Abstract
Tea plants prefer NH4+-N to NO3−-N, and thus nitrification would be detrimental to the N uptake of tea. However, the effects of different stand ages on nitrification and nitrogen oxide (NO and N2O) emissions in tropical and subtropical regions remain unclear. We performed an incubation experiment with tea field soils from different stand ages (5, 15, and 30 years) under different water contents in subtropical (Changsha, Hunan; C5L, C15L, C30L, C5H, C15H, C30H) and tropical regions (Baisha, Hainan; B5L, B15L, B30L, B5H, B15H, B30H). The results showed that the highest net nitrification rate was in C15L and B15. The results indicated that there was more NO3−-N loss in the 15-y tea field soil in both regions. The highest nitrogen oxide emissions from the subtropical and tropical plots were in C15H and B30H. Available K was the key variable for NO and N2O emissions in Changsha county, whereas SOM, pH, and available P were the key factors affecting NO and N2O emissions in Baisha county. Our findings suggest that more attention should be paid to NO3−-N loss in middle-aged (10–30 years) tea fields. Similarly, the focus should be given to nitrogen oxide emissions from middle-aged tea plantations in subtropical regions and old tea plantations (≥30 stand years) in tropical regions.
1. Introduction
In agricultural ecosystems, the increased use of anthropogenic nitrogen fertilizer to meet food production demands impacts the nitrogen cycle [1]. Nitrification, the oxidation of ammonia into nitrate, is a critical process in the nitrogen cycle [2]. NO3−-N produced from nitrification would be vulnerable to losses via runoff, leaching, and denitrification. This process regulates the balance of soil inorganic nitrogen, crop fertilizer use efficiency, nitrate leaching into the subsoil or groundwater [3], and the emissions of nitrogen oxides (NO and N2O) from both nitrification and denitrification [4]. Thus, regulating nitrification is important for improving nitrogen-use efficiency and food production as well as limiting environmental damage. As such, nitrification has received increasing global attention over the past few decades [5].
As the largest tea plantation country in the world, China accounts for 61% of the world’s total tea plantations with over 2.98 million hm2 of tea cultivation areas in 2018 [6]. Tea is an economically important crop that is widely planted in subtropical and tropical China. Nitrogen is not only an important element for tea growth, but is also an important cultivation measure to regulate tea quality and yield. Tea farmers often apply a large amount of nitrogen fertilizer to tea field soils [7]. Some studies have estimated the application rates of nitrogen fertilizer applied to tea field soil to be around 450–1200 kg N hm−2 a−1 [8]. Excessive nitrogen fertilizer application stimulates the primary productivity of the tea field soil and acidifies the tea field soil, which affects the nitrification rate [9]. This impacts the related metabolic activities, which eventually seriously damages tea plant yield and quality, as well as increases N loss [10,11]. The high nitrogen application rate and the decrease in soil pH also promote the production of NO and N2O through microbial and abiotic mechanisms, resulting in nitrogen loss [12,13]. Agricultural soil is an important source of NO and N2O emissions, accounting for 10% and 60% of global anthropogenic emissions, respectively [14]. Stehfest et al. [15] indicated that the annual amount of NO-N and N2O-N released from the soil after fertilization can reach 1.4 Tg and 3.3 Tg, respectively. The average N2O emission factor of tea field soils in China is approximately 2.72%, which is more than twice that of upland soils (1.05%) [16]. A number of studies have indicated that the direct emission factor of NO in tea field soil is as high as 3.15–3.68% [17]. Yao et al. [18] indicated that fertilization significantly increased NO emissions in subtropical tea field soils. Tokuda et al. [19] suggested that long-term over-fertilization promoted N2O emissions in tea plantations. N2O emissions were mainly regulated by the nitrification and denitrification processes [20], while denitrification was reported to be the dominant process generating N2O in tea field soil [12]. Thus, regulating the nitrification process to control the NO3−-N production would be an effective way to lower denitrification and N2O emissions in tea fields. The most important variables that influence nitrification rate and nitrogen gas emissions from tea fields include climate conditions, soil types, fertilization regimes, water content, and the stand ages of tea plants [11,17,18,21].
Increases in tea stand age led to changes in soil properties, such as the decrease in the soil pH value, the relative lack of base ions and trace elements, the increase in SOC, the change of soil aggregates, and the acceleration of net mineralization and nitrification [11,22,23]. These differences have an impact on soil microbial communities and lead to changes in nitrification and NO and N2O emissions [24]. Previous studies have suggested that nitrification and nitrogen gas emissions vary with stand age [8,18]; these studies considered the nitrification rate and nitrogen gas emissions in young stand ages (less than 10-y) [18], or old stand ages (more than 30-y) [9,11,21,25]. Wang et al. [26] reported that soil structure in a 23-y tea field was more stable than that in other plantations, and that soil microbial biomass and activity decreased after 23 years of tea planting. Wang et al. [23] also suggested that large aggregates were concentrated in the 17-y tea field soil, which provided relatively favorable soil conditions for the growth and proliferation of bacteria. Previous studies also showed that bacteria and fungi abundance were higher in 20-y tea field soil compared to other stand ages [27,28]. Higher microbial activity in this middle stand age (10–30-y) may also promote nitrification and nitrogen gas emissions. Therefore, it is necessary to understand nitrification rates, NO, and N2O in stand ages between 10-y and 30-y stands to grasp the characteristics of the nitrogen transformation process in tea plantations. Due to the limited distribution of research sites and the limited number of field observations, few studies have analyzed the nitrification rate and nitrogen gas emissions from tea soil in both subtropical and tropical regions.
Soil moisture content is also a sensitive environmental factor that affects the distribution of soil inorganic nitrogen, microbial activity, and enzyme activity during nitrification, as well as the emissions of NO and N2O [29]. Lan et al. [30] showed that the nitrification rate increased with an increase in soil moisture in forests. Water contents vary by season; high water contents often occur after heavy rain in spring and summer. As such, understanding how high and low water contents affect nitrification is critical.
Soil N2O emissions are positively correlated with soil moisture within a certain range [31]. Johansson et al. [32] observed that after long periods of drought, small amounts of rainfall can lead to large soil NO emissions, whereas high amounts of rainfall can prevent NO production. Under high-moisture conditions, the anaerobic environment promotes the microbial denitrification process, and the loss of gaseous nitrogen mainly comes from N2O and N2, which may weaken NO emissions [33]. A more thorough understanding of the impact of stand year and soil moisture on the nitrification rate and soil NO and N2O emissions can improve our evaluation of nitrogen transformation in tea soils and help us take measures to mitigate the related N loss.
An incubation experiment was performed on nitrification and nitrogen gas emissions from tea field soils with different stand ages (5, 15, and 30-y) in subtropical (Changsha, Hunan) and tropical (Baisha, Hainan) China. The objectives of the study were as follows: (I) to examine the potential of nitrification and nitrogen gas (NO and N2O) emissions in response to stand ages and moisture contents in tea fields in subtropical and tropical China; and (II) to identify the underlying factors that influence nitrification and nitrogen gas emissions in different stand ages and moisture contents in these regions.
2. Materials and Methods
2.1. Site Description and Soil Sampling
We sampled tea field soil from Changsha county, Hunan province and Baisha county, Hainan Province. Changsha county (113°19′58′′ E, 28°32′50′′ N) is located in a subtropical region with a subtropical monsoon climate and abundant precipitation. The annual mean precipitation is 1339.2 mm, and the annual mean temperature is 17.5 °C. The selected soils in this area are derived from highly granite-weathered parent material and are classified as Haplic Alfisol (Soil Taxonomy, Washington, DC, USA). Soil samples were collected randomly from tea plantations that had been cultivated for 5 years (5-y), 15 years (15-y), and 30 years (30-y) (C5, C15, and C30, respectively). Baisha county (109°28′18′′ E, 19°11′56′′ N) is located in tropical China, with an annual average rainfall of 1870 mm and an annual mean temperature of 22.7 °C. The stand ages of the tea fields were 5, 15, and 30 years (B5, B15, and B30, respectively). The Baisha tea field soil was Latosol (US soil Taxonomy). About 2kg of soil samples (including twelve soil cores and different placements of the tea tree, in-tree-row space, under-tree space, and fertilization point) were collected at a depth of 0–20 cm from multiple points of the selected field. The soils were air-dried at room temperature and passed through a 2 mm sieve. The basic properties are shown in Table 1.

Table 1.
Basic properties of tea field soils.
The experiment included 12 treatments with different stand ages and moisture contents: C5L, C5H, C15L, C15H, C30L, C30H, B5L, B5H, B15L, B15H, B30L, and B30H (L and H represent low and high moisture content, respectively). A total of 150 g of dry soil was mixed in a 500 mL polypropylene jar. We adjusted the soil water content to 50% water-filled pore space (WFPS), and pre-incubated the soil at 25 °C for 7 days to activate soil microorganisms. After pre-incubation, the different stand age soils were treated with urea (200 mg N kg−1 dry soil) and separated into two moisture contents (50% WFPS-L and 80% WFPS-H) for 71 days at 25 °C. To prevent moisture loss, the polypropylene jars were sealed with cling film, into which pinholes were pierced to allow gas exchange. The cling film was made from PVC. We maintained the moisture content of the incubated soil samples by adding deionized water every 2 days.
2.2. NO and N2O Fluxes Measurement
After 1, 3, 5, 7, 9, 11, 13, 15, 19, 23, 27, 31, 35, 43, 47, 51, 55, 63, 67, and 71 days of incubation, gas samples were taken at 0 min and 40 min after the sealing of the jars in a non-destructive set of triplicates to analyze N2O concentration using a gas chromatograph (GC; 7890A GC System, Agilent Technologies, USA) fitted with an electron capture detector (ECD). NO samples were taken at 0 min and 6 h incubation from the headspace of the jars to analyze NO concentration using a 42i NO-NO-NOX analyzer (Thermo Environmental Instruments Inc., Franklin, MA, USA). The calculation formula for NO and N2O emissions rate is as follows [34]:
where, F is NO and N2O emission flux [μg N·(kg·h)−1]; ρ is the gas density under the standard condition (kg·m−3); v is the gas volume in the incubation flask (mL); w is the weight of air-dried soil (kg); Δc/Δt is the change rate of gas mole fraction in the bottle during gas production; and T is the average temperature in the bottle at the time of sampling (°C).
The cumulative NO and N2O emissions are calculated as follows:
where f is the cumulative emissions (mg N·kg−1); n and i are the sampling times; and ti+1 − ti is the number of days between the two samplings.
2.3. Soil Sampling and Measurements
Soil pH, NH4+-N, and NO3−-N were sampled at 7, 15, 23, 31, 47, and 63 days of incubation in separate destructive triplicates. Soil pH was measured in the supernatant suspension of 1:5 soil and H2O solution using a pH meter (Mettler Toledo, FiveEasy, FE 20). Soil concentrations of NH4+-N and NO3−-N in 2 M KCl solution were determined using a Continuous Flow Analyzer (Skalar Analytical, Breda, The Netherlands). Net nitrification rates were calculated from the difference in NO3−-N concentrations between the final and the initial sampling divided by the incubation period [35]. Other soil basic properties were determined according to Bao et al. [36]. Soil organic matter (SOM) was analyzed by wet digestion with H2SO4-K2Cr2O7, and the Total N (TN) was determined by semi-micro Kjeldahl digestion using Se, CuSO4, and K2SO4 as catalysts. Soil-available Phosphorus (AP) was extracted by 0.5 M NaHCO3 and measured colorimetrically. Available Potassium (AK) was extracted by 1M CH3COONH4 and quantified with a flame photometer (Inesa Instrument, Shanghai, China). Cation exchange capacity (CEC) was determined using the ammonium acetate method at pH 7.0.
2.4. Statistical Analyses
All statistical analyses were conducted in SPSS 26.0 software(SPSS Inc., Chicago, CA, USA). A one-way analysis of ANOVA was used to analyze the differences in the soil properties among the different regions with the three stand ages (three replicates for different stand ages), and the differences were tested using least-significant differences (LSDs) at a level of p < 0.05. In addition, a muti-factor ANOVA was used to evaluate the main and interaction effects of moisture content (low and high), tea stand age (5-y, 15-y, and 30-y), and region (Changsha county and Baisha county) on NO emissions, N2O emissions, and net nitrification. Redundancy analyzes (RDA) were carried out using CANOCO 5 (Microcomputer Power Inc., Ithaca, NY, USA) version. All variables were examined for normality and homogeneity of variance.
3. Results
3.1. Soil Properties
The older the tea field soil, the lower the soil pH. Following the application of urea, the soil pH increased by about 1 unit compared with the beginning of incubation. The 7th day of incubation had the highest soil pH at 6.47. After this peak, the pH gradually decreased to the end of the incubation period. At the end of the incubation period, the soil pH from the two regions decreased the most in 15-y treatments: C15L and B15L decreased to 3.47 and 3.99, respectively. (Figure 1). The NH4+-N content of the tea field soil decreased the most in 15-y treatments. The NH4+-N content of the 30-y treatments was the highest in the treatments, and the highest NH4+-N concentration was observed under low moisture content. The NH4+-N of Changsha county tea field soil showed a decreasing trend during the incubation period. The NH4+-N content was significantly higher in the C15H treatment than in other treatments (p < 0.05), reaching a maximum (361.3 mg N kg−1) on day 7 (Figure 2a). Moreover, the 15-y treatment had the highest net nitrification in Changsha county tea field soil. Additionally, the net nitrification rate was greater with lower water content than with high water content. The highest net nitrification rate of C15L was 2.3 mg N kg−1 d−1 (Figure 3). The NH4+-N content in the tea field soil of Baisha county first increased and then gradually decreased. There was no significant difference on day 15. The NH4+-N content in B5 was higher than it was in B15 after day 23 (Figure. 2b). Soil NO3−-N content was higher in the 15-y treatments than in the 5- and 30-y treatments. Additionally, NO3−-N concentrations were higher under low-moisture conditions when compared to high-moisture conditions. The average NO3−-N content of tea field soil in Changsha county was greatest in C15, followed by C5 and then C30 (Figure 2c). The average NO3−-N content in the tea field soil of Baisha county was greatest in B15, followed by B30, and then B5. The NO3−-N content in the soil of B15L was as high as 223.8 mg N kg−1 and 224.8 mg N kg−1 at day 15 and day 63, respectively. The NO3−-N content in this region showed a trend of increasing and then decreased gradually, except in B30H, in which NO3−-N increased during the entire incubation period (Figure 2d). The net nitrification rate was also higher in B15L than in B5L and B30L. The maximum nitrification rate of B15L was 3.4 mg N kg−1 d−1. However, the soil nitrification rate increased with the increasing age of the tea stands planting years under high-water-content treatments, and reached 2.3 mg N kg−1 d−1 under the B30H treatment (Figure 3).
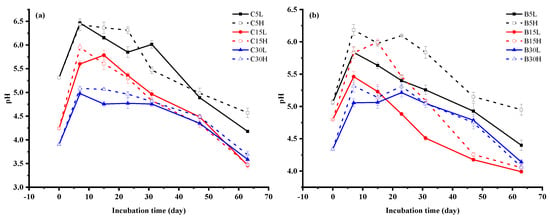
Figure 1.
Soil pH during 71-day incubation period; (a) Changsha, (b) Baisha; L: 50% WFPS, H: 80% WFPS. 5, 15, and 30 represent soil under 5-y, 15-y, and 30-y tea plantations. Error bars indicate standard deviation.
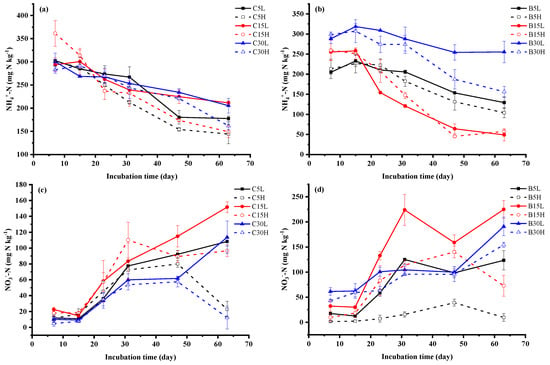
Figure 2.
Soil NH4+-N and NO3−-N concentration during 71-day incubation period; (a,c) Changsha and (b,d) Baisha; L: 50% WFPS, H: 80% WFPS. 5, 15, and 30 represent soil under 5-y, 15-y, and 30-y tea plantations. Error bars indicate standard deviation.
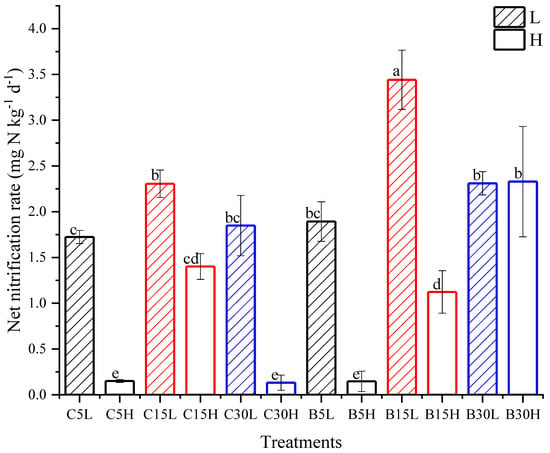
Figure 3.
Soil net nitrification rate during the incubation period. C: Changsha tea field soil, B: Baisha tea field soil. L: 50% WFPS, H: 80% WFPS. 5, 15, and 30 represent soil under 5-y, 15-y, and 30-y tea plantations. Error bars indicate standard deviation. Different letters refer to significant differences between the treatments at the statistical level of 0.05.
3.2. NO and N2O Emissions
During the incubation period, the NO flux of the tea field soil from Changsha county increased significantly after 15 days of incubation, and the peak value of NO emissions in each treatment appeared from day 43 to day 55, and then decreased (Figure 4a). The cumulative NO emissions in different stand years were greatest in C15, followed by C5 and then C30. Cumulative NO emissions under low-water-content treatments were higher than the high-water-content treatments. The NO cumulative emissions from each treatment were in the order of C15L > C5L > C15H > C30L > C5H > C30H, ranging from 1.56 mg N kg−1 in C15L to 0.15 mg N kg−1 in C30H (Figure 5a). B5 and B15 had high NO fluxes under high moisture content at the initial stage of incubation. With increased incubation time, NO fluxes in low-moisture-content treatments were gradually higher than in high-moisture-content treatments, except for B30L and B30H. B15L and B30H had higher NO emissions than other treatments, and the peak values occurred between days 43–47 of incubation (Figure 4b). The cumulative NO emissions from each treatment were in the order of B15L > B30H > B15H > B30L > B5H > B5L, in which the cumulative NO emissions of B15L and B30H were as high as 0.61 mg N kg−1 and 0.53 mg N kg−1, respectively. All treatments, with the exception of B30H, showed that NO emissions in Changsha county were higher than those in Baisha county. Moreover, under the low-moisture-content treatments, the NO cumulative emissions were the highest in the 15-y treatments (Figure 5a).
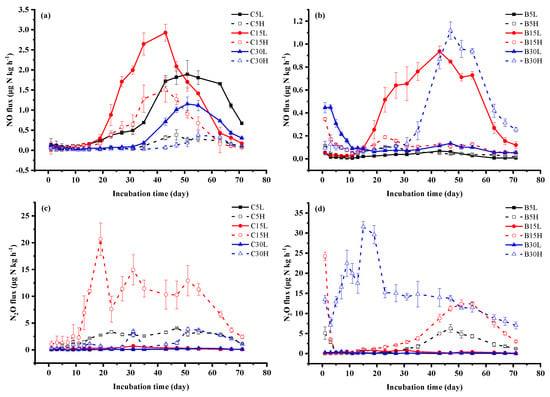
Figure 4.
Soil NO (a,b) and N2O (c,d) fluxes during 71-day incubation period in (a,c) Changsha tea field soil, and Baisha tea field soil (b,d). L: 50% WFPS, H: 80% WFPS; 5, 15, and 30 represent soil under 5-y, 15-y, and 30-y tea plantations. Error bars indicate standard deviation.
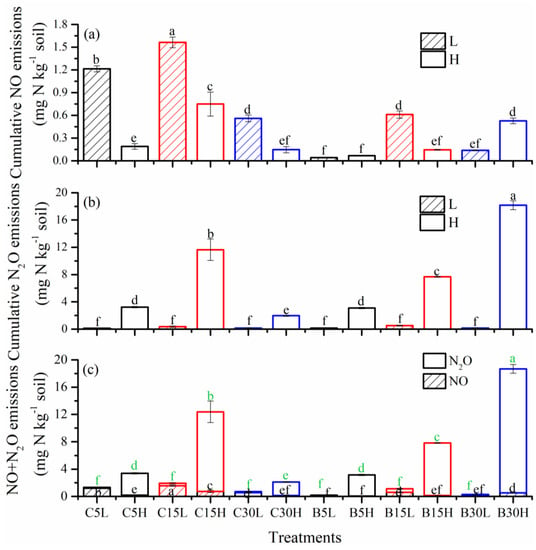
Figure 5.
Soil cumulative NO (a), N2O (b) and NO+N2O (c) emissions during 71-day incubation period. L: 50% WFPS, H: 80% WFPS. 5, 15, and 30 represent soil under 5-y, 15-y, and 30-y tea plantations. Error bars indicate standard deviation. Different letters refer to significant differences between the treatments at the statistical level of 0.05.
The N2O fluxes of Changsha county tea field soil increased after 11 days of incubation. Under the same moisture content condition, the soil N2O emissions were the highest in the 15-y treatments, but N2O emissions were greater with a high water content compared to low water content (Figure 4c). The N2O cumulative emissions of each treatment were in the order of C15H > C5H > C30H > C15L > C30L > C5L, of which the maximum figure for cumulative emissions was 11.64 mg N kg−1 in C15H (Figure 5b). The dynamic variation of N2O fluxes with moisture content in Baisha county under different stand ages was consistent with our findings in Changsha county. The N2O emissions were the highest in B15L, while the N2O fluxes of high-moisture-content treatments first decreased, then increased, and then decreased to the end of the incubation experiment. The N2O emissions increased with the increase in plantation age. The N2O flux peak in B5H and B15H occurred on days 47–55 of incubation, while N2O fluxes peaked at days 11–19 in the B30H treatment, and then decreased to the end of incubation (Figure 4d). Cumulative N2O emissions from different treatments were in the order of B30H > B15H > B5H > B15L > B30L > B5L. Significant differences in N2O emissions were only found under high-moisture-content treatments, among which the maximum figure for cumulative emissions was 18.16 mg N kg−1 in the B30H treatment (Figure 5b).
There was no significant difference in N2O emissions under low-moisture-content treatments, but there was a significant difference in N2O emissions under high-moisture-content treatments (Figure 5b). At the end of the incubation period, the cumulative emissions of NO+N2O in Changsha county tea field soil followed the same trend under different water content treatments, showing that NO and N2O emissions were highest in the 15-y treatments. The emissions in C15L and C15H reached 1.92 and 12.38 mg N kg−1, respectively. NO emissions accounted for 78.29–90.99% of the total emissions from the two gases in the low-moisture-content treatments, while N2O emissions accounted for 93.09–94.44% of the total emissions from the two gases in the high-moisture-content treatments. The highest total emissions of NO+N2O from Baisha tea field soil under low moisture content were 1.11 mg N kg−1 in B15L. The total emissions of soil NO+N2O in high moisture content increased with the increase in plantation age, and gas emissions in B30H were as high as 18.69 mg N kg−1. The total emissions of NO+N2O from tea field soil with different stand ages were higher under high moisture content relative to low moisture content. Except for in the 30-y treatments with high moisture content, the cumulative nitrogen oxide (NO+N2O) emissions were higher in Changsha county tea field soil than in Baisha county tea field soil under the same stand age and water content (Figure 5c).
3.3. The Correlation between Nitrification Rate and Nitrogen Oxide Emissions with Soil Properties
There was a significantly negative correlation between tea field soil NO emissions and NH4+-N in different stand ages in Changsha county (p < 0.05). We observed a significant negative correlation between soil NO emissions and pH, except in C15L and C15H (p < 0.01). Only NO emissions from C5L, C15H, and C30L had a significantly positive correlation with NO3−-N content (p < 0.05). N2O emissions from Changsha county tea field soil were negatively correlated with pH and NH4+-N except for in C15L and C15H. N2O emissions were positively correlated with NO3−-N content except for in C15L and C30H (p < 0.05). There was a significantly negative correlation between soil NO emissions and pH and NH4+-N in B15L and B30H (p < 0.05). NO emissions were positively correlated with NO3−-N only in the B15L treatment (p < 0.01). NO emissions were negatively correlated with NO3−-N in B30L (p < 0.05). N2O emissions from B5H and B15H were negatively correlated with pH and NH4+-N (p < 0.05), but positively correlated with NO3−-N (p < 0.01). That said, B30H showed a contrary correlation compared with B5H and B15H (Table 2). Moisture content, stand age, region, and their interactions significantly (p < 0.05) affected NO, N2O, and the net nitrification rate, with the exception of the impact of region on net nitrification (Table 3). We performed the redundancy analyses (RDA) to explore the soil properties that related to cumulative NO and N2O emissions (Figure 6). In treatments from Changsha county, the first principal component explained 67.35% of the observed variation, while the second principal component accounted for 24.42% of the observed variation. Soil NO and N2O emissions were positively correlated with available K (AK). (Figure 6a). In Baisha county, the first principal component explained about 34.21% of the observed variation, while the second principal component accounted for 6.53%. Both soil NO and N2O emissions were negatively correlated with soil pH and available P (AP). Moreover, soil NO emissions were positively correlated with soil organic matter (SOM), total nitrogen (TN), and AK (Figure 6b). There was a positive correlation between the soil net nitrification rate and cumulative NO emissions (p < 0.01, Figure 6 and Figure 7).

Table 2.
Correlations between soil Nitrogen oxide (NO or N2O) emissions and soil pH and mineral nitrogen.

Table 3.
Results of multi-factor ANOVA with the factors of soil moisture, stand age, and region.
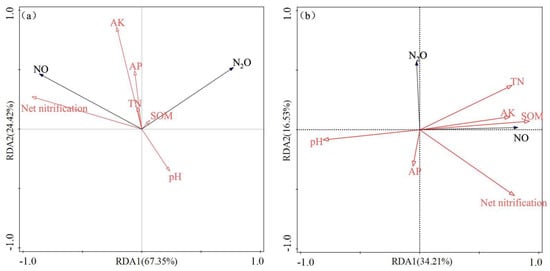
Figure 6.
Redundancy analyses (RDA) of the correlation between NO emissions, N2O emissions, and soil properties in (a) Changsha tea field soil and (b) Baisha tea field soil.
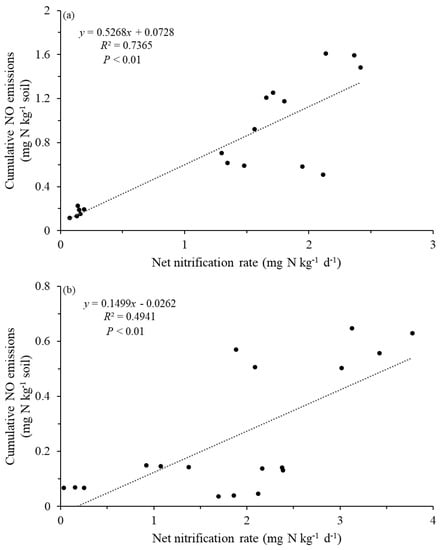
Figure 7.
Correlations of soil net nitrification and NO cumulative emissions; (a) Changsha, (b) Baisha.
4. Discussion
4.1. Effect of Different Stand Ages on Net Nitrification Rate and Nitrogen Gas Emissions in Tea Field Soil
A number of studies reported the nitrification rate in tea field soil, and the nitrification rate was calculated under low water content after N fertilization [11]. Our results showed that the nitrification rate was the highest in the 15-y-stand treatments under 50% WFPS (Figure 3). Nitrate production in tea soil occurs mainly through autotrophic and heterotrophic nitrification [11,37,38]. The autotrophic and heterotrophic nitrification processes were performed by different nitrifiers [39,40]. A significant relationship between the net nitrification rate and NO emissions was observed in both regions (Figure 7), while NO fluxes had a significantly negative correlation with pH in Changsha county except for in the C15 treatment (Table 2). This result indicated that there may be differences in the microbial community of the C15L treatment relative to the C5L and C30L treatments, which can undertake the nitrification process under a wide range of pH [41]. Only the B15L treatment had a significantly negative correlation with pH relative to the B5L and B30L treatments (Table 2). This result also suggested that there may be a different microbial community in the B15L treatment. Previous studies demonstrated that mid-age tea fields had the highest microbial activity [9,42]. Higher amounts of bacteria and fungi have been observed in 20-y tea fields relative to other stand years [27,28]. The soil NH4+-N content was the lowest, and the NO3−-N content was the highest in the C15L and B15L treatments (Figure 2), which may be due to the highest microbial biomass and activity in the tea field soil of 15-y stands relative to 5- and 30-y tea field soil [43]. However, no information on the underlying microbial mechanisms of nitrifiers regulating nitrification was provided in this study, so microbial communities of nitrifiers require further study.
Previous studies suggested that the highest nitrification rates existed in 30-y or 36-y tea plantations [9,11,42]. Our results indicated that 10–30 y tea fields may have the highest nitrification rate, especially under low-water-content conditions. Liu et al. [29] suggested that NO3−-N leaching was greater in winter (low water content) than in spring or summer (high water content) due to increased tea tree N uptake in warmer seasons. This information suggests that avoiding nitrate loss requires specific practices to lower the nitrification rate in tea fields, especially in middle-aged tea plantations ranging from 10 to 30 years old. Ammonium can be used as a substrate for ammonia oxidation, microbial ammonium immobilization, and plant uptake for growth [44]. Given that tea trees prefer to absorb ammonium, organic fertilizers with a high C/N ratio would enhance ammonium immobilization and reduce the ammonia utilization rate of nitrifiers [45], improving overall nitrogen-use efficiency and promoting tea yield.
Tea is an acidophilic crop; with long-term non-rotation cultivation, soil will gradually acidify [9]. Severe soil acidification affects the cycling and absorption of nitrogen by plants. Stand ages also change the physicochemical and biochemical properties of tea field soil, thus affecting soil N cycling [11,22]. In this experiment, the NO and N2O emissions of Changsha county tea field soil in the 15-y treatments were the highest among the treatments under the same moisture content. Meanwhile, the cumulative emissions of the two nitrogen gases in the tea field soil of Baisha county were consistent with those of the same stand age in Changsha county under low moisture content (Figure 5). Higher NO emissions result from higher nitrification rates in the 15-y treatments than in the other treatments (Figure 3). High nitrification rates also produce significant amounts of NO3−-N for denitrification and N2O production [11,46]. However, under high water content, we observed higher NO and N2O emissions in the B30H treatment than in the B5H and B15H treatments, in line with the highest net nitrification in the B30H treatment under high water content (Figure 3). This may result from the higher fungal heterotrophic nitrification and fungal denitrification in this treatment [11,21]. Available K was significantly correlated with NO and N2O emissions in the Changsha county tea field soil (Figure 6). The highest available K content in the C15 treatment is consistent with previous studies showing that available K content was highest in 16-y and 20-y tea field soil relative to other stand ages [28,47]. K fertilizer can stimulate N2O emissions and improve the activity of acidic-tolerant nitrifiers and denitrifiers [48]. K can also inhibit the soil fixation of NH4+-N in soil by competing for the soil fixation site [49]. NO emissions were significantly correlated with SOM content in the Baisha county region (Figure 6), so we proposed that microbes that can utilize the ammonia produced by the mineralization of SOM may play an important role in NO emissions in tropical tea fields [37,50]. N2O emissions were also significantly correlated with AP in the Baisha tea field. Tang et al. [51] showed that the fungi community was more sensitive to AP content than the bacteria counterpart, so we speculated that fungi (through fungi denitrification) may play an important role in N2O emissions in tropical tea plantations.
4.2. Effects of Different Moisture on Net Nitrification Rate and Nitrogen Gas Emissions in Tea Field Soils
The autotrophic nitrification process was favored under aerobic conditions, and high water content can inhibit this process [50]. Thus, higher nitrification rates were reported under low water content compared to high water content, except for in the B30 treatment. Comparable net nitrification rates in B30H relative to B30L may result from facultative anaerobic nitrifiers, which can grow in oxygen-limited conditions [52,53]. This means that different water contents had little impact on net nitrification rates in the B30 treatment. In our experiment, except for in B30, the NO emissions of treatments under low moisture content exceeded those of high moisture content, which was similar to other studies [18,54]. The NO emissions of B30H were higher than that of B30L, and a higher nitrification rate was observed in B30H compared with B30L (Figure 3). Many studies showed that NO emissions were produced by autotrophic and heterotrophic nitrification processes [12,55]. Heterotrophic nitrification may also dominate NO and N2O production under certain conditions [56]. Under high-moisture conditions, NO and N2O emissions in Baisha county tea field soil increased with stand ages, which corresponds to the high net nitrification rate in the B30H treatment (Figure 3). In this experiment, the correlation between soil N2O emissions and pH and mineral nitrogen in B30H was contrary to that of the other treatments (Table 2). N2O emissions showed a significantly positive correlation with pH and NH4+-N, and a significantly negative correlation with NO3−-N in the B30H treatment, which differed from the positive correlation between N2O emissions and NO3−-N found in the other treatments (Table 2). This may be due to the high rate of soil mineralization in B30H, as supported by high NH4+-N contents during the incubation period, which provided a substantial substrate for nitrifiers and denitrifiers, resulting in high N2O emissions [57,58]. Considering the abundant rainfall in Baisha county, high NO and N2O emissions may occur in 30-y tea field soil.
5. Conclusions
An incubation experiment was conducted to explore the response of different stand ages on net nitrification and nitrogen oxides in tropical and subtropical tea field soil. Our findings highlighted that the highest net nitrification was in 15-y tea field soil under low water content irrespective of region. Nitrogen oxide emissions responded differently to stand ages in the tea fields of different regions, the highest nitrogen oxide emissions were in the 15-y subtropical tea field and 30-y tropical tea field soils. Given that the tea tree prefers ammonium over nitrate as its nitrogen source, nitrification would be detrimental to the growth of tea, while the NO3−-N produced by nitrification would be vulnerable to losses in middle-stand-age tea fields (10–30-y). Measures such as nitrification inhibitors or organic fertilizer with a high C/N ratio can be used to reduce N loss and improve tea yield in tea fields, especially for middle-stand-age tea fields. The different impacts of stand ages on nitrogen oxide emissions in tropical and subtropical tea fields should be considered in order to obtain an accurate estimation of nitrogen oxide levels in tea fields. To better understand the changes in nitrification rates and nitrogen oxide among different stand ages of tea fields, the soil functional microorganisms involved in the nitrification and denitrification processes require further investigation.
Author Contributions
L.M. and Y.W. designed the research; R.T. and Y.H. performed trials; R.T. and T.H. performed statistical analysis; R.T. and Y.Z. wrote the manuscript; L.M. and Y.W. commented on and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Hainan Provincial Natural Science Foundation of China (320QN196, 320RC493), National Natural Science Foundation of China (41807044) and Priming Scientific Research Foundation of Hainan University (KYQD (ZR) 1847) to Yanzheng Wu.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Elizabeth for her assistance with English language and grammatical editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.C.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of thenitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Hink, L.; Rangin, G.C.; Nicol, G.W.; Prosser, J.I.W. The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J. 2018, 12, 1084–1093. [Google Scholar] [CrossRef]
- Pilegaard, K. Processes regulating nitric oxide emissions from soils. Philos. Trans. R. Soc. B 2013, 368, 20130126. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2007, 3, 17074. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization. FAOSTAT Database Collections; FAO: Rome, Italy, 2018; Available online: www.fao.org/faostat/ (accessed on 16 March 2022).
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Han, W.Y. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef]
- Han, W.; Xu, J.; Wei, K.; Shi, Y.; Ma, L. Estimation of N2O emission from tea garden soils, their adjacent vegetable garden and forest soils in eastern China. Environ. Earth Sci. 2013, 70, 2495–2500. [Google Scholar] [CrossRef]
- Han, W.Y.; Kemmitt, S.J.; Brookes, P.C. Soil microbial biomass and activity in Chinese tea gardensof varying stand age and productivity. Soil Biol. Biochem. 2007, 39, 1468–1478. [Google Scholar] [CrossRef]
- Fu, X.Q.; Li, Y.; Su, W.J.; Shen, J.L.; Xiao, R.L.; Tong, C.L.; Wu, J. annual dynamics of N2O emissions from a tea field in southern subtropical China. Plant Soil Environ. 2012, 58, 73–378. [Google Scholar] [CrossRef]
- Zhu, T.B.; Zhang, J.B.; Meng, T.; Zhang, Y.; Yang, J.; Müller, C.; Cai, Z.C. Tea plantation destroys soil retention of NO3− and increases N2O emissions in subtropical China. Soil Biol. Biochem. 2014, 73, 106–114. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Wang, C.; Fu, X.; Liu, X.; Shen, J.; Wang, Y.; Xiao, R.; Liu, D.L.; Wu, J. Measurement and modeling of nitrous and nitric oxide emissions from a tea field in subtropical central China. Nutr. Cycl. Agroecosyst. 2017, 107, 157–173. [Google Scholar] [CrossRef]
- Dannenmann, M.; Butterbach-Bahl, K.; Gasche, R.; Willibald, G.; Papen, H. Dinitrogen emissions and the N2:N2O emission ratio of a Rendzic Leptosol as influenced by pH and forest thinning. Soil Biol. Biochem. 2008, 40, 2317–2323. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, UK, 2014. [Google Scholar]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosyst. 2006, 74, 207–228. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Fu, X.; Wu, Y. Is green tea still ‘green’? Geo-Geogr. Environ. 2016, 3, e00021. [Google Scholar] [CrossRef]
- Yao, Z.; Wei, Y.; Liu, C.; Zheng, X.; Xie, B. Organically fertilized tea plantation stimulates N2O emissions and lowers NO fluxes in subtropical China. Biogeosciences 2015, 12, 5915–5928. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Liu, C.; Wang, R.; Xie, B.; Butterbach-Bahl, K. Stand age amplifies greenhouse gas and NO releases following conversion of rice paddy to tea plantations in subtropical China. Agric. Forest Meteorol. 2018, 248, 386–396. [Google Scholar] [CrossRef]
- Tokuda, S.; Hayatsu, M. Nitrous oxide production from strongly acid tea field soils. Soil Sci. Plant Nutr. 2000, 46, 835–844. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Zheng, N.; Yu, Y.; Wang, J.; Chapman, S.J.; Yao, H.; Zhang, Y. The conversion of subtropical forest to tea plantation changes the fungal community and the contribution of fungi to N2O production. Environ. Pollut. 2020, 265, 115106. [Google Scholar] [CrossRef] [PubMed]
- Kamau, D.M.; Spiertz, J.H.J.; Oenema, O. Carbon and nutrient stocks of tea plantations differing in age, genotype and plant population density. Plant Soil. 2008, 307, 29–39. [Google Scholar] [CrossRef]
- Wang, S.; Yao, X.; Ye, S. Soil aggregate-related organic carbon and relevant enzyme activities as affected by tea (Camellia sinensis L.) planting age in hilly region of southern Guangxi, China. Appl. Soil Ecol. 2020, 150, 103444. [Google Scholar] [CrossRef]
- Frostegård, Å.; Vick, S.H.W.; Lim, N.Y.N.; Bakken, L.R.; Shapleigh, J.P. Linking meta-omics to the kinetics of denitrification intermediates reveals pH-dependent causes of N2O emissions and nitrite accumulation in soil. ISME J. 2022, 16, 26–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chapman, S.J.; Yao, H.; Zheng, N.; Müller, C. Tea plantation affects soil nitrogen transformations in subtropical China. J. Soil Sediment. 2020, 21, 441–451. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Zheng, Z. Distribution of microbial biomass and activity within soil aggregates as affected by tea plantation age. Catena 2017, 153, 1–8. [Google Scholar] [CrossRef]
- Chen, P.F.; Liu, Y.Z.; Mo, C.Y.; Jiang, Z.H.; Yang, J.P.; Lin, J.D. Microbial mechanism of biochar addition on nitrogen leaching and retention in tea soils from different plantation ages. Sci. Total Environ. 2020, 757, 143817. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.X.; Li, Y.C.; Li, Y.F.; Chen, Z.H. Effects of different tea plantation ages on soil microbial community structure and diversity. Ying Yong Sheng Tai Xue Bao = Chin. J. Appl. Ecol. 2020, 31, 2749–2758. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, Q.; Zhou, Z.; Liao, K.; Lai, X. Soil nitrate leaching of tea plantation and its responses to seasonal drought and wetness scenarios. Agric. Water Manag. 2022, 260, 107325. [Google Scholar] [CrossRef]
- Lan, T.; Liu, R.; Deng, O.; Chen, D. Stimulation of heterotrophic nitrification and N2O production, inhibition of autotrophic nitrification in soil by adding readily degradable carbon. J. Soil Sediment. 2019, 20, 81–90. [Google Scholar] [CrossRef]
- Kachenchart, B.; Jones, D.L.; Gajaseni, N.; Edwards-Jones, G.; Limsakul, A. Seasonal nitrous oxide emissions from different land uses and their controlling factors in a tropical riparian ecosystem. Agric. Ecosyst. Environ. 2012, 158, 15–30. [Google Scholar] [CrossRef]
- Johansson, C.; Rodhe, H. Emission of NO in a tropical savanna and a cloud forest during the dry season. J. Geophys. Res. 1988, 93, 7180–7192. [Google Scholar] [CrossRef]
- del-Prado, A.; Merino, P.; Estavillo, J.M.; Pinto, M.; González-Murua, C. N2O and NO emissions from different N sources and under a range of soil water contents. Nutr. Cycl. Agroecosyst. 2006, 74, 229–243. [Google Scholar] [CrossRef]
- Yu, J.; Lin, S.; Shaaban, M.; Ju, W.; Fang, L. Nitrous oxide emissions from tea garden soil following the addition of urea and rapeseed cake. J. Soil Sediment. 2020, 20, 3330–3339. [Google Scholar] [CrossRef]
- Wang, J.; Tu, X.; Zhang, H.; Cui, J.; Ni, K.; Chen, J.; Cheng, Y.; Zhang, J.B.; Chang, S.X. Effects of ammonium-based nitrogen addition on soil nitrification and nitrogen gas emissions depend on fertilizer-induced changes in pH in a tea plantation soil. Sci. Total Environ. 2020, 747, 141340. [Google Scholar] [CrossRef]
- Bao, S.D. Agricultural and Chemistry Analysis of Soil; Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Medinets, S.; Skiba, U.; Rennenberg, H.; Butterbach-Bahl, K. A review of soil NO transformation: Associated processes and possible physiological significance on organisms. Soil Biol. Biochem. 2015, 80, 92–117. [Google Scholar] [CrossRef]
- Qiao, C.; Xu, B.; Han, Y.; Wang, J.; Wang, X.; Liu, L.; Liu, W.; Wan, S.; Tan, H.; Liu, Y.; et al. Synthetic nitrogen fertilizers alter the soil chemistry, production and quality of tea. A meta-analysis. Agron. Sustain Dev. 2018, 38, 10. [Google Scholar] [CrossRef]
- He, J.Z.; Hu, H.W.; Zhang, L.M. Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils. Soil Biol. Biochem. 2012, 55, 146–154. [Google Scholar] [CrossRef]
- Yokoyama, K.; Jinnai, K.; Sakiyama, Y.; Touma, M. Contribution of fungi to acetylene-tolerant and high ammonia availability-dependent nitrification potential in tea field soils with relatively neutral pH. Appl. Soil Ecol. 2012, 62, 37–41. [Google Scholar] [CrossRef]
- Yang, X.; Ni, K.; Shi, Y.; Yi, X.; Ji, L.; Ma, L.; Ruan, J. Heavy nitrogen application increases soil nitrification through ammonia-oxidizing bacteria rather than archaea in acidic tea (Camellia sinensis L.) plantation soil. Sci. Total Environ. 2020, 717, 137248. [Google Scholar] [CrossRef]
- Xue, D.; Yao, H.Y.; Huang, C.Y. Study on soil microial properties and enzyme activities in tea gardens. J. Soil Water Conserv. 2005, 2, 84–87. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Tian, Y.; Zhang, H.; Cheng, Y.; Zhang, J.B. A soil management strategy for ameliorating soil acidification and reducing nitrification in tea plantations. Eur. J. Soil Biol. 2018, 88, 36–40. [Google Scholar] [CrossRef]
- Dan, X.; Meng, L.; He, M.; He, X.; Zhao, C.; Chen, S.; Zhang, J.; Cai, Z.; Müller, C. Regulation of nitrogen acquisition in vegetables by different impacts on autotrophic and heterotrophic nitrification. Plant Soil 2022, 474, 581–594. [Google Scholar] [CrossRef]
- Chen, H.H.; Li, X.C.; Hu, F.; Shi, W. Soil nitrous oxide emissions following crop residue addition: A meta-analysis. Glob. Change Biol. 2013, 19, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Weier, K.L.; Doran, J.W.; Power, J.F.; Walters, D.T. Denitrification and the dinitrogen nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Sci. Soc. Am. J. 1993, 57, 66–72. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Zheng, Z.; Zhang, X.; Chen, H.Y. Soil organic carbon and nutrients associated with aggregate fractions in a chronosequence of tea plantations. Ecol. Indic. 2019, 101, 444–452. [Google Scholar] [CrossRef]
- Li, Z.; Xia, S.; Zhang, R.; Zhang, R.; Chen, F.; Liu, Y. N2O emissions and product ratios of nitrification and denitrification are altered by K fertilizer in acidic agricultural soils. Environ. Pollut. 2020, 265, 115065. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soils 2010, 47, 1–14. [Google Scholar] [CrossRef]
- Ji, C.; Li, S.; Geng, Y.; Zou, J. Decreased N2O and NO emissions associated with stimulated denitrification following biochar amendment in subtropical tea plantations. Geoderma 2020, 365, 114223. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, J.; Pan, W.; Tang, R.; Ma, Q.; Xu, M.; Qi, T.; Ma, Z.; Fu, H.; Wu, L. Impact of N application rate on tea (Camellia sinensis) growth and soil bacterial and fungi communities. Plant Soil 2022, 475, 343–359. [Google Scholar] [CrossRef]
- Lu, S.; Liu, X.; Ma, Z.; Liu, Q.; Wu, Z.; Zeng, X.; Shi, X.; Gu, Z. Vertical segregation and phylogenetic characterization of ammonia-oxidizing bacteria and archaea in the sediment of a freshwater aquaculture pond. Front. Microbiol. 2016, 6, 1539. [Google Scholar] [CrossRef]
- Li, P.P.; Han, Y.L.; He, J.Z.; Zhang, S.Q.; Zhang, L.M. Soil aggregate size and long-term fertilization effects on the function and community of ammonia oxidizers. Geoderma 2019, 338, 107–117. [Google Scholar] [CrossRef]
- Yu, J.; Meixner, F.X.; Sun, W.; Mamtimin, B.; Wang, G.; Qi, X.; Xia, C.; Xie, W. Nitric oxide emissions from black soil, northeastern China: A laboratory study revealing significantly lower rates than hitherto reported. Soil Biol. Biochem. 2010, 42, 1784–1792. [Google Scholar] [CrossRef]
- Loick, N.; Dixon, E.R.; Abalos, D.; Vallejo, A.; Matthews, G.P.; McGeough, K.L.; Well, R.; Watson, C.J.; Laughlin, R.J.; Cardenas, L.M. Denitrification as a source of nitric oxide emissions from incubated soil cores from a UK grassland soil. Soil Biol. Biochem. 2016, 95, 1–7. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, J.; Zhu, T.; Cheng, Y. Stimulation of NO and N2O emissions from soils by SO2 deposition. Glob. Change Biol. 2012, 18, 2280–2291. [Google Scholar] [CrossRef]
- Chen, C.F.; Lin, J.Y. Estimating the gross budget of applied nitrogen and phosphorus in tea plantations. Sustain. Environ. Res. 2016, 26, 124–130. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Chang, S.X.; Cai, Z.C.; Müller, C.; Zhang, J.B. Nitrogen deposition affects both net and gross soil nitrogen transformations in forest ecosystems: A review. Environ. Pollut. 2019, 244, 608–616. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).