Utility of Comprehensive GC×GC Gas Chromatography in Finding Varietal Markers among Volatile Compounds in Non-Aromatic Red Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wine Samples
2.2. Standards, Chemicals, and Consumables
2.3. GC-MS and GC×GC-TOF-MS Analysis of Volatile Aroma Compounds
2.4. Statistical Data Elaboration
3. Results and Discussion
3.1. Standard Physico-Chemical Parameters
3.2. GC-MS Volatile Metabolome
3.3. GC×GC-TOF-MS Volatile Metabolome
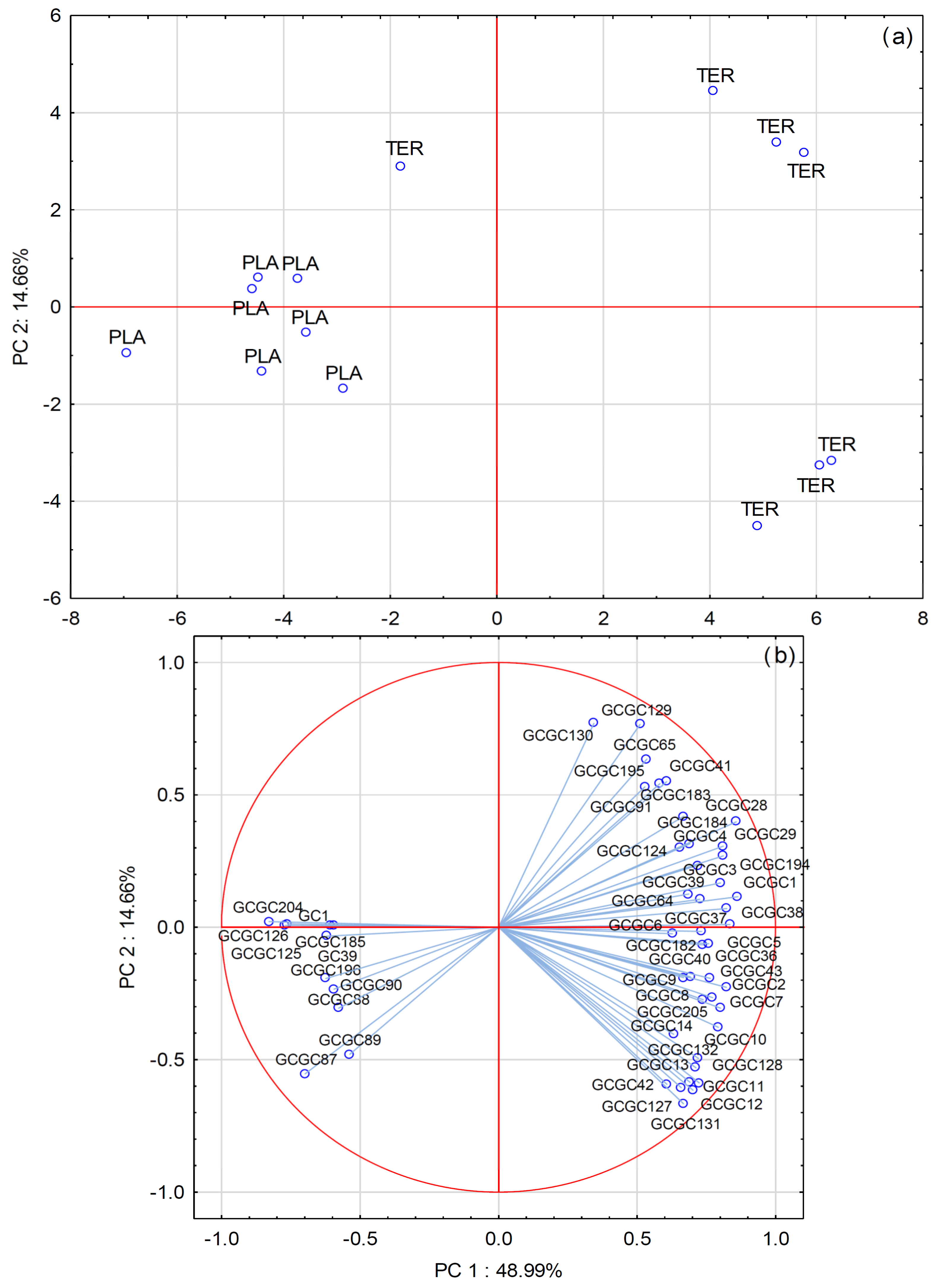
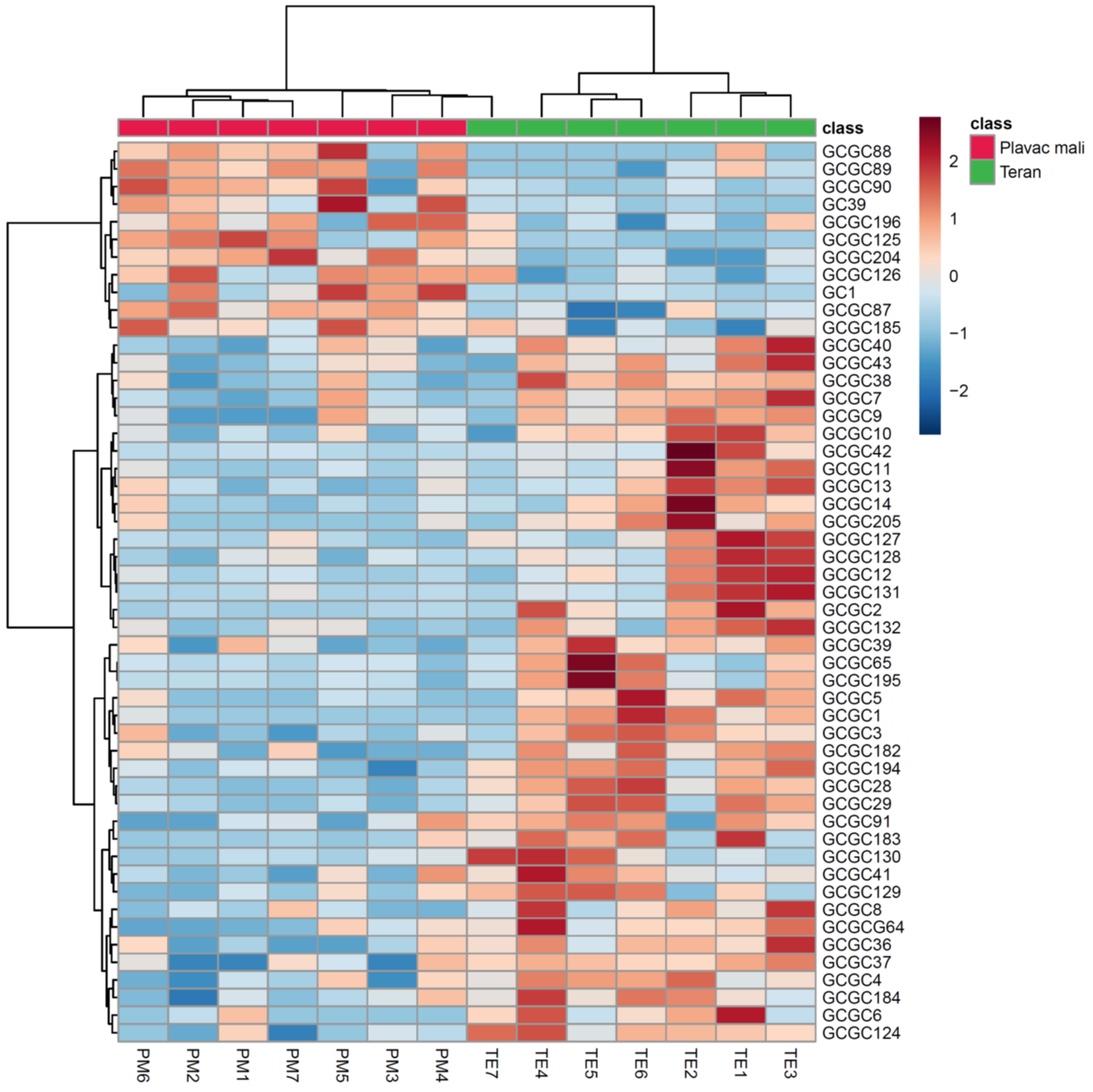
3.4. Multivariate Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Organisation of Vine and Wine (OIV) State of the World Vitivinicultural Sector in 2020. Available online: https://www.oiv.int/public/medias/7909/oiv-state-of-the-world-vitivinicultural-sector-in-2020.pdf (accessed on 23 August 2022).
- Valls Fonayet, J.; Loupit, G.; Richard, T. MS- and NMR-metabolomic tools for the discrimination of wines: Applications for authenticity. In Advances in Botanical Research: Plant Metabolomics in Full Swing, 1st ed.; Petriacq, P., Bouchereau, A., Eds.; Academic Press: San Diego, CA, USA, 2021; Volume 98, pp. 297–357. [Google Scholar]
- Cadot, Y.; Caillé, S.; Samson, A.; Barbeau, G.; Cheynier, V. Sensory representation of typicality of Cabernet franc wines related to phenolic composition: Impact of ripening stage and maceration time. Anal. Chim. Acta 2012, 732, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, Y.; Jaffre, J.; Valentin, D. Sensory space of typical Chardonnay wines and its relation to volatile composition. In Proceedings of the 8th Pangborn Sensory Science Symposium, Florence, Italy, 26–30 July 2009; p. 23. [Google Scholar]
- Lukić, I.; Carlin, S.; Vrhovsek, U. Comprehensive 2D Gas Chromatography with TOF-MS Detection Confirms the Matchless Discriminatory Power of Monoterpenes and Provides In-Depth Volatile Profile Information for Highly Efficient White Wine Varietal Differentiation. Foods 2020, 9, 1787. [Google Scholar] [CrossRef] [PubMed]
- Parr, W.V.; Green, J.A.; White, K.G.; Sherlock, R.R. The distinctive flavor of New Zealand Sauvignon blanc: Sensory characterization by wine professionals. Food Qual. Prefer. 2007, 18, 849–861. [Google Scholar] [CrossRef]
- Schüttler, A.; Friedel, M.; Jung, R.; Rauhut, D.; Darriet, P. Characterizing aromatic typicality of Riesling wines: Merging volatile compositional and sensory aspects. Food Res. Int. 2015, 69, 26–37. [Google Scholar] [CrossRef]
- Holmberg, L. Wine fraud. Int. J. Wine Res. 2010, 2, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Stupak, M.; Goodall, I.; Tomaniova, M.; Pulkrabova, J.; Hajslova, J. A novel approach to assess the quality and authenticity of Scotch whisky based on gas chromatography coupled to high resolution mass spectrometry. Anal. Chim. Acta 2018, 1042, 60–70. [Google Scholar] [CrossRef]
- Villano, C.; Lisanti, M.T.; Gambuti, A.; Vecchio, R.; Moio, L.; Frusciante, L.; Aversano, R.; Carputo, D. Wine varietal authentication based on phenolics, volatiles and DNA markers: State of the art, perspectives and drawbacks. Food Control 2017, 80, 1–10. [Google Scholar] [CrossRef]
- Ribérau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine—Stabilization and Treatments, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; Volume 2. [Google Scholar]
- Del Barrio-Galán, R.; del Valle-Herrero, H.; Bueno-Herrera, M.; López-de-la-Cuesta, P.; Pérez-Magariño, S. Volatile and Non-Volatile Characterization of White and Rosé Wines from Different Spanish Protected Designations of Origin. Beverages 2021, 7, 49. [Google Scholar] [CrossRef]
- Fabani, M.P.; Ravera, M.J.A.; Wunderlin, D.A. Markers of typical red wine varieties from the Valley of Tulum (San Juan-Argentina) based on VOCs profile and chemometrics. Food Chem. 2013, 141, 1055–1062. [Google Scholar] [CrossRef]
- Gómez García-Carpintero, E.; Sánchez-Palomo, E.; Gómez Gallego, M.A.; González-Viñas, M.A. Free and bound volatile compounds as markers of aromatic typicalness of Moravia Dulce, Rojal and Tortosí red wines. Food Chem. 2012, 131, 90–98. [Google Scholar] [CrossRef]
- Valentin, L.; Barroso, L.P.; Barbosa, R.M.; de Paulo, G.A.; Castro, I.A. Chemical typicality of South American red wines classified according to their volatile and phenolic compounds using multivariate analysis. Food Chem. 2020, 302, 125340. [Google Scholar] [CrossRef]
- Ziółkowska, A.; Wąsowicz, E.; Jeleń, H.H. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chem. 2016, 213, 714–720. [Google Scholar] [CrossRef]
- Purcaro, G.; Cordero, C.; Liberto, E.; Bicchi, C.; Conte, L.S. Toward a definition of blueprint of virgin olive oil by comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2014, 1334, 101–111. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Donato, P.; Cacciola, F.; Beccaria, M.; Dugo, P.; Mondello, L. Potential of comprehensive chromatography in food analysis. Trends Anal. Chem. 2013, 52, 186–205. [Google Scholar] [CrossRef]
- Barbará, J.A.; Primieri Nicolli, K.; Souza-Silva, É.A.; Telles Biasoto, A.C.; Welke, J.E.; Alcaraz Zini, C. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2020, 308, 125552. [Google Scholar] [CrossRef]
- Carlin, S.; Vrhovsek, U.; Lonardi, A.; Landi, L.; Mattivi, F. Aromatic complexity in Verdicchio wines: A case study. OENO One 2019, 53, 597–610. [Google Scholar] [CrossRef]
- Šuklje, K.; Carlin, S.; Antalick, G.; Blackman, J.W.; Deloire, A.; Vrhovsek, U.; Schmidtke, L.M. Regional Discrimination of Australian Shiraz Wine Volatome by Two-Dimensional Gas Chromatography Coupled to Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 10273–10284. [Google Scholar] [CrossRef]
- Šuklje, K.; Carlin, S.; Stanstrup, J.; Antalick, G.; Blackman, J.W.; Meeks, C.; Deloire, A.; Schmidtke, L.M.; Vrhovsek, U. Unravelling wine volatile evolution during shiraz grape ripening by untargeted HS-SPME-GC×GC-TOFMS. Food Chem. 2019, 277, 753–765. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. Influence of Yeast Strain, Canopy Management, and Site on the Volatile Composition and Sensory Attributes of Cabernet Sauvignon Wines from Western Australia. J. Agric. Food Chem. 2011, 59, 3273–3284. [Google Scholar] [CrossRef]
- Ryan, D.; Watkins, P.; Smith, J.; Allen, M.; Marriott, P. Analysis of methoxypyrazines in wine using headspace solid phase microextraction with isotope dilution and comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2005, 28, 1075–1082. [Google Scholar] [CrossRef]
- Weldegergis, B.T.; de Villiers, A.; McNeish, C.; Seethapathy, S.; Mostafa, A.; Górecki, T.; Crouch, A.M. Characterisation of volatile components of Pinotage wines using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC × GC–TOFMS). Food Chem. 2011, 129, 188–199. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazarotto, M.; Alcaraz Zini, C. Characterization of the volatile profile of Brazilian Merlot wines through comprehensive two-dimensional gas chromatography time-of-flight mass spectrometric detection. J. Chromatogr. A 2012, 1226, 124–139. [Google Scholar] [CrossRef] [Green Version]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Differentiation of wines according to grape variety using multivariate analysis of comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection dana. Food Chem. 2013, 141, 3897–3905. [Google Scholar] [CrossRef] [Green Version]
- Bubola, M.; Lukić, I.; Radeka, S.; Sivilotti, P.; Grozić, K.; Vanzo, A.; Bavčar, D.; Lisjak, K. Enhancement of Istrian Malvasia wine aroma and hydroxycinnamate composition by hand and mechanical leaf removal. J. Food Sci. Agric. 2019, 99, 904–914. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 53. [Google Scholar] [CrossRef]
- Carlin, S.; Vrhovsek, U.; Franceschi, P.; Lotti, C.; Bontempo, L.; Camin, F.; Toubiana, D.; Zottele, F.; Toller, G.; Fait, A.; et al. Regional features of northern Italian sparkling wines, identified using solid-phase microextraction and comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. Food Chem. 2016, 208, 68–80. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Lukić, I.; Radeka, S.; Budić-Leto, I.; Bubola, M.; Vrhovsek, U. Targeted UPLC-QqQ-MS/MS profiling of phenolic compounds for differentiation of monovarietal wines and corroboration of particular varietal typicity concepts. Food Chem. 2019, 300, 125251. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Zalacain, A.; Marín, J.; Alonso, G.L.; Salinas, M.R. Analysis of wine primary aroma compounds by stir bar sorptive extraction. Talanta 2007, 71, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Marais, J.; van Wyk, C.J.; Rapp, A. Effect of sunlight and shade on norisoprenoids levels in maturing Weisser Riesling and Chenin blanc grapes and Weisser Riesling wines. S. Afr. J. Enol. Vitic. 1992, 13, 23–32. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M. Carotenoid breakdown products—The norisoprenoids—In wine aroma. Arch. Biochem. Biophys. 2009, 483, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Kotseridis, Y.; Baumes, R.L.; Bertrand, A.; Skouroumounis, G.K. Quantitative Determination of β-Ionone in Red Wines and Grapes of Bordeaux Using a Stable Isotope Dilution Assay. J. Chromatogr. A 1999, 848, 317–325. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Which impact for β-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Pinilla, O.; Guadalupe, Z.; Ayestarán, B.; Pérez-Magariño, S.; Ortega-Heras, M. Characterization of volatile compounds and olfactory profile of red minority varietal wines from La Rioja. J. Sci. Food Agric. 2013, 93, 3720–3729. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.A.; Zea, L.; Moyano, L.; Medina, M. Aroma compounds as markers of the changes in sherry wines subjected to biological ageing. Food Control 2005, 16, 333–338. [Google Scholar] [CrossRef]
- Stilo, F.; Del Pilar Segura Borrego, M.; Bicchi, C.; Battaglino, S.; Callejón Fernadez, R.M.; Morales, M.L.; Reichenbach, S.E.; McCurry, J.; Peroni, D.; Cordero, C. Delineating the extra-virgin olive oil aroma blueprint by multiple headspace solid phase microextraction and differential-flow modulated comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2021, 1650, 462232. [Google Scholar] [CrossRef]
- Belancic, A.; Agosin, E.; Ibacache, A.; Bordeu, E.; Baumes, R.; Razungles, A.; Bayonove, C. Influence of sun exposure on the aromatic composition of chilean Muscat grape cultivars Moscatel de Alejandria and Moscatel rosada. Am. J. Enol. Vitic. 1997, 48, 181–186. [Google Scholar]
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (Vitis vinifera) fruit. BMC Plant Biol. 2014, 14, 108. [Google Scholar] [CrossRef] [Green Version]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Torres, R.; Ferrara, G.; Soto, F.; López, J.A.; Sanchez, F.; Mazzeo, A.; Pérez-Pastor, A.; Domingo, R. Effects of soil and climate in a table grape vineyard with cover crops. Irrigation management using sensors networks. Ciênc. Téc. Vitiviníc. 2017, 32, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Bedbabis, S.; Ben Rouina, B.; Boukhris, M.; Ferrara, G. Effects of irrigation with treated wastewater on root and fruit mineral elements of Chemlali olive cultivar. Sci. World J. 2014, 2014, 973638. [Google Scholar] [CrossRef]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Odor potency of aroma compounds in Riesling and Vidal blanc table wines and icewines by gas chromatography-olfactometry mass spectrometry. J. Agric. Food Chem. 2012, 60, 2874–2883. [Google Scholar] [CrossRef]
- Pietra Torres, M.; Cabrita, M.; Gomes Da Silva, M.; Palma, V.; Costa Freitas, A. The impact of malolactic fermentation on the volatile composition of the Trincadeira wine variety. J. Food Biochem. 2011, 35, 898–913. [Google Scholar] [CrossRef]
- Louw, L.; Tredoux, A.G.J.; Van Rensburg, P.; Kidd, M.; Naes, T.; Nieuwoudt, H.H. Fermentation derived aroma compounds in varietal young wines from South Africa. S. Afr. J. Enol. Vitic. 2010, 31, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Verstrepen, K.J.; Van Laere, S.D.M.; Vanderhaegen, B.M.P.; Derdelinckx, G.; Dufour, J.-P.; Pretorius, I.S.; Winderickx, J.; Thevelein, J.M.; Delvaux, F.R. Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl. Environ. Microbiol. J. 2003, 69, 5228–5237. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Changes in volatile composition of Madeira wines during their oxidative ageing. Anal. Chim. Acta 2006, 563, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Lorenzo, R.; Bloem, A.; Farines, V.; Sablayrolles, J.-M.; Camarasa, C. How to modulate the formation of negative volatile sulfur compounds during wine fermentation? FEMS Yeast Res. 2021, 21, foab038. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Bekker, M.Z.; Smith, P.A.; Wilkes, E.N. Sources of volatile sulfur compounds in wine. Aust. J. Grape Wine Res. 2015, 21, 705–712. [Google Scholar] [CrossRef]
- Moreira, N.; Guedes de Pinho, P.; Santos, C.; Vasconcelos, I. Volatile sulphur compounds composition of monovarietal white wines. Food Chem. 2010, 123, 1198–1203. [Google Scholar] [CrossRef]

| Physico-Chemical Parameter | Variety | |
|---|---|---|
| PM | TE | |
| Specific gravity 20 °C/20 °C | 0.9944 ± 0.0016 | 0.9950 ± 0.0010 |
| Alcoholic strength (vol. %) | 14.2 ± 0.5 a | 13.0 ± 0.9 b |
| Total acidity (as mg/L tartaric acid) | 5.3 ± 0.3 | 6.7 ± 1.8 |
| Volatile acidity (as mg/L acetic acid) | 0.27 ± 0.08 | 0.35 ± 0.09 |
| Total dry extract without reducing sugars (g/L) | 29.9 ± 3.7 | 29.0 ± 3.8 |
| pH | 3.63 ± 0.12 | 3.44 ± 0.28 |
| No. | Volatile Compounds | tR | ID | LRIexp | LRIlit | F-Ratio | Variety | |
|---|---|---|---|---|---|---|---|---|
| (min:sec) | PM | TE | ||||||
| Terpenes | ||||||||
| 1 | Limonene | 15:39 | MS, LRI | 1191 | 1196 | 7.00 | 0.43 ± 0.18 a | 0.26 ± 0.02 b |
| 2 | Linalool | 33:08 | S, MS, LRI | 1542 | 1542 | 3.93 | 8.40 ± 3.84 | 11.98 ± 2.85 |
| 3 | 4-Terpineol | 35:37 | MS, LRI | 1594 | 1596 | 1.62 | 0.85 ± 0.67 | 2.05 ± 2.41 |
| 4 | β-Pinene | 14:06 | MS, LRI | 1146 | 1145 | 1.37 | 0.54 ± 0.25 | 0.41 ± 0.16 |
| 5 | trans-Nerolidol | 54:34 | MS, LRI | 2031 | 2031 | 0.58 | 2.86 ± 2.30 | 2.14 ± 0.99 |
| 6 | Geraniol | 46:35 | S, MS, LRI | 1838 | 1838 | 0.48 | 12.3 ± 6.6 | 18.3 ± 9.1 |
| 7 | Geranyl acetone | 46:59 | MS, LRI | 1845 | 1845 | 0.43 | 3.20 ± 1.29 | 3.87 ± 2.36 |
| 8 | Citronellol | 43:10 | S, MS, LRI | 1758 | 1758 | 0.37 | 9.81 ± 1.57 | 10.51 ± 2.63 |
| 9 | α-Terpineol | 39:58 | S, MS, LRI | 1684 | 1684 | 0.18 | 3.02 ± 0.56 | 4.37 ± 1.49 |
| C13-Norisoprenoids | ||||||||
| 10 | Vitispirane I | 32:10 | MS, LRI | 1521 | 1526 | 2.76 | 0.46 ± 0.16 | 0.28 ± 0.25 |
| 11 | β-Ionone | 50:15 | S, MS, LRI | 1923 | 1923 | 2.27 | 0.12 ± 0.05 | 0.16 ± 0.05 |
| 12 | β-Damascenone | 45:25 | MS, LRI | 1809 | 1809 | 1.30 | 1.05 ± 0.39 | 0.84 ± 0.30 |
| 13 | Actinidol ethyl ether II | 41:47 | MS, LRI | 1723 | 1723 | 0.64 | 0.12 ± 0.07 | 0.20 ± 0.25 |
| 14 | Actinidol ethyl ether I | 40:24 | MS, LRI | 1690 | 1690 | 0.60 | 0.21 ± 0.12 | 0.34 ± 0.42 |
| 15 | Vitispirane II | 32:18 | MS, LRI | 1523 | 1529 | 0.33 | 0.19 ± 0.09 | 0.23 ± 0.16 |
| 16 | Actinidol I | 49:53 | MS, LRI | 1914 | 1914 | 0.09 | 0.10 ± 0.04 | 0.11 ± 0.10 |
| 17 | Actinidol II | 50:25 | MS, LRI | 1927 | 1927 | 0.02 | 0.18 ± 0.07 | 0.19 ± 0.16 |
| Alcohols | ||||||||
| 18 | trans-3-Hexen-1-ol | 23:57 | S, MS, LRI | 1361 | 1361 | 0.36 | 55.0 ± 25.2 | 47.3 ± 22.4 |
| 19 | 1-Hexanol | 23:29 | S, MS, LRI | 1356 | 1357 | 0.19 | 1385 ± 441 | 1466 ± 228 |
| 20 | cis-3-Hexen-1-ol | 24:57 | S, MS, LRI | 1379 | 1379 | 0.03 | 72.4 ± 25.6 | 80.9 ± 20.1 |
| 21 | 2-Phenylethanol | 48:55 | S, MS, LRI | 1891 | 1893 | 0.00 | 41,647 ± 12,069 | 41,712 ± 7813 |
| Acids | ||||||||
| 22 | Octanoic Acid | 54:51 | S, MS, LRI | 2043 | 2042 | 3.70 | 1356 ± 283 | 1086 ± 240 |
| 23 | Decanoic Acid | 62:47 | S, MS, LRI | 2257 | 2258 | 2.65 | 199.1 ± 73.6 | 144.3 ± 50.1 |
| 24 | Butyric acid | 36:25 | S, MS, LRI | 1612 | 1612 | 1.98 | 934 ± 109 | 1078 ± 248 |
| 25 | Hexanoic acid | 46:08 | S, MS, LRI | 1830 | 1828 | 0.00 | 1588 ± 377 | 1597 ± 321 |
| Esters | ||||||||
| 26 | Isoamyl lactate II | 34:04 | MS, LRI | 1562 | 1570 | 4.96 | 8.59 ± 4.40 b | 16.01 ± 7.63 a |
| 27 | Isoamyl lactate I | 33:57 | MS, LRI | 1561 | 1570 | 3.88 | 1.52 ± 1.08 | 3.05 ± 1.74 |
| 28 | 2-Phenethyl acetate | 45:02 | S, MS, LRI | 1803 | 1801 | 3.38 | 1369 ± 556 | 912 ± 351 |
| 29 | Isoamyl acetate | 12:29 | S, MS, LRI | 1120 | 1122 | 2.03 | 879 ± 286 | 659 ± 291 |
| 30 | Ethyl decanoate | 37:43 | S, MS, LRI | 1637 | 1638 | 1.97 | 55.0 ± 23.5 | 40.9 ± 12.4 |
| 31 | Ethyl butyrate | 09:24 | S, MS, LRI | 1030 | 1030 | 1.75 | 236.0 ± 58.2 | 197.1 ± 51.9 |
| 32 | Hexyl acetate | 19:30 | S, MS, LRI | 1272 | 1272 | 1.31 | 26.26 ± 33.67 | 11.14 ± 9.40 |
| 33 | Ethyl-3-methylbutyrate | 10:28 | S, MS, LRI | 1065 | 1065 | 1.20 | 17.29 ± 5.72 | 23.57 ± 8.60 |
| 34 | Ethyl octanoate | 27:48 | S, MS, LRI | 1435 | 1435 | 1.10 | 153.7 ± 141.5 | 96.5 ± 28.1 |
| 35 | Ethyl hexanoate | 17:38 | S, MS, LRI | 1236 | 1236 | 0.99 | 284.0 ± 313.1 | 165.1 ± 50.6 |
| 36 | Diethyl succinate | 39:06 | S, MS, LRI | 1667 | 1669 | 0.28 | 3375 ± 2094 | 4069 ± 2750 |
| 37 | Ethyl 2-methylbutyrate | 09:57 | S, MS, LRI | 1049 | 1049 | 0.28 | 11.15 ± 4.06 | 15.83 ± 7.39 |
| 38 | Ethyl lactate | 22:48 | S, MS, LRI | 1341 | 1341 | 0.17 | 77,793 ± 27,760 | 71,695 ± 28,375 |
| Benzenoids and phenols | ||||||||
| 39 | Ethyl cinnamate | 57:31 | S, MS, LRI | 2111 | 2122 | 11.32 | 0.36 ± 0.16 a | 0.14 ± 0.04 b |
| 40 | Eugenol | 58:45 | S, MS, LRI | 2148 | 2146 | 1.12 | 0.79 ± 0.72 | 1.09 ± 0.25 |
| No. | Volatile Compounds | tR (1D) | tR (2D) | ID | LRIexp | LRIlit | F-Ratio | Variety | |
|---|---|---|---|---|---|---|---|---|---|
| (min:sec) | (min:sec) | PM | TE | ||||||
| Terpenes | |||||||||
| 1 | Ho-trienol | 17:07.0 | 00:01.0 | MS, LRI | 1607 | 1612 | 17.13 | 0.07 ± 0.18 b | 1.12 ± 0.65 a |
| 2 | 10,11-Epoxycalamenene | 22:14.3 | 00:01.7 | MS, LRI | 1893 | 1900 | 12.11 | 0.04 ± 0.06 b | 0.86 ± 0.62 a |
| 3 | Linalool | 15:50.0 | 00:01.1 | S, MS, LRI | 1541 | 1541 | 10.72 | 27.1 ± 11.2 b | 47.3 ± 11.9 a |
| 4 | Dihydro-γ-terpineol | 17:42.0 | 00:01.1 | MS, LRI | 1644 | 1650 | 10.42 | 1.43 ± 0.34 b | 1.95 ± 0.25 a |
| 5 | cis-Ocimene | 09:22.7 | 00:01.6 | MS, LRI | 1253 | 1255 | 9.99 | 0.62 ± 0.90 b | 3.22 ± 1.98 a |
| 6 | Pinocarvone | 16:15.7 | 00:01.5 | MS, LRI | 1563 | 1561 | 8.39 | 0.04 ± 0.08 b | 0.21 ± 0.14 a |
| 7 | α-Curcumene | 20:16.0 | 00:01.8 | MS, LRI | 1787 | 1782 | 7.97 | 0.11 ± 0.07 b | 0.23 ± 0.08 a |
| 8 | Cadalene | 27:48.0 | 00:01.5 | MS, LRI | >2100 | 2256 | 7.56 | 0.27 ± 0.20 b | 0.68 ± 0.34 a |
| 9 | α-Farnesene | 19:48.0 | 00:01.9 | S, MS, LRI | 1763 | 1762 | 5.97 | 0.18 ± 0.18 b | 0.40 ± 0.17 a |
| 10 | α-Terpineol | 18:48.0 | 00:01.2 | S, MS, LRI | 1711 | 1709 | 5.72 | 6.32 ± 2.43 b | 11.14 ± 4.75 a |
| 11 | Geranyl ethyl ether | 15:08.0 | 00:01.8 | MS, LRI | 1506 | 1506 | 5.64 | 1.37 ± 0.76 b | 3.69 ± 2.47 a |
| 12 | o-Cymene | 09:48.5 | 00:01.6 | S, MS, LRI | 1272 | 1268 | 5.55 | 1.94 ± 0.74 b | 5.11 ± 3.48 a |
| 13 | Linalool ethyl ether | 11:02.0 | 00:01.9 | MS, LRI | 1329 | 1331 | 5.45 | 3.71 ± 1.79 b | 6.95 ± 3.20 a |
| 14 | trans-Alloocimene | 12:12.5 | 00:01.6 | MS, LRI | 1384 | 1388 | 5.27 | 0.29 ± 0.16 b | 0.63 ± 0.36 a |
| 15 | β-Cyclocitral | 17:21.0 | 00:01.5 | S, MS, LRI | 1622 | 1629 | 4.36 | 1.19 ± 0.51 | 1.68 ± 0.34 |
| 16 | p-Cymenene | 13:37.0 | 00:01.4 | MS, LRI | 1443 | 1438 | 3.76 | 11.52 ± 2.69 | 16.06 ± 5.58 |
| 17 | Nerolidol | 25:03.0 | 00:01.3 | S, MS, LRI | 2040 | 2039 | 3.26 | 0.71 ± 0.82 | 1.45 ± 0.71 |
| 18 | β-Myrcene | 07:17.4 | 00:01.6 | MS, LRI | 1158 | 1162 | 2.61 | 1.14 ± 1.04 | 2.10 ± 1.18 |
| 19 | 4-Terpineol | 17:00.0 | 00:01.3 | S, MS, LRI | 1600 | 1606 | 2.52 | 8.42 ± 5.58 | 24.75 ± 26.66 |
| 20 | γ-Isogeraniol | 20:30.0 | 00:01.0 | MS, LRI | 1799 | 1800 | 2.43 | 1.43 ± 0.98 | 2.22 ± 0.92 |
| 21 | Geraniol | 21:33.0 | 00:01.0 | S, MS, LRI | 1856 | 1857 | 1.88 | 3.99 ± 2.73 | 6.33 ± 3.59 |
| 22 | trans-Menthone | 14:05.5 | 00:01.6 | MS, LRI | 1463 | 1464 | 1.30 | 1.01 ± 0.34 | 1.34 ± 0.68 |
| 23 | 1-Terpineol | 16:25.0 | 00:01.2 | MS, LRI | 1571 | 1565 | 1.19 | 0.61 ± 0.51 | 0.32 ± 0.46 |
| 24 | o-Cymenene | 13:16.0 | 00:01.5 | MS | 1429 | na | 1.10 | 0.87 ± 0.25 | 0.98 ± 0.15 |
| 25 | Citronellol | 20:01.5 | 00:01.1 | S, MS, LRI | 1775 | 1777 | 0.67 | 14.94 ± 3.13 | 18.34 ± 10.51 |
| 26 | Cuparene enantiomer | 21:05.0 | 00:02.0 | MS, LRI | 1831 | 1830 | 0.63 | 0.17 ± 0.03 | 0.20 ± 0.08 |
| 27 | Sesquiterpene (n.i.) | 19:41.5 | 00:01.1 | MS, LRI | 1757 | na | 0.35 | 0.21 ± 0.12 | 0.18 ± 0.09 |
| Norisoprenoids | |||||||||
| 28 | Theaspirane I | 14:54.0 | 00:02.1 | S, MS, LRI | 1496 | 1482 | 37.49 | 0.54 ± 0.34 b | 3.03 ± 1.02 a |
| 29 | Theaspirane II | 15:43.0 | 00:02.0 | S, MS, LRI | 1536 | 1520 | 17.70 | 0.46 ± 0.35 b | 2.18 ± 1.02 a |
| 30 | trans-1-(2,3,6-Trimethylphenyl)buta-1,3-diene (TPB) | 21:12.0 | 00:01.5 | MS, LRI | 1837 | 1816 | 3.88 | 0.13 ± 0.10 | 0.35 ± 0.28 |
| 31 | 1,2-Dihydro-1,1,6-trimethylnaphthalene (TDN) | 19:41.0 | 00:01.6 | S, MS, LRI | 1757 | 1751 | 3.09 | 0.57 ± 0.24 | 1.02 ± 0.64 |
| 32 | cis-β-Damascenone | 20:01.0 | 00:01.6 | MS, LRI | 1774 | 1791 | 1.90 | 0.52 ± 0.23 | 0.75 ± 0.37 |
| 33 | Actinidol ethyl ether II | 18:52.0 | 00:01.9 | MS, LRI | 1714 | 1723 | 1.73 | 0.63 ± 0.51 | 2.17 ± 3.06 |
| 34 | α-Isophorone | 16:46.0 | 00:01.3 | S, MS, LRI | 1588 | 1593 | 1.35 | 1.60 ± 0.62 | 1.99 ± 0.63 |
| 35 | trans-β-Damascenone | 21:05.0 | 00:01.5 | S, MS, LRI | 1831 | 1838 | 1.01 | 20.92 ± 7.83 | 26.33 ± 11.89 |
| 36 | Safranal | 17:49.0 | 00:01.4 | S, MS, LRI | 1651 | 1648 | 0.63 | 0.80 ± 0.27 | 0.90 ± 0.20 |
| 37 | Vitispirane I | 15:24.6 | 00:02.0 | MS, LRI | 1520 | 1526 | 0.31 | 0.22 ± 0.41 | 0.12 ± 0.24 |
| 38 | Vitispirane II | 15:29.0 | 00:01.9 | MS, LRI | 1524 | 1529 | 0.25 | 38.2 ± 16.7 | 46.6 ± 41.2 |
| Benzenoids | |||||||||
| 39 | Ethyl salicylate | 20:51.0 | 00:01.2 | S, MS, LRI | 1818 | 1820 | 10.75 | 0.11 ± 0.12 b | 0.32 ± 0.11 a |
| 40 | Estragole | 18:17.0 | 00:01.3 | MS, LRI | 1681 | 1683 | 9.92 | 0.12 ± 0.11 b | 0.26 ± 0.04 a |
| 41 | 1-Methoxy-4,4a,5,6,7,8-hexahydro-2(3H)-naphthalenone | 19:34.0 | 00:01.5 | MS | 1751 | na | 7.93 | 9.92 ± 2.69 b | 14.08 ± 2.84 a |
| 42 | 2,3-Dihydrobenzofuran | 16:46.0 | 00:01.2 | MS | 1588 | na | 6.74 | 0.47 ± 0.12 b | 0.63 ± 0.11 a |
| 43 | 4-Ethylbenzaldehyde | 19:34.5 | 00:01.2 | MS, LRI | 1751 | 1747 | 6.46 | 0.47 ± 0.13 b | 0.66 ± 0.15 a |
| 44 | Benzenemethanol | 22:01.0 | 00:00.9 | S, MS, LRI | 1881 | 1877 | 5.38 | 16.7 ± 10.3 b | 29.5 ± 10.5 a |
| 45 | Ethyl benzeneacetate | 20:30.0 | 00:01.2 | S, MS, LRI | 1799 | 1788 | 4.89 | 35.4 ± 13.8 b | 169.0 ± 159.2 a |
| 46 | 3-Ethylbenzaldehyde | 19:01.0 | 00:01.2 | MS, LRI | 1722 | 1732 | 4.85 | 0.76 ± 0.10 b | 0.92 ± 0.17 a |
| 47 | 2-Methylbenzaldehyde | 17:27.5 | 00:01.1 | MS, LRI | 1629 | 1622 | 3.48 | 0.81 ± 0.31 | 1.10 ± 0.26 |
| 48 | 1,2-Dimethylbenzene | 07:40.0 | 00:05.1 | MS, LRI | 1175 | 1181 | 3.18 | 6.48 ± 2.25 | 8.52 ± 2.04 |
| 49 | Benzeneethanol | 22:40.1 | 00:01.0 | S, MS, LRI | 1916 | 1897 | 2.97 | 0.93 ± 1.30 | 0.08 ± 0.12 |
| 50 | trans-Anethole | 21:12.0 | 00:01.3 | S, MS, LRI | 1837 | 1834 | 2.88 | 2.32 ± 1.60 | 3.79 ± 1.64 |
| 51 | cis-Anethole | 19:55.0 | 00:01.3 | S, MS, LRI | 1769 | 1759 | 2.68 | 0.09 ± 0.05 | 0.13 ± 0.05 |
| 52 | Benzaldehyde | 15:22.0 | 00:01.1 | S, MS, LRI | 1518 | 1509 | 2.53 | 26.5 ± 11.4 | 78.2 ± 85.4 |
| 53 | 2,3-Dihydro-4-methyl-1H-indene | 14:54.0 | 00:01.6 | MS, LRI | 1496 | 1506 | 2.39 | 0.40 ± 0.28 | 0.57 ± 0.11 |
| 54 | Ethyl hydroxycinnamate | 22:15.0 | 00:01.3 | MS, LRI | 1894 | 1897 | 2.36 | 1.27 ± 0.62 | 2.40 ± 1.84 |
| 55 | Methyl salicylate | 20:16.0 | 00:01.2 | S, MS, LRI | 1787 | 1789 | 2.01 | 19.1 ± 11.8 | 30.5 ± 17.7 |
| 56 | Ethyl benzoate | 18:17.0 | 00:01.2 | MS, LRI | 1681 | 1678 | 1.55 | 10.11 ± 2.78 | 13.47 ± 6.57 |
| 57 | Benzeneacetaldehyde | 17:49.0 | 00:01.1 | S, MS, LRI | 1651 | 1648 | 1.53 | 139.6 ± 39.1 | 167.8 ± 45.8 |
| 58 | Benzonitrile | 17:00.5 | 00:01.0 | MS, LRI | 1601 | 1591 | 1.53 | 0.49 ± 0.18 | 0.60 ± 0.17 |
| 59 | 1,4-Dimethoxybenzene | 19:34.5 | 00:01.2 | MS, LRI | 1751 | 1752 | 1.43 | 1.44 ± 0.48 | 1.69 ± 0.28 |
| 60 | Azulene | 19:34.0 | 00:01.3 | MS, LRI | 1751 | 1740 | 1.37 | 1.99 ± 0.53 | 2.26 ± 0.30 |
| 61 | m-Dimethoxybenzene | 19:48.0 | 00:01.2 | MS, LRI | 1763 | 1761 | 0.63 | 0.35 ± 0.26 | 0.71 ± 1.15 |
| 62 | Benzoic Acid | 30:48.5 | 00:00.8 | S, MS, LRI | >2100 | 2433 | 0.62 | 4.05 ± 1.05 | 4.50 ± 1.11 |
| 63 | Butylated hydroxytoluene | 22:43.0 | 00:01.5 | MS, LRI | 1919 | 1914 | 0.20 | 3.27 ± 2.23 | 2.84 ± 1.25 |
| 64 | 2-Ethyl-m-xylene | 12:00.0 | 00:01.6 | MS, LRI | 1374 | 1372 | 0.16 | 1.32 ± 0.65 | 1.21 ± 0.39 |
| 65 | Styrene | 09:28.5 | 00:05.0 | MS, LRI | 1257 | 1257 | 0.12 | 14.14 ± 5.49 | 15.16 ± 5.80 |
| 66 | Ethylbenzene | 06:26.5 | 00:05.1 | MS, LRI | 1119 | 1116 | 0.04 | 3.30 ± 5.22 | 2.72 ± 5.80 |
| Aldehydes and ketones | |||||||||
| 67 | 3-Ethoxy-2-butanone | 05:55.0 | 00:01.1 | MS | 1095 | na | 9.52 | 23.3 ± 20.1 b | 59.0 ± 23.1 a |
| 68 | 3,4-Dihydroxy-3-cyclobutene-1,2-dione | 18:10.0 | 00:01.1 | MS | 1673 | na | 4.89 | 0.48 ± 0.12 b | 0.98 ± 0.59 a |
| 69 | 2-Dodecanone | 18:59.0 | 00:01.6 | MS, LRI | 1720 | 1709 | 4.69 | 0.47 ± 0.35 | 0.81 ± 0.24 |
| 70 | 2-Octanone | 10:16.9 | 00:01.4 | S, MS, LRI | 1294 | 1294 | 4.38 | 14.51 ± 16.07 | 1.76 ± 1.31 |
| 71 | 3-Hydroxy-4-phenyl-2-butanone | 29:06.4 | 00:01.0 | MS, LRI | >2100 | 2259 | 4.27 | 0.01 ± 0.01 | 0.24 ± 0.30 |
| 72 | Acetophenone | 17:56.0 | 00:01.1 | S, MS, LRI | 1659 | 1660 | 4.09 | 3.28 ± 0.82 | 4.24 ± 0.94 |
| 73 | Decanal | 14:54.0 | 00:01.5 | S, MS, LRI | 1496 | 1497 | 3.90 | 4.49 ± 1.21 | 6.94 ± 3.05 |
| 74 | 6-Methyl-5-hepten-2-one | 11:24.0 | 00:01.3 | S, MS, LRI | 1346 | 1343 | 3.04 | 5.46 ± 5.31 | 1.80 ± 1.62 |
| 75 | 3-Hydroxybutanone | 10:08.1 | 00:00.8 | MS, LRI | 1287 | 1287 | 2.75 | 0.89 ± 0.46 | 1.67 ± 1.16 |
| 76 | 2-Hydroxy-5-methylacetophenone | 27:25.5 | 00:01.0 | MS, LRI | >2100 | 2188 | 1.81 | 1.86 ± 0.94 | 1.11 ± 1.15 |
| 77 | p-tert-Butylcyclohexanone | 17:35.0 | 00:01.5 | MS, LRI | 1637 | 1645 | 1.36 | 0.4 ± 0.31 | 1.82 ± 3.21 |
| 78 | Octanal | 10:21.0 | 00:01.4 | S, MS, LRI | 1297 | 1297 | 1.07 | 5.61 ± 3.04 | 7.42 ± 3.49 |
| 79 | 3-Penten-2-one | 06:39.8 | 00:01.0 | MS, LRI | 1129 | 1133 | 0.99 | 0.33 ± 0.28 | 0.64 ± 0.77 |
| 80 | 2,3-Pentanedione | 05:22.7 | 00:00.9 | MS, LRI | 1067 | 1072 | 0.91 | 5.00 ± 3.60 | 7.74 ± 6.69 |
| 81 | 2-Undecanone | 16:53.5 | 00:01.5 | MS, LRI | 1595 | 1598 | 0.59 | 2.62 ± 2.01 | 2.02 ± 0.44 |
| 82 | trans-2-Decenal | 17:50.6 | 00:01.4 | S, MS, LRI | 1651 | 1647 | 0.59 | 0.19 ± 0.40 | 0.35 ± 0.38 |
| 83 | 2-Heptanone | 07:48.2 | 00:01.3 | S, MS, LRI | 1181 | 1180 | 0.57 | 14.26 ± 14.47 | 9.15 ± 10.59 |
| 84 | Undecanal | 17:06.5 | 00:01.5 | S, MS, LRI | 1607 | 1610 | 0.56 | 0.42 ± 0.23 | 0.52 ± 0.30 |
| 85 | 2,2,6-Trimethylcyclohexanone | 10:49.6 | 00:01.6 | MS, LRI | 1319 | 1323 | 0.52 | 2.80 ± 2.00 | 2.12 ± 1.54 |
| 86 | Nonanal | 12:44.2 | 00:01.5 | S, MS, LRI | 1408 | 1404 | 0.45 | 217 ± 178 | 273 ± 129 |
| 87 | Dodecanal | 19:06.0 | 00:01.6 | S, MS, LRI | 1727 | 1722 | 0.04 | 0.50 ± 0.39 | 0.46 ± 0.19 |
| Alcohols | |||||||||
| 88 | 2,3-Butadienol | 15:43.0 | 00:00.8 | MS, LRI | 1536 | 1542 | 17.16 | 71.96 ± 9.45 a | 42.82 ± 16.04 b |
| 89 | 4-Octanol | 12:20.0 | 00:01.0 | MS, LRI | 1390 | 1376 | 11.41 | 0.13 ± 0.07 a | 0.02 ± 0.05 b |
| 90 | 1-Octen-3-ol | 13:51.0 | 00:01.0 | S, MS, LRI | 1453 | 1452 | 9.93 | 260.5 ± 79.0 a | 146.5 ± 54.1 b |
| 91 | 4-Hepten-1-ol | 14:51.0 | 00:00.9 | MS, LRI | 1493 | 1487 | 9.21 | 26.09 ± 12.48 a | 11.41 ± 2.83 b |
| 92 | 2-(1-Ethoxyethoxy)-3-methyl-1,4-butanediol | 15:57.0 | 00:01.0 | MS | 1547 | na | 5.06 | 1.08 ± 1.17 b | 2.49 ± 1.18 a |
| 93 | 1-Pentylcyclopentanol | 13:23.0 | 00:01.6 | MS | 1434 | na | 3.44 | 1.01 ± 0.55 | 0.53 ± 0.39 |
| 94 | 1-Penten-3-ol | 07:27.2 | 00:00.8 | MS, LRI | 1165 | 1162 | 3.07 | 5.47 ± 4.84 | 13.84 ± 11.67 |
| 95 | 2-Heptanol | 10:56.0 | 00:01.0 | S, MS, LRI | 1324 | 1320 | 2.42 | 36.0 ± 20.3 | 61.2 ± 37.7 |
| 96 | 3-Octanol | 12:37.0 | 00:01.0 | MS, LRI | 1403 | 1408 | 2.11 | 30.81 ± 18.54 | 19.79 ± 7.71 |
| 97 | 2-Penten-1-ol | 10:56.0 | 00:00.8 | MS, LRI | 1324 | 1321 | 2.06 | 0.96 ± 0.35 | 1.54 ± 1.00 |
| 98 | 3-Ethoxy-1-propanol | 12:13.0 | 00:00.9 | MS, LRI | 1384 | 1389 | 2.00 | 1.29 ± 1.42 | 4.05 ± 4.95 |
| 99 | 2-Nonanol | 15:15.0 | 00:01.1 | S, MS, LRI | 1512 | 1510 | 1.96 | 9.53 ± 5.19 | 14.87 ± 8.63 |
| 100 | 2-Octen-1-ol | 17:18.0 | 00:00.9 | S, MS, LRI | 1619 | 1617 | 1.68 | 8.50 ± 3.49 | 6.62 ± 1.59 |
| 101 | 5-Methyl-2-heptanol | 12:15.0 | 00:00.9 | MS, LRI | 1386 | 1394 | 1.57 | 8.70 ± 5.68 | 5.50 ± 3.68 |
| 102 | 2-Undecanol | 19:13.0 | 00:01.2 | MS, LRI | 1733 | 1738 | 1.45 | 0.11 ± 0.08 | 0.17 ± 0.11 |
| 103 | 4-Methyl-3-penten-2-ol | 13:02.0 | 00:01.7 | MS | 1420 | na | 1.40 | 0.93 ± 0.59 | 0.59 ± 0.47 |
| 104 | 3-Pentanol | 06:24.2 | 00:00.9 | MS, LRI | 1117 | 1111 | 1.22 | 1.02 ± 1.47 | 2.88 ± 4.20 |
| 105 | 6-Methyl-1-heptanol | 15:35.4 | 00:01.0 | MS, LRI | 1529 | 1524 | 0.96 | 0.37 ± 0.31 | 0.23 ± 0.21 |
| 106 | 1-Decanol | 20:15.4 | 00:01.1 | S, MS, LRI | 1787 | 1783 | 0.83 | 4.09 ± 3.77 | 2.51 ± 2.63 |
| 107 | 3-Methylhexanol | 12:59.7 | 00:00.9 | MS, LRI | 1418 | 1413 | 0.63 | 2.65 ± 3.47 | 1.50 ± 1.57 |
| 108 | 6-Methyl-5-hepten-2-ol | 14:05.5 | 00:01.0 | S, MS, LRI | 1463 | 1466 | 0.48 | 1.42 ± 1.33 | 1.03 ± 0.63 |
| 109 | 2,6-Dimethyl-5-hepten-1-ol | 17:57.6 | 00:01.0 | MS, LRI | 1660 | 1654 | 0.41 | 2.54 ± 2.71 | 1.75 ± 1.80 |
| 110 | cis-3-Hexen-1-ol | 12:20.0 | 00:00.9 | S, MS, LRI | 1390 | 1384 | 0.38 | 46.90 ± 44.71 | 57.47 ± 8.79 |
| 111 | cis-3-Nonen-1-ol | 18:32.6 | 00:01.0 | MS, LRI | 1697 | 1693 | 0.11 | 3.78 ± 5.49 | 4.54 ± 2.46 |
| 112 | 3-Methylpentanol | 11:06.0 | 00:00.9 | MS, LRI | 1332 | 1332 | 0.09 | 46.7 ± 28.4 | 42.7 ± 21.4 |
| 113 | cis-2-Hexen-1-ol | 13:02.0 | 00:00.9 | S, MS, LRI | 1420 | 1420 | 0.06 | 7.69 ± 2.52 | 7.38 ± 2.41 |
| 114 | 1-Hepten-4-ol | 16:53.5 | 00:01.0 | MS, LRI | 1595 | 1585 | 0.05 | 0.53 ± 0.15 | 0.50 ± 0.31 |
| 115 | 4-Methylpentanol | 10:49.0 | 00:00.9 | MS, LRI | 1319 | 1319 | 0.00 | 42.3 ± 23.1 | 42.6 ± 18.4 |
| Acids | |||||||||
| 116 | Isobutyric acid | 16:18.0 | 00:00.7 | S, MS, LRI | 1565 | 1561 | 3.78 | 63.9 ± 34.4 | 93.5 ± 20.9 |
| 117 | trans-2-Hexenoic acid | 23:39.0 | 00:00.8 | MS, LRI | 1967 | 1967 | 3.03 | 1.01 ± 0.65 | 1.64 ± 0.71 |
| 118 | Pentanoic acid | 19:01.3 | 00:00.7 | S, MS, LRI | 1722 | 1723 | 2.35 | 0.51 ± 0.64 | 2.02 ± 2.52 |
| 119 | Sorbic acid | 26:46.7 | 00:00.8 | MS, LRI | >2100 | 2150 | 2.13 | 6.36 ± 10.91 | 0.33 ± 0.32 |
| 120 | Isohexanoic acid | 20:44.0 | 00:00.8 | MS, LRI | 1812 | 1813 | 1.60 | 0.04 ± 0.05 | 0.07 ± 0.03 |
| 121 | Butyric acid | 17:28.0 | 00:00.7 | S, MS, LRI | 1629 | 1626 | 0.86 | 104.3 ± 15.8 | 112.2 ± 15.8 |
| 122 | Propanoic acid | 15:43.0 | 00:00.7 | S, MS, LRI | 1536 | 1540 | 0.29 | 15.27 ± 2.72 | 16.09 ± 2.99 |
| 123 | Heptanoic Acid | 23:23.5 | 00:00.8 | S, MS, LRI | 1954 | 1949 | 0.01 | 1.89 ± 0.34 | 1.87 ± 0.57 |
| 124 | Isovaleric acid | 18:31.0 | 00:00.8 | S, MS, LRI | 1695 | 1680 | 0.00 | 295.8 ± 69.1 | 296.0 ± 117.2 |
| Esters | |||||||||
| 125 | Ethyl methylhexanoate | 10:35.0 | 00:01.6 | MS | 1308 | na | 17.54 | 0.24 ± 0.17 b | 0.59 ± 0.14 a |
| 126 | Ethyl pentanoate | 06:44.0 | 00:01.4 | MS, LRI | 1132 | 1136 | 10.74 | 8.32 ± 3.39 a | 3.67 ± 1.61 b |
| 127 | Isoamyl 2-methyl-2-propenoate | 07:26.5 | 00:01.2 | MS | 1165 | na | 7.27 | 24.23 ± 8.04 a | 12.88 ± 7.71 b |
| 128 | Diethyl oxalate | 14:33.0 | 00:01.1 | MS, LRI | 1481 | 1496 | 7.07 | 0.31 ± 0.08 b | 0.56 ± 0.24 a |
| 129 | Diethyl fumarate | 17:56.0 | 00:01.2 | MS, LRI | 1659 | 1660 | 6.43 | 0.13 ± 0.10 b | 0.37 ± 0.24 a |
| 130 | Isoamyl lactate | 16:18.0 | 00:01.0 | MS, LRI | 1565 | 1570 | 5.59 | 21.4 ± 16.4 b | 49.9 ± 27.4 a |
| 131 | Butyl ethyl succinate | 20:37.0 | 00:01.3 | MS, LRI | 1806 | 1820 | 5.40 | 6.16 ± 4.48 b | 24.01 ± 19.84 a |
| 132 | Diethyl malate | 25:36.6 | 00:00.9 | MS, LRI | 2069 | 2060 | 4.97 | 1.39 ± 1.76 b | 10.08 ± 10.15 a |
| 133 | Isoamyl isobutyrate | 08:02.4 | 00:01.7 | MS, LRI | 1192 | 1194 | 4.81 | 1.12 ± 1.13 b | 3.63 ± 2.81 a |
| 134 | Isobutyl hexanoate | 11:45.0 | 00:01.8 | MS, LRI | 1362 | 1357 | 4.63 | 2.39 ± 1.20 | 4.90 ± 2.84 |
| 135 | Ethyl 9-decenoate | 18:45.0 | 00:01.6 | S, MS, LRI | 1708 | 1707 | 4.51 | 18.17 ± 15.45 | 5.63 ± 2.39 |
| 136 | Isoamyl hexanoate | 14:05.0 | 00:01.8 | MS, LRI | 1462 | 1458 | 4.29 | 5.93 ± 2.78 | 11.55 ± 6.63 |
| 137 | Ethyl 2-hexenoate | 11:35.5 | 00:01.4 | S, MS, LRI | 1355 | 1357 | 3.72 | 21.8 ± 11.3 | 40.1 ± 22.4 |
| 138 | Heptyl acetate | 12:13.5 | 00:01.5 | S, MS, LRI | 1385 | 1385 | 3.64 | 4.00 ± 2.20 | 1.84 ± 2.02 |
| 139 | Ethyl 2-methylbutyrate | 05:07.0 | 00:01.4 | MS, LRI | 1053 | 1050 | 3.63 | 17.0 ± 14.9 | 36.1 ± 21.9 |
| 140 | Ethyl methyl succinate | 17:36.0 | 00:01.1 | MS, LRI | 1638 | 1631 | 3.29 | 7.29 ± 4.57 | 12.88 ± 6.76 |
| 141 | Isoamyl isoamylate | 10:25.5 | 00:01.8 | MS, LRI | 1300 | 1294 | 3.27 | 1.12 ± 0.62 | 1.74 ± 0.68 |
| 142 | Ethyl isoamyl succinate | 22:36.5 | 00:01.3 | MS, LRI | 1913 | 1907 | 3.22 | 14.5 ± 11.6 | 60.0 ± 66.0 |
| 143 | Ethyl propyl succinate | 20:02.0 | 00:01.2 | MS, LRI | 1775 | 1767 | 3.17 | 2.10 ± 1.82 | 6.43 ± 6.18 |
| 144 | Amyl formate | 08:32.0 | 00:05.6 | MS, LRI | 1214 | 1187 | 3.12 | 12.45 ± 7.31 | 6.86 ± 4.09 |
| 145 | Ethyl 3-methylbutyrate | 05:27.0 | 00:01.3 | S, MS, LRI | 1070 | 1068 | 2.73 | 13.91 ± 7.14 | 21.11 ± 9.06 |
| 146 | Ethyl 3-hydroxybutyrate | 15:15.6 | 00:00.9 | MS, LRI | 1513 | 1512 | 2.59 | 7.56 ± 3.79 | 3.94 ± 4.60 |
| 147 | Phenylmethyl acetate | 19:27.0 | 00:01.2 | S, MS, LRI | 1745 | 1747 | 2.58 | 0.25 ± 0.08 | 0.53 ± 0.47 |
| 148 | Diethyl glutarate | 20:23.0 | 00:01.2 | MS, LRI | 1793 | 1780 | 2.51 | 0.48 ± 0.69 | 1.19 ± 0.95 |
| 149 | Diethyl 2-methylsuccinate | 17:49.0 | 00:01.3 | MS | 1651 | na | 2.26 | 0.17 ± 0.12 | 0.29 ± 0.17 |
| 150 | Isoamyl propanoate | 07:58.5 | 00:01.5 | MS, LRI | 1189 | 1188 | 2.23 | 8.58 ± 3.90 | 13.29 ± 7.38 |
| 151 | Ethyl 2,4-hexadienoate | 14:36.2 | 00:01.3 | MS, LRI | 1483 | 1501 | 1.77 | 0.53 ± 0.77 | 0.14 ± 0.12 |
| 152 | Hexyl acetate | 09:58.0 | 00:01.4 | S, MS, LRI | 1279 | 1277 | 1.76 | 164.9 ± 210.9 | 58.0 ± 30.4 |
| 153 | Ethyl pyruvate | 09:46.0 | 00:04.8 | MS, LRI | 1270 | 1267 | 1.68 | 83.5 ± 59.8 | 49.2 ± 36.4 |
| 154 | Isoamyl butyrate | 09:45.5 | 00:01.6 | S, MS, LRI | 1270 | 1266 | 1.67 | 12.04 ± 6.42 | 16.46 ± 6.37 |
| 155 | Octyl formate | 16:04.9 | 00:01.0 | MS, LRI | 1554 | 1560 | 1.66 | 53.2 ± 27.0 | 38.9 ± 11.6 |
| 156 | 2-Phenylethyl formate | 20:30.0 | 00:01.1 | MS, LRI | 1799 | 1806 | 1.50 | 2.31 ± 0.85 | 2.98 ± 1.18 |
| 157 | trans-3-Hexenyl acetate | 10:42.0 | 00:01.3 | MS, LRI | 1313 | 1316 | 1.47 | 4.52 ± 7.31 | 1.05 ± 1.98 |
| 158 | Ethyl 2-octenoate | 16:04.0 | 00:01.6 | MS, LRI | 1553 | 1557 | 1.43 | 0.19 ± 0.07 | 0.16 ± 0.02 |
| 159 | Ethyl 4-hydroxybutyrate | 20:50.0 | 00:00.9 | MS, LRI | 1817 | 1819 | 1.22 | 42.0 ± 19.0 | 31.2 ± 17.9 |
| 160 | Isobutyl acetate | 04:30.5 | 00:01.1 | S, MS, LRI | 1021 | 1009 | 0.89 | 17.00 ± 8.29 | 22.05 ± 11.48 |
| 161 | Propyl octanoate | 15:22.0 | 00:01.8 | MS, LRI | 1518 | 1504 | 0.88 | 0.85 ± 0.92 | 1.22 ± 0.51 |
| 162 | cis-3-Hexenyl acetate | 10:57.0 | 00:01.3 | MS, LRI | 1325 | 1320 | 0.67 | 15.77 ± 33.94 | 5.23 ± 3.11 |
| 163 | Ethyl heptanoate | 11:17.0 | 00:01.6 | MS, LRI | 1341 | 1342 | 0.64 | 56.0 ± 24.8 | 68.8 ± 34.4 |
| 164 | Isoamyl octanoate | 18:08.0 | 00:01.9 | S, MS, LRI | 1671 | 1671 | 0.63 | 9.32 ± 3.41 | 10.99 ± 4.40 |
| 165 | Propyl hexanoate | 10:58.6 | 00:01.7 | MS, LRI | 1326 | 1319 | 0.54 | 0.27 ± 0.28 | 0.44 ± 0.53 |
| 166 | Ethyl hydrogen succinate | 30:09.0 | 00:00.8 | MS, LRI | >2100 | 2367 | 0.46 | 182 ± 128 | 224 ± 102 |
| 167 | Isobutyl octanoate | 15:57.5 | 00:01.9 | MS, LRI | 1548 | 1551 | 0.45 | 0.77 ± 0.69 | 0.97 ± 0.38 |
| 168 | Ethyl 6-heptenoate | 12:27.0 | 00:01.4 | MS | 1395 | na | 0.28 | 1.48 ± 0.60 | 1.28 ± 0.78 |
| 169 | Isobutyl 2-methyl-2-propenoate | 11:00.2 | 00:00.9 | MS | 1327 | na | 0.25 | 0.60 ± 0.61 | 0.76 ± 0.57 |
| 170 | Ethyl trans-3-hexenoate | 10:37.5 | 00:01.4 | MS, LRI | 1310 | 1295 | 0.22 | 9.86 ± 8.47 | 11.44 ± 2.73 |
| 171 | Methyl decanoate | 16:53.0 | 00:01.6 | MS, LRI | 1594 | 1599 | 0.20 | 3.10 ± 1.55 | 2.78 ± 1.09 |
| 172 | Ethyl isobutyrate | 03:49.0 | 00:01.1 | MS, LRI | <1000 | 962 | 0.19 | 4.29 ± 2.13 | 5.63 ± 7.96 |
| 173 | Diethyl malonate | 16:32.0 | 00:01.1 | MS, LRI | 1577 | 1574 | 0.18 | 4.37 ± 6.46 | 3.32 ± 1.03 |
| 174 | Ethyl propanoate | 03:42.0 | 00:01.1 | MS, LRI | <1000 | 949 | 0.09 | 58.2 ± 31.2 | 52.0 ± 44.7 |
| 175 | Ethyl trans-4-heptenoate | 12:15.8 | 00:01.5 | MS, LRI | 1387 | 1382 | 0.06 | 0.95 ± 0.84 | 0.82 ± 1.00 |
| 176 | Methyl hexanoate | 08:10.3 | 00:01.3 | S, MS, LRI | 1198 | 1184 | 0.03 | 11.44 ± 5.68 | 11.99 ± 5.59 |
| 177 | Methyl octanoate | 12:34.0 | 00:01.5 | MS, LRI | 1401 | 1404 | 0.00 | 95.7 ± 73.2 | 96.1 ± 47.3 |
| Volatile phenols | |||||||||
| 178 | 2-Methoxyphenol | 21:47.0 | 00:00.9 | MS, LRI | 1869 | 1869 | 2.42 | 3.10 ± 1.53 | 2.06 ± 0.89 |
| 179 | 4-Methylphenol | 24:14.0 | 00:00.8 | MS, LRI | 1998 | 2000 | 2.19 | 0.46 ± 0.12 | 0.60 ± 0.22 |
| 180 | 4-Ethylguaiacol | 24:42.0 | 00:01.0 | S, MS, LRI | 2022 | 2024 | 1.94 | 1.33 ± 1.99 | 13.19 ± 22.42 |
| 181 | 4-Ethylphenol | 26:56.0 | 00:00.8 | MS, LRI | >2100 | 2161 | 1.30 | 6.99 ± 8.43 | 23.60 ± 37.59 |
| 182 | Phenol | 24:14.0 | 00:00.8 | S, MS, LRI | 1998 | 1995 | 0.01 | 10.04 ± 1.76 | 9.94 ± 3.16 |
| Furanoids and lactones | |||||||||
| 183 | Pantolactone | 24:42.0 | 00:00.8 | MS, LRI | 2022 | 2029 | 8.38 | 3.01 ± 1.27 b | 4.95 ± 1.25 a |
| 184 | γ-Ethoxybutyrolactone | 19:20.0 | 00:01.0 | MS, LRI | 1739 | 1728 | 8.12 | 2.57 ± 2.48 b | 9.09 ± 5.53 a |
| 185 | γ-Octalactone | 22:50.0 | 00:01.1 | S, MS, LRI | 1925 | 1923 | 8.05 | 2.63 ± 1.63 b | 5.15 ± 1.69 a |
| 186 | α-Methyl-γ-crotonolactone | 19:13.6 | 00:01.0 | MS, LRI | 1733 | 1726 | 6.87 | 0.39 ± 0.12 a | 0.19 ± 0.16 b |
| 187 | Ethyl 2-furoate | 17:21.0 | 00:01.1 | MS, LRI | 1622 | 1621 | 3.22 | 22.5 ± 11.3 | 48.3 ± 36.4 |
| 188 | γ-Crotonolactone | 19:57.0 | 00:00.9 | MS, LRI | 1771 | 1787 | 2.90 | 0.83 ± 0.04 | 0.97 ± 0.22 |
| 189 | δ-Hexalactone | 20:37.0 | 00:01.1 | MS, LRI | 1806 | 1816 | 1.75 | 1.79 ± 0.91 | 2.28 ± 0.34 |
| 190 | Furfural | 14:12.0 | 00:00.9 | S, MS, LRI | 1467 | 1469 | 0.92 | 26.8 ± 12.1 | 19.9 ± 14.6 |
| 191 | 2-Furanmethanol | 18:04.4 | 00:00.8 | MS, LRI | 1668 | 1671 | 0.35 | 6.96 ± 15.56 | 3.36 ± 4.09 |
| 192 | γ-Pentalactone | 17:07.0 | 00:01.0 | MS, LRI | 1607 | 1605 | 0.31 | 0.58 ± 0.36 | 0.68 ± 0.31 |
| 193 | γ-Nonalactone | 25:07.1 | 00:01.1 | S, MS, LRI | 2044 | 2057 | 0.05 | 6.60 ± 7.34 | 5.93 ± 1.80 |
| 194 | γ-Butyrolactone | 17:28.0 | 00:01.0 | MS, LRI | 1629 | 1626 | 0.02 | 102.6 ± 33.3 | 100.6 ± 24.3 |
| Sulfur compounds | |||||||||
| 195 | Ethyl 3-(methylsulfanyl)propanoate | 16:18.0 | 00:01.2 | MS, LRI | 1565 | 1562 | 20.46 | 7.58 ± 4.20 b | 19.39 ± 5.48 a |
| 196 | Dihydro-2-methylthiophen-3-one | 15:27.5 | 00:01.1 | MS, LRI | 1523 | 1523 | 6.99 | 7.15 ± 1.66 b | 14.72 ± 7.39 a |
| 197 | Ethyl methanesulfonate | 18:31.0 | 00:00.9 | MS | 1695 | na | 5.13 | 1.44 ± 0.24 a | 1.18 ± 0.19 b |
| 198 | Methionol | 19:08.0 | 00:00.9 | S, MS, LRI | 1728 | 1733 | 2.73 | 56.4 ± 19.8 | 80.2 ± 32.6 |
| 199 | Isothiocyanatocyclohexane | 18:17.0 | 00:01.6 | MS, LRI | 1681 | 1670 | 1.30 | 0.11 ± 0.15 | 0.21 ± 0.17 |
| 200 | 2-Thiophenecarboxaldehyde | 18:45.0 | 00:01.0 | MS, LRI | 1708 | 1701 | 1.25 | 0.66 ± 0.41 | 0.96 ± 0.58 |
| 201 | 3-(Methylthio)propyl acetate | 17:29.6 | 00:01.2 | MS, LRI | 1631 | 1633 | 1.03 | 1.14 ± 0.75 | 1.67 ± 1.17 |
| 202 | Methyl isocyanate | 18:17.9 | 00:00.8 | MS, LRI | 1682 | na | 0.67 | 0.12 ± 0.19 | 0.23 ± 0.30 |
| 203 | 3-(Methylthio)propanal | 13:58.5 | 00:01.0 | MS, LRI | 1458 | 1455 | 0.30 | 1.41 ± 0.70 | 1.68 ± 1.09 |
| 204 | 2-(Methylthio)ethanol | 15:29.0 | 00:00.8 | S, MS, LRI | 1524 | 1523 | 0.22 | 4.21 ± 0.60 | 3.97 ± 1.24 |
| Miscellaneous | |||||||||
| 205 | Tridecane | 10:33.5 | 00:02.7 | S, MS, LRI | 1306 | 1300 | 21.07 | 18.29 ± 3.01 a | 11.40 ± 2.59 b |
| 206 | 1,5,5,6-Tetramethyl-1,3-cyclohexadiene | 12:41.0 | 00:01.6 | MS | 1405 | na | 7.08 | 0.12 ± 0.21 b | 0.57 ± 0.40 a |
| 207 | Tetradecane | 12:47.5 | 00:02.7 | S, MS | 1410 | na | 4.51 | 0.88 ± 0.34 | 0.48 ± 0.36 |
| 208 | 1,2-Benzisothiazole | 23:25.7 | 00:01.1 | MS, LRI | 1956 | 1955 | 0.30 | 0.11 ± 0.12 | 0.15 ± 0.16 |
| 209 | cis-5-Hydroxy-2-methyl-1,3-dioxane | 14:48.2 | 00:00.9 | MS, LRI | 1492 | 1494 | 0.01 | 0.65 ± 0.36 | 0.62 ± 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukić, I.; Carlin, S.; Vrhovsek, U. Utility of Comprehensive GC×GC Gas Chromatography in Finding Varietal Markers among Volatile Compounds in Non-Aromatic Red Wines. Agronomy 2022, 12, 2512. https://doi.org/10.3390/agronomy12102512
Lukić I, Carlin S, Vrhovsek U. Utility of Comprehensive GC×GC Gas Chromatography in Finding Varietal Markers among Volatile Compounds in Non-Aromatic Red Wines. Agronomy. 2022; 12(10):2512. https://doi.org/10.3390/agronomy12102512
Chicago/Turabian StyleLukić, Igor, Silvia Carlin, and Urska Vrhovsek. 2022. "Utility of Comprehensive GC×GC Gas Chromatography in Finding Varietal Markers among Volatile Compounds in Non-Aromatic Red Wines" Agronomy 12, no. 10: 2512. https://doi.org/10.3390/agronomy12102512
APA StyleLukić, I., Carlin, S., & Vrhovsek, U. (2022). Utility of Comprehensive GC×GC Gas Chromatography in Finding Varietal Markers among Volatile Compounds in Non-Aromatic Red Wines. Agronomy, 12(10), 2512. https://doi.org/10.3390/agronomy12102512








