species | Essential Oils | Extracts | |
|---|---|---|---|
| Acetone | n-Hexane | ||
| Origanum syriacum | 4.0 | 13.8 | 14.3 |
| Origanum onites | 4.5 | 14.2 | 14.7 |
| Origanum majorana | 5.0 | 14.3 | 14.8 |
| Compound | Essential Oil Compounds (%) | ||
|---|---|---|---|
| O. syriacum | O. onites | O. majorana | |
| α-Pinene | 0.30 | - | 0.40 |
| Myrcene | 0.21 | - | 0.57 |
| 3-Octanol | 0.28 | - | 0.42 |
| 3-Octanone | - | - | 0.17 |
| α-Terpinene | 0.37 | 0.19 | 0.85 |
| p-Cymene | 5.71 | 1.94 | 9.02 |
| 1,8-Cineole | - | - | 2.20 |
| γ-Terpinene | 1.63 | 0.95 | 5.80 |
| Terpinen-4-ol | 0.65 | 0.58 | 2.15 |
| α-Terpineol | 0.27 | 0.21 | 29.28 |
| Thymol | 0.35 | 30.97 | - |
| Carvacrol | 88.49 | 58.65 | 40.57 |
| Carvacrol methyl ether | - | - | 3.46 |
| β-Caryoplhyllene | 1.48 | 0.98 | 1.76 |
| Linalool | - | 4.17 | 0.85 |
| Borneol | - | 0.64 | - |
| Class composition (%) | |||
| Monoterpene hydrocarbons | 8.22 | 3.08 | 16.64 |
| Oxygenated monoterpenes | 89.76 | 95.22 | 78.51 |
| Sesquiterpene hydrocarbons | 1.48 | 0.98 | 1.76 |
| Oxygenated hydrocarbons | - | - | - |
| Aliphatic compounds | 0.28 | - | 0.59 |
| Total | 99.74 | 99.28 | 97.50 |
| Species | Applications | 24 h | 48 h |
|---|---|---|---|
| Origanum onites | Essential oil | 50.00 ± 4.00 bcd* | 73.33 ± 1.15 b |
| Acetone | 37.33 ± 4.16 ef | 61.33 ± 2.31 d | |
| Hexane | 36.00 ± 2.00 f | 58.67 ± 2.31 d | |
| Origanum syriacum | Essential oil | 55.33 ± 1.15 bc | 79.33 ± 1.15 a |
| Acetone | 44.00 ± 4.00 de | 68.00 ± 2.00 c | |
| Hexane | 42.00 ± 4.00 ef | 66.67 ± 1.15 c | |
| Origanum majorana | Essential oil | 56.67 ± 0.58 b | 79.33 ± 1.15 a |
| Acetone | 49.33 ± 1.15 cd | 68.67 ± 1.15 c | |
| Hexane | 50.00 ± 2.00 bcd | 69.33 ± 1.15 c | |
| Control | 0.00 ± 0.00 g | 0.00 ± 0.00 e | |
| Trifluralin | 69.33 ± 3.06 a | 76.67 ± 3.06 ab |
| Species | Applications | 24 h | 48 h |
|---|---|---|---|
| Origanum onites | Essential oil | 62.67 ± 1.15 b* | 90.00 ± 0.00 ab |
| Acetone | 52.67 ± 1.15 c | 74.00 ± 2.00 c | |
| Hexane | 49.33 ± 1.15 cd | 76.00 ± 0.00 c | |
| Origanum syriacum | Essential oil | 63.33 ± 3.06 b | 87.33 ± 5.03 b |
| Acetone | 50.67 ± 1.15 cd | 74.67 ± 1.15 c | |
| Hexane | 45.33 ± 2.31 d | 70.00 ± 0.00 c | |
| Origanum majorana | Essential oil | 71.33 ± 1.15 a | 84.67 ± 1.15 b |
| Acetone | 49.33 ± 1.15 cd | 70.67 ± 2.31 c | |
| Hexane | 26.00 ± 5.29 e | 48.67 ± 6.11 d | |
| Control | 0.00 ± 0.00 f | 0.00 ± 0.00 e | |
| Trifluralin | 70.67 ± 1.15 a | 94.00 ± 2.00 a |
| Species | Applications | 24 h | 48 h |
|---|---|---|---|
| Origanum onites | Essential oil | 56.67 ± 0.58 b* | 81.33 ± 1.15 b |
| Acetone | 49.33 ± 1.15 bc | 68.67 ± 1.15 c | |
| Hexane | 47.33 ± 1.15 c | 68.67 ± 1.15 c | |
| Origanum syriacum | Essential oil | 55.33 ± 1.15 bc | 71.33 ± 1.15 c |
| Acetone | 52.67 ± 4.16 bc | 70.67 ± 1.15 c | |
| Hexane | 46.67 ± 1.15 c | 66.67 ± 3.06 c | |
| Origanum majorana | Essential oil | 52.67 ± 6.43 bc | 67.33 ± 6.11 c |
| Acetone | 51.67 ± 4.73 bc | 69.33 ± 3.06 c | |
| Hexane | 50.67 ± 6.11 bc | 58.67 ± 2.31 d | |
| Control | 0.00 ± 0.00 d | 0.00 ± 0.00 e | |
| Trifluralin | 77.33 ± 1.15 a | 91.33 ± 1.15 a |
| Species | Applications | 24 h | 48 h |
|---|---|---|---|
| Origanum onites | Essential oil | 56.67 ± 0.58 b | 80.67 ± 1.15 ab |
| Acetone | 46.67 ± 2.31 ef | 66.67 ± 1.15 f | |
| Hexane | 48.67 ± 1.15 de | 67.33 ± 1.15 f | |
| Origanum syriacum | Essential oil | 56.67 ± 0.58 b | 78.00 ± 2.00 bc |
| Acetone | 44.00 ± 2.00 f | 68.67 ± 1.15 ef | |
| Hexane | 50.67 ± 1.15 cde | 71.33 ± 1.15 de | |
| Origanum majorana | Essential oil | 54.67 ± 1.15 bc | 77.33 ± 1.15 c |
| Acetone | 49.33 ± 1.15 de | 72.67 ± 2.31 d | |
| Hexane | 52.67 ± 1.15 bcd | 70.67 ± 1.15 de | |
| Control | 0.00 ± 0.00 g | 0.00 ± 0.00 g | |
| Trifluralin | 72.00 ± 4.00 a | 83.33 ± 1.15 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordali, S.; Kabaagac, G.; Sen, İ.; Yilmaz, F.; Najda, A. Phytotoxic Effects of Three Origanum Species Extracts and Essential Oil on Seed Germinations and Seedling Growths of Four Weed Species. Agronomy 2022, 12, 2581. https://doi.org/10.3390/agronomy12102581
Kordali S, Kabaagac G, Sen İ, Yilmaz F, Najda A. Phytotoxic Effects of Three Origanum Species Extracts and Essential Oil on Seed Germinations and Seedling Growths of Four Weed Species. Agronomy. 2022; 12(10):2581. https://doi.org/10.3390/agronomy12102581
Chicago/Turabian StyleKordali, Saban, Gulbahar Kabaagac, İsmail Sen, Ferah Yilmaz, and Agnieszka Najda. 2022. "Phytotoxic Effects of Three Origanum Species Extracts and Essential Oil on Seed Germinations and Seedling Growths of Four Weed Species" Agronomy 12, no. 10: 2581. https://doi.org/10.3390/agronomy12102581
APA StyleKordali, S., Kabaagac, G., Sen, İ., Yilmaz, F., & Najda, A. (2022). Phytotoxic Effects of Three Origanum Species Extracts and Essential Oil on Seed Germinations and Seedling Growths of Four Weed Species. Agronomy, 12(10), 2581. https://doi.org/10.3390/agronomy12102581








