Rapid Diagnosis and Visual Detection of Potato Cyst Nematode (Globodera rostochiensis) Using Recombinase Polymerase Amplification Combination with Lateral Flow Assay Method (RPA-LFA)
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematode Populations
2.2. DNA Extraction
2.3. RPA Primer and Probe Design and Testing
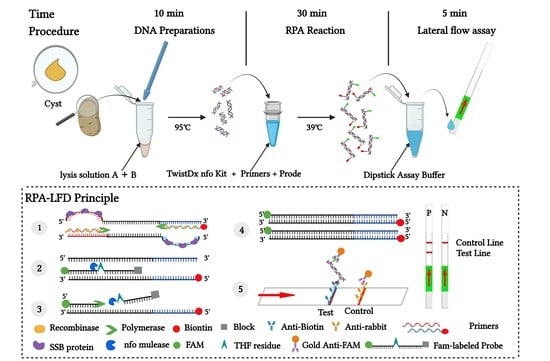
2.4. RPA-LFA Reaction
2.4.1. Specificity Test
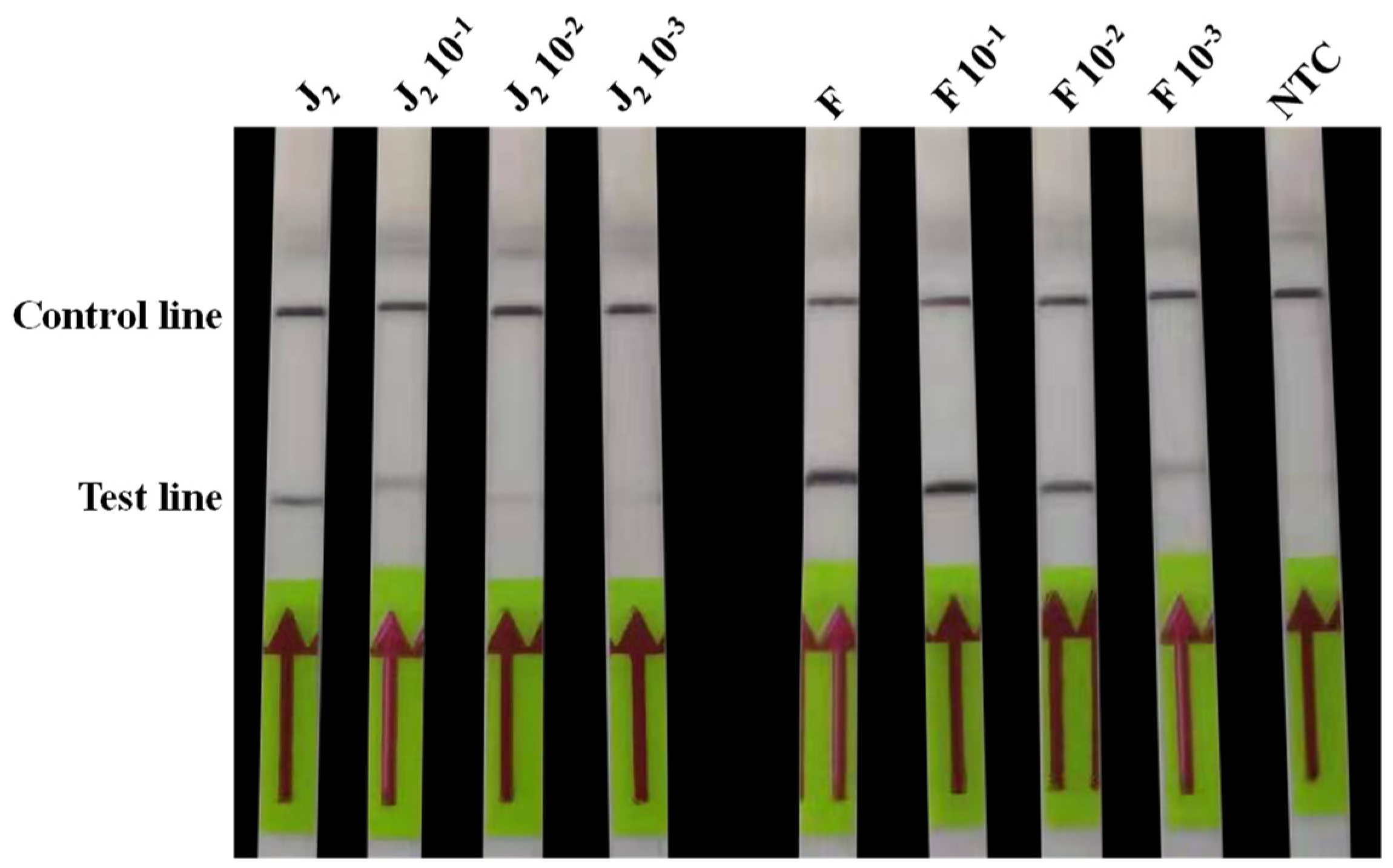
2.4.2. Sensitivity Test
2.5. Detection of Globodera rostochiensis from Actual Field Soil
3. Results
3.1. Primer and Probe Design
3.2. RPA Detection
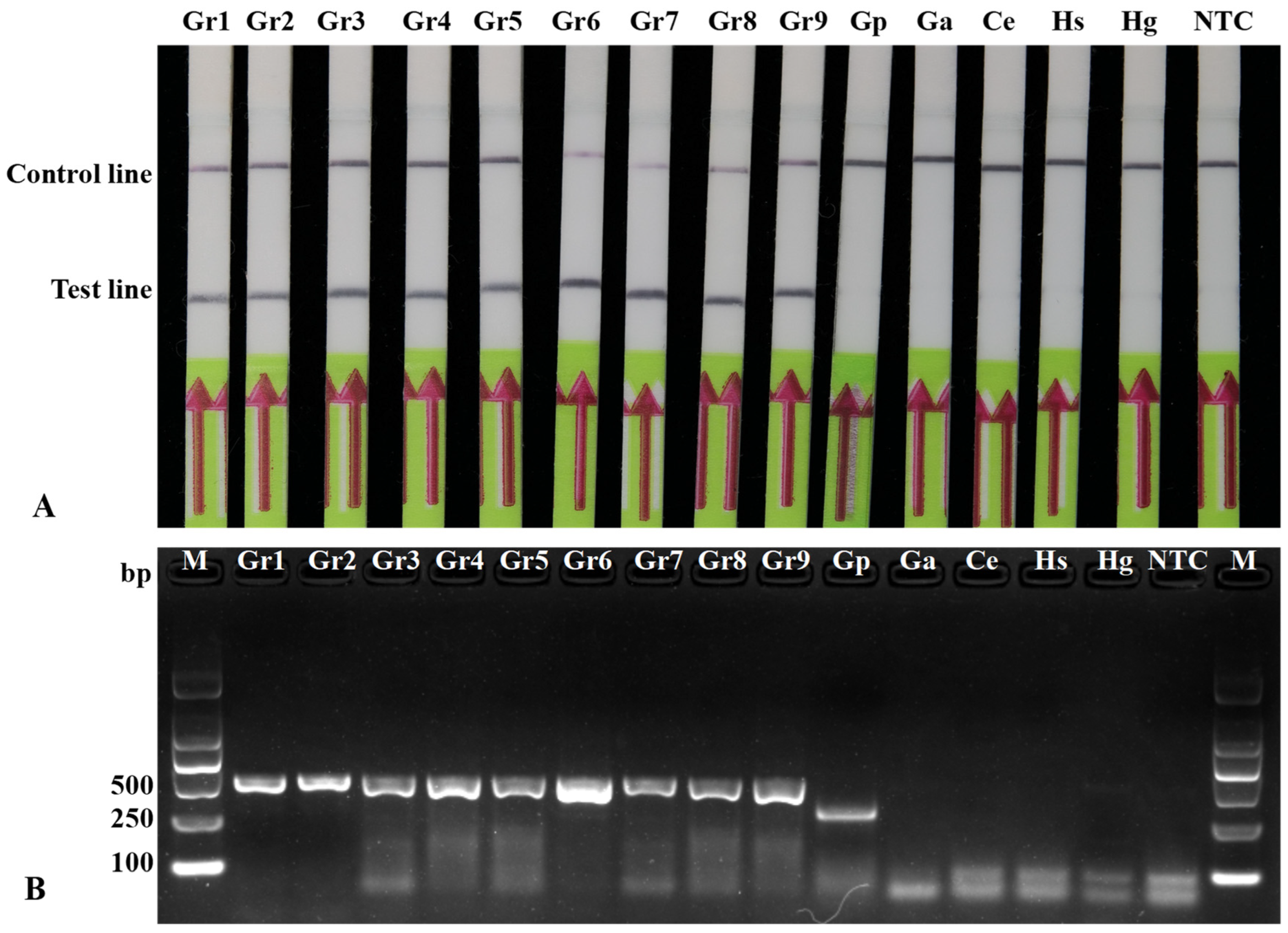
3.3. Specificity Test
3.4. Sensitivity Test
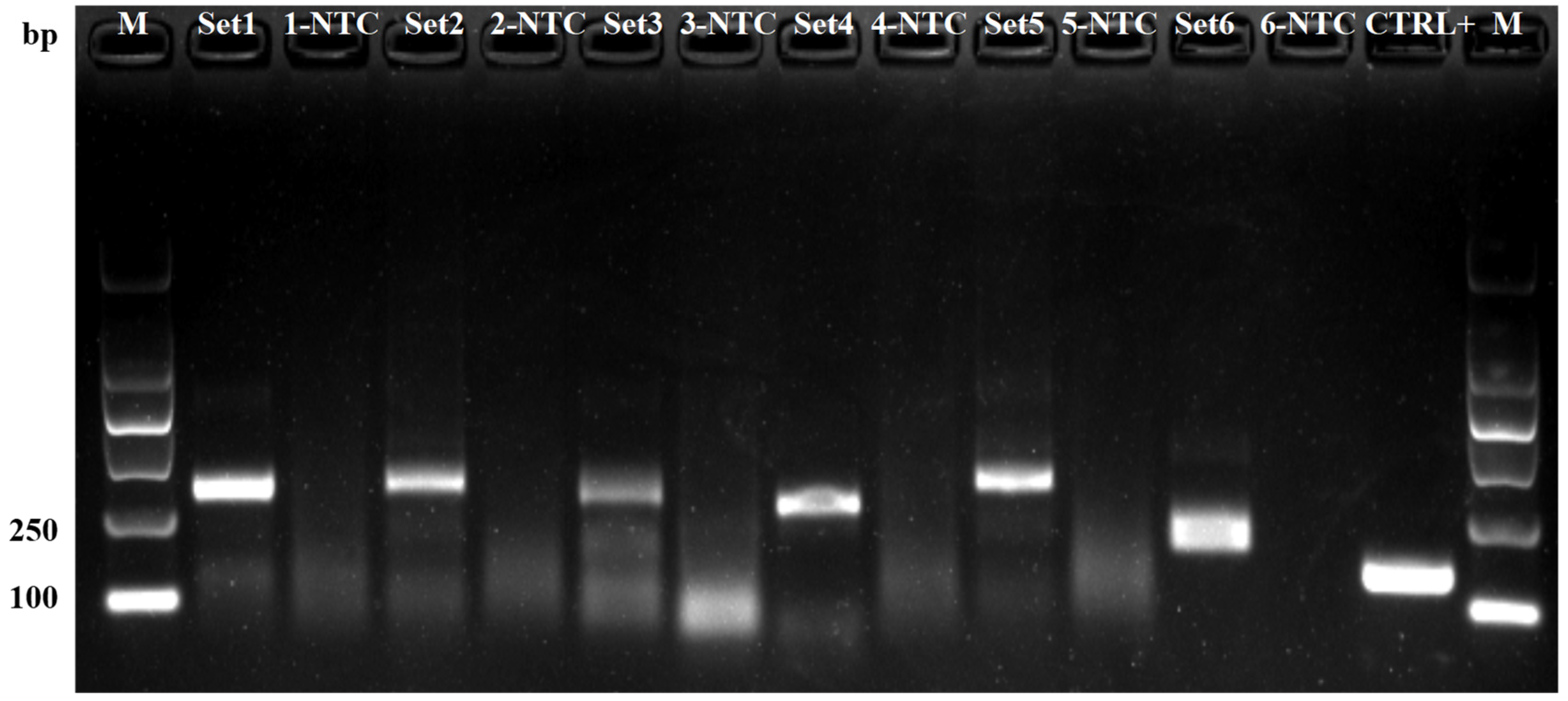
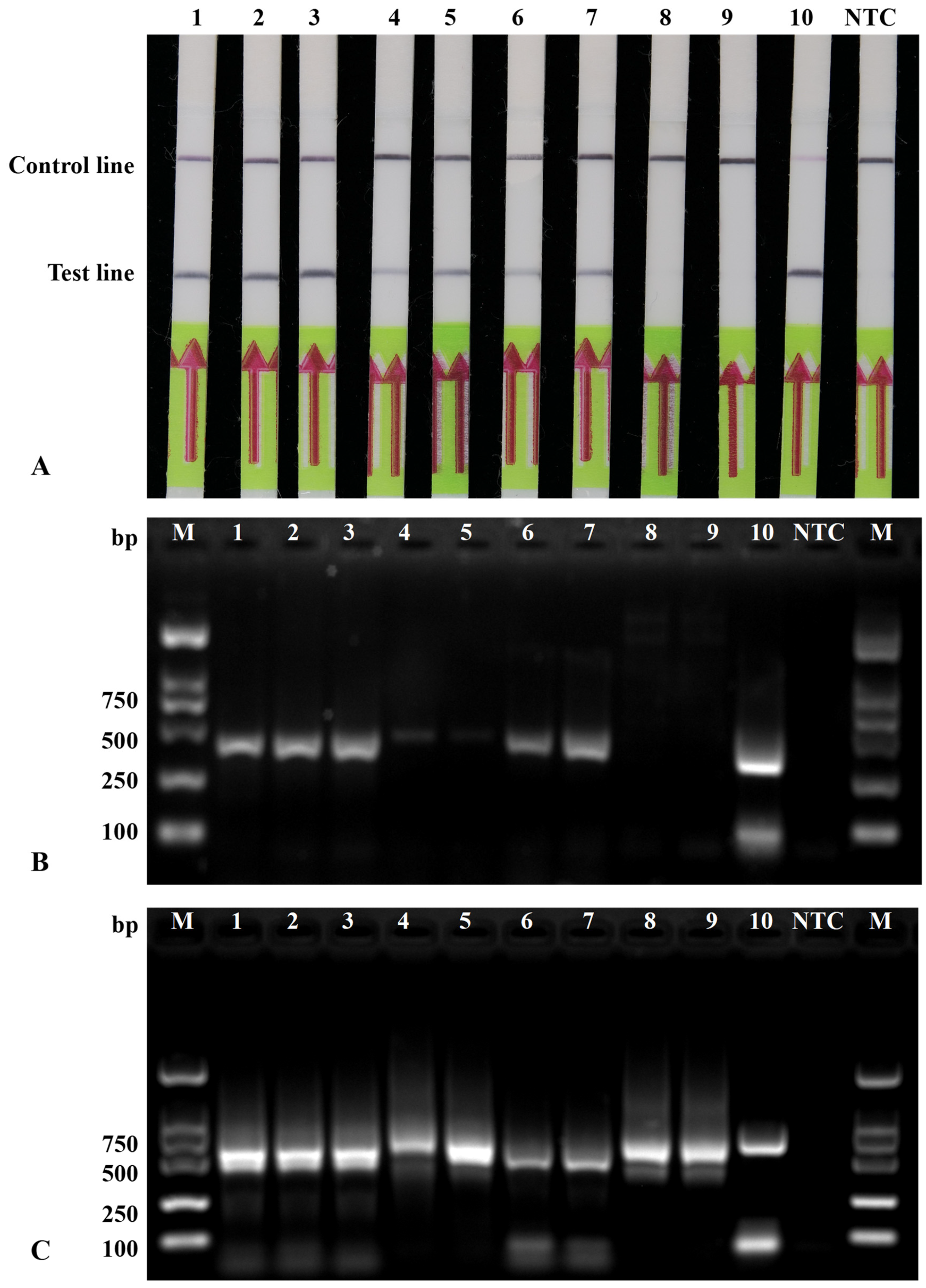
3.5. Detection of Globodera rostochiensis in Actual Field Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abad, P.; Favery, B.; Rosso, M.N.; Castagnone-Sereno, P. Root-knot nematode parasitism and host response: Molecular basis of a sophisticated interaction. Mol. Plant Pathol. 2003, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, G.J.; Jone, M.G.K.; Kikuchi, T.; Manzanila-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.R. CIH Descriptions of Plant-Parasitic Nematodes No. 16 Globodera rostochiensis; CAB International: Wallingford, UK, 1973; p. 3. [Google Scholar]
- Evans, K.; Stone, A.R. A review of the distribution and biology of the potato cyst-nematodes Globodera rostochiensis and G. pallida. Pans 1977, 23, 178–189. [Google Scholar] [CrossRef]
- Evans, K.; Brodie, B.B. The origin and distribution of the golden nematode and its potential in the USA. Am. Potato J. 1980, 57, 79–89. [Google Scholar] [CrossRef]
- Wainer, J.; Dinh, Q. Taxonomy, morphological and molecular identification of the potato cyst nematodes, Globodera pallida and G. rostochiensis. Plants 2021, 10, 184. [Google Scholar] [CrossRef]
- Jatala, P.; Bridge, J. Nematode parasites of root and tuber crops. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Luc, M.R., Sikora, A., Bridge, J., Eds.; CAB International: Wallingford, UK, 1990; pp. 137–180. [Google Scholar]
- Price, J.A.; Coyne, D.; Blok, V.C.; Jones, J.T. Potato cyst nematodes Globodera rostochiensis and G. pallida. Mol. Plant Pathol. 2021, 22, 495–507. [Google Scholar] [CrossRef]
- Perry, R.N. Hatching. In The Biology of Nematodes; Lee, D.L., Ed.; Taylor and Francis: Oxfordshire, UK, 2002; pp. 147–169. [Google Scholar]
- Evans, K. Reviews: New approaches for potato cyst nematode management. Nematropica 1993, 23, 221–231. [Google Scholar]
- Lehman, P. Cost–benefits of nematode management through regulatory programs. In Nematology Advances and Perspectives: Nematode Management and Utilization; Chen, Z.X., Chen, S.Y., Dickson, D.W., Eds.; CAB International: Wallingford, UK, 2004; pp. 1133–1177. [Google Scholar]
- EPPO. PM 9/26(1) National regulatory control system for Globodera pallida and Globodera rostochiensis. EPPO Bull. 2018, 48, 516–532. [Google Scholar]
- Jiang, R.; Peng, H.; Li, Y.Q.; Liu, H.; Zhao, S.Q.; Long, H.B.; Hu, X.Q.; Ge, J.J.; Li, X.Y.; Liu, M.Y.; et al. First record of the golden potato nematode Globodera rostochiensis in Yunnan and Sichuan provinces of China. J. Integr. Agric. 2022, 21, 898–899. [Google Scholar] [CrossRef]
- Peng, D.L.; Liu, H.; Peng, H.; Jiang, R.; Li, Y.Q.; Wang, X.; Ge, J.J.; Zhao, S.Q.; Feng, X.D.; Feng, M.Y. First detection of the potato cyst nematode (Globodera rostochiensis) in a major potato production region of China. Plant Dis. 2022. [Google Scholar] [CrossRef]
- Colin, C.F.; Thomas, O.P. Potato cyst nematode diagnostics: Morphology, differential hosts and biochemical techniques. In Potato Cyst Nematodes: Biology, Distribution and Control; Marks, R.J., Brodie, B.B., Eds.; CAB International: Wallingford, UK, 1998; pp. 91–114. [Google Scholar]
- EPPO. PM 7/40 (4) Globodera rostochiensis and Globodera pallida. EPPO Bull. 2017, 47, 174–197. [Google Scholar] [CrossRef]
- Shields, R.; Fleming, C.C.; Stratford, R. Identification of potato cyst nematodes using the polymerase chain reaction. Fundam. Appl. Nematol. 1996, 19, 167–173. [Google Scholar]
- Mulholland, V.; Carde, L.; Donnell, K.O.; Fleming, C.C.; Powers, T.O. Use of the polymerase chain reaction to discriminate potato cyst nematode at the species level. In BCPC Proceedings No. 65, Diagnostics in Crop Production; Marshall, G., Ed.; The British Crop Protection Council: Farnham, UK, 1996; pp. 247–252. [Google Scholar]
- Bulman, S.R.; Marshall, J.W. Differentiation of Australasian potato cyst nematode (PCN) populations using the polymerase chain reaction (PCR). N. Z. J. Crop Hortic. Sci. 1997, 25, 123–129. [Google Scholar] [CrossRef]
- Fullaondo, A.; Barrena, E.; Viribay, M.; Viribay, M.; Barrena, I.; Salazar, A.; Ritter, E. Identification of potato cyst nematode species Globodera rostochiensis and G. pallida by PCR using specific primer combinations. Nematology 1999, 1, 157–163. [Google Scholar] [CrossRef]
- Picard, D.; Sempere, T.; Plantard, O. A northward colonisation of the Andes by the potato cyst nematode during geological times suggests multiple host-shifts from wild to cultivated potatoes. Mol. Phylogenet. Evol. 2007, 42, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.; Evans, F.; Mulholland, V.; Cole, Y.; Pickup, J. High-throughput diagnosis of potato cyst nematodes in soil samples. Methods Mol. Biol. 2015, 1302, 137. [Google Scholar]
- Nowaczyk, K.; Dobosz, R.; Kornobis, S.; Obrepalska-Steplowska, A. Taqman real-time PCR-based approach for differentiation between Globodera rostochiensis (golden nematode) and Globodera artemisiae species. Parasitol. Res. 2008, 103, 577. [Google Scholar] [CrossRef]
- Beniers, J.E.; Been, T.H.; Mendes, O. Quantification of viable eggs of the potato cyst nematodes (Globodera spp.) using either trehalose or RNA-specific Real-Time PCR. Nematology 2014, 16, 1219–1232. [Google Scholar] [CrossRef]
- Gamel, S.; Letort, A.; Fouville, D.; Folcher, L.; Grenier, E. Development and validation of real-time PCR assays based on novel molecular markers for the simultaneous detection and identification of Globodera pallida, G. rostochiensis and Heterodera schachtii. Nematology 2017, 19, 789–804. [Google Scholar] [CrossRef]
- EPPO. PM 7/129(1) DNA barcoding as an identification tool for a number of regulated pests. EPPO Bull. 2016, 46, 501–537. [Google Scholar] [CrossRef]
- Ahuja, A.; Joshi, V.; Singh, G.; Kundu, A.; Bhat, C.G.; Kumar, S.; Rao, U.; Somvanshi, V.S. Rapid and sensitive detection of potato cyst nematode Globodera rostochiensis by loop-mediated isothermal amplification assay. 3 Biotech 2021, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Macdonald, J.; Stetten, F. Review: A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 2019, 144, 31–67. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Kong, J.; Qiu, Y.H.; Chen, N.Z.; Zhu, S.F.; Wang, X.Y.; Wu, P.S. Rapid detection of the pathogenic fungi causing blackleg of Brassica napus using a portable real-time fluorescence detector. Food Chem. 2019, 288, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Wu, P.S.; Li, L.M.; Huang, Q.X.; Wang, J.Y.; Zhang, D.; Li, M.F.; Chen, N.Z.; Wang, X.Y. Ultrasensitive isothermal detection of a plant pathogen by using a gold nanoparticle-enhanced microcantilever sensor. Sens. Actuators B Chem. 2021, 338, 129874. [Google Scholar] [CrossRef]
- Li, C.; Ju, Y.; Shen, P.; Wu, X.; Cao, L.; Zhou, B.; Yan, X.; Pan, Y. Development of Recombinase Polymerase Amplification Combined with Lateral Flow Detection Assay for Rapid and Visual Detection of Ralstonia solanacearum in Tobacco. Plant Dis. 2021, 105, 3985–3989. [Google Scholar] [CrossRef]
- Kumar, R.; Kaundal, P.; Tiwari, R.K.; Siddappa, S.; Kumari, H.; Lal, M.K.; Naga, K.C.; Sharma, S.; Sagar, V.; Kumar, M. Establishment of a one-step reverse transcription recombinase polymerase amplification assay for the detection of potato virus S. J. Virol. Methods 2022, 307, 114568. [Google Scholar] [CrossRef] [PubMed]
- Subbotin, S.A. Recombinase polymerase amplification assay for rapid detection of the root-knot nematode Meloidogyne enterolobii. Nematology 2019, 21, 243–251. [Google Scholar] [CrossRef]
- Ju, Y.L.; Lin, Y.; Yang, G.G.; Wu, H.P.; Pan, Y.M. Development of recombinase polymerase amplification assay for rapid detection of Meloidogyne incognita, M. javanica, M. arenaria, and M. enterolobii. Eur. J. Plant Pathol. 2019, 155, 1155–1163. [Google Scholar] [CrossRef]
- Chi, Y.K.; Zhao, W.; Ye, M.D.; Ali, F.; Wang, T.; Qi, R.D. Evaluation of recombinase polymerase amplifition assay for detecting Meloidogyne javanica. Plant Dis. 2020, 104, 801–807. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Burbridge, J. Sensitive, accurate and rapid detection of the northern root-knot nematode, Meloidogyne hapla, using recombinase polymerase amplification assays. Plants 2021, 10, 336. [Google Scholar] [CrossRef]
- Cha, D.J.; Kim, D.S.; Lee, S.K.; Han, H.R. A new on-site detection method for Bursaphelenchus xylophilus in infected pine trees. For. Pathol. 2019, 49, e12503. [Google Scholar] [CrossRef]
- Cha, D.J.; Kim, D.S.; Choi, W.; Park, S.; Han, H.R. Point-of-care diagnostic (POCD) method for detecting Bursaphelenchus xylophilus in pinewood using recombinase polymerase amplification (RPA) with the portable optical isothermal device (POID). PLoS ONE 2020, 15, e0227476. [Google Scholar]
- Yao, K.; Peng, D.L.; Jiang, C.; Zhao, W.; Li, G.K.; Huang, W.K.; Kong, L.A.; Gao, H.F.; Zheng, J.W.; Peng, H. Rapid and visual detection of Heterodera schachtii using recombinase polymerase amplification combined with Cas12a-mediated technology. Int. J. Mol. Sci. 2021, 22, 12577. [Google Scholar] [CrossRef] [PubMed]
- Courtright, E.M.; Wall, D.H.; Virginia, R.A.; Frisse, L.M.; Vida, L.M.; Thomas, W.K. Nuclear and mitochondrial DNA sequence diversity in the antarctic nematode Scottnema lindsayae. J. Nematol. 2000, 32, 143. [Google Scholar]
- Szalansky, A.L.; Sui, D.D.; Harris, T.S.; Powers, T.O. Identification of cyst nematodes of agronomic and regulatory concern with PCR RFLP of ITS1. J. Nematol. 1997, 29, 255–267. [Google Scholar]
- Powers, T.O.; Todd, T.C.; Burnell, A.M.; Murray, P.C.; Fleming, C.C.; Szalanski, A.L.; Adams, B.A.; Harris, T.S. The rDNA internal transcribed spacer region as a taxonomic marker for nematodes. J. Nematol. 1997, 29, 441–450. [Google Scholar]
- Song, Z.; Yang, X.; Zhang, X.; Luan, M.; Mei, S. Rapid and visual detection of Meloidogyne hapla using recombinase polymerase amplification combined with a lateral flow dipstick assay. Plant Dis. 2020, 105, 2697–2703. [Google Scholar] [CrossRef]
- Bairwa, A.; Venkatasalam, E.P.; Sudha, R.; Umamaheswari, R.; Singh, B.P. Techniques for characterization and eradication of potato cyst nematode: A review. J. Parasit. Dis. 2017, 41, 607–620. [Google Scholar] [CrossRef]
| Code | Species | Host | Origin |
|---|---|---|---|
| Gr1 | G.rostochiensis | Potato | Hezhang county, Guizhou, China |
| Gr2 | Hezhang county, Guizhou, China | ||
| Gr3 | Weining, Guizhou, China | ||
| Gr4 | Ludian county, Yunnan, China | ||
| Gr5 | Zhaojue county, Sichuan, China | ||
| Gr6 | Norway | ||
| Gr7 | Norway | ||
| Gr8 | Norway | ||
| Gr9 | Norway | ||
| Ga | G.artemisiae | Artemisia argyi | Guizhou, China |
| Gp | G.pallida | Potato | Belgium |
| Ce | Cactodera estonica | Potato | Neimenggu, China |
| Hs | H.schachtii | Sugar beet | Xinyuan county, Xinjiang, China |
| Hg | H.glycines | Soybean | Langfang, Hebei, China |
| Code | Host | Location | Sampling Date |
|---|---|---|---|
| 1 | Potato | Hezhang county, Guizhou, China | August 2020 |
| 2 | Hezhang county, Guizhou, China | August 2020 | |
| 3 | Weining, Guizhou, China | August 2020 | |
| 4 | Ludian county, Yunnan, China | September 2020 | |
| 5 | Yunnan, China | September 2020 | |
| 6 | Zhaojue county, Sichuan, China | September 2020 | |
| 7 | Sichuan, China | September 2020 | |
| 8 | Huludao, Liaoning, China | September 2020 | |
| 9 | Linyi, Shandong, China | July 2020 |
| Name | Sequences (5′–3′) | Usage | Reference |
|---|---|---|---|
| GrF4 | CTGTGTATGGGCTGGCACATTGACCAACA | G. rostochiensis-specific RPA-LFA primers and probe | This study |
| GrR4 | [Biotin] TACGGCACGTACAACATGGAGTAGCAGCTAC | ||
| GrP | [Fam] 1 CGGAGGAAGCACGCCCACAGGGCACCCTAACG [THF] 2 CTGTGCTGGCGTCTGT [C3-spacer] 3 | ||
| ITS5 | GGAAGTAAAAGTCGTAACAAGG | G. rostochiensis-specific PCR primers | [19] |
| PITSr3 PITSp4 | AGCGCAGACATGCCGCAA ACAACAGCAATCGTCGAG | ||
| D2A | ACAAGTACCGTGAGGGAAAGTTG | 28S rDNA universal PCR primers | [40] |
| D3B | TCGGAAGGAACCAGCTACTA |
| Code | Density of Cysts 1 | Detection Results | ||
|---|---|---|---|---|
| RPA-LFA | PCR (ITS5/PITSr3) | PCR (D2A/D3B) | ||
| 1 | 437 ± 28 | + | + | + |
| 2 | 344 ± 26 | + | + | + |
| 3 | 542 ± 36 | + | + | + |
| 4 | 280 ± 18 | + | + | + |
| 5 | 251 ± 7 | + | + | + |
| 6 | 379 ± 29 | + | + | + |
| 7 | 361 ± 16 | + | + | + |
| 8 | 0 | − | − | + |
| 9 | 0 | − | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lei, R.; Peng, H.; Jiang, R.; Shao, H.; Ge, J.; Peng, D. Rapid Diagnosis and Visual Detection of Potato Cyst Nematode (Globodera rostochiensis) Using Recombinase Polymerase Amplification Combination with Lateral Flow Assay Method (RPA-LFA). Agronomy 2022, 12, 2580. https://doi.org/10.3390/agronomy12102580
Wang X, Lei R, Peng H, Jiang R, Shao H, Ge J, Peng D. Rapid Diagnosis and Visual Detection of Potato Cyst Nematode (Globodera rostochiensis) Using Recombinase Polymerase Amplification Combination with Lateral Flow Assay Method (RPA-LFA). Agronomy. 2022; 12(10):2580. https://doi.org/10.3390/agronomy12102580
Chicago/Turabian StyleWang, Xu, Rong Lei, Huan Peng, Ru Jiang, Hudie Shao, Jianjun Ge, and Deliang Peng. 2022. "Rapid Diagnosis and Visual Detection of Potato Cyst Nematode (Globodera rostochiensis) Using Recombinase Polymerase Amplification Combination with Lateral Flow Assay Method (RPA-LFA)" Agronomy 12, no. 10: 2580. https://doi.org/10.3390/agronomy12102580
APA StyleWang, X., Lei, R., Peng, H., Jiang, R., Shao, H., Ge, J., & Peng, D. (2022). Rapid Diagnosis and Visual Detection of Potato Cyst Nematode (Globodera rostochiensis) Using Recombinase Polymerase Amplification Combination with Lateral Flow Assay Method (RPA-LFA). Agronomy, 12(10), 2580. https://doi.org/10.3390/agronomy12102580