Suaeda glauca and Suaeda salsa Employ Different Adaptive Strategies to Cope with Saline–Alkali Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Determination of Soil Properties and Ion Contents
2.3. Elemental Analysis
2.4. Primary Metabolite Analysis
2.5. Determination of Phenol Metabolites
2.6. Statistical Analysis
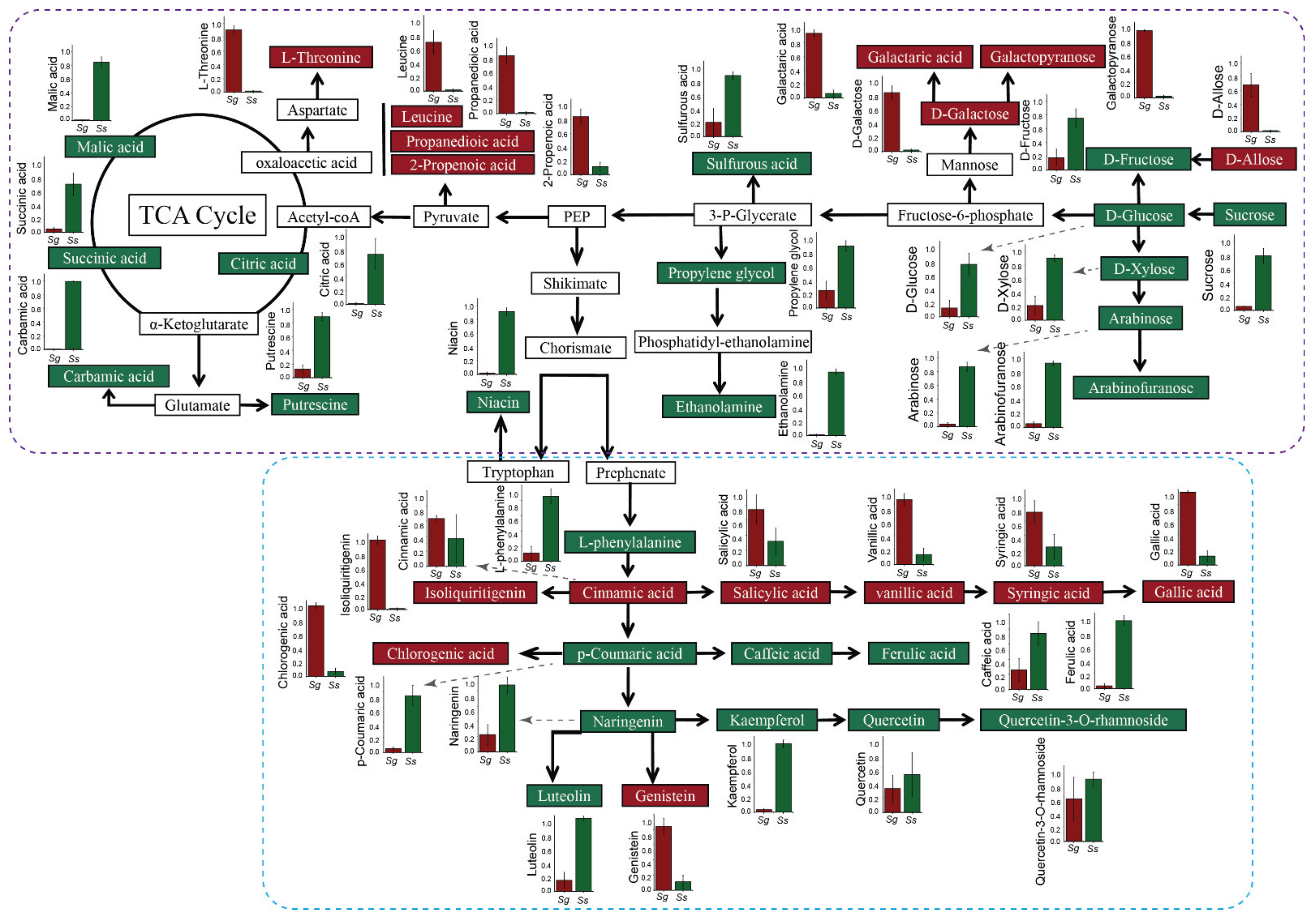
3. Results
3.1. Differential Distribution of Mineral Elements in Leaves of S. glauca and S. salsa
3.2. Primary Metabolite Profiles of Leaves in S. glauca and S. salsa
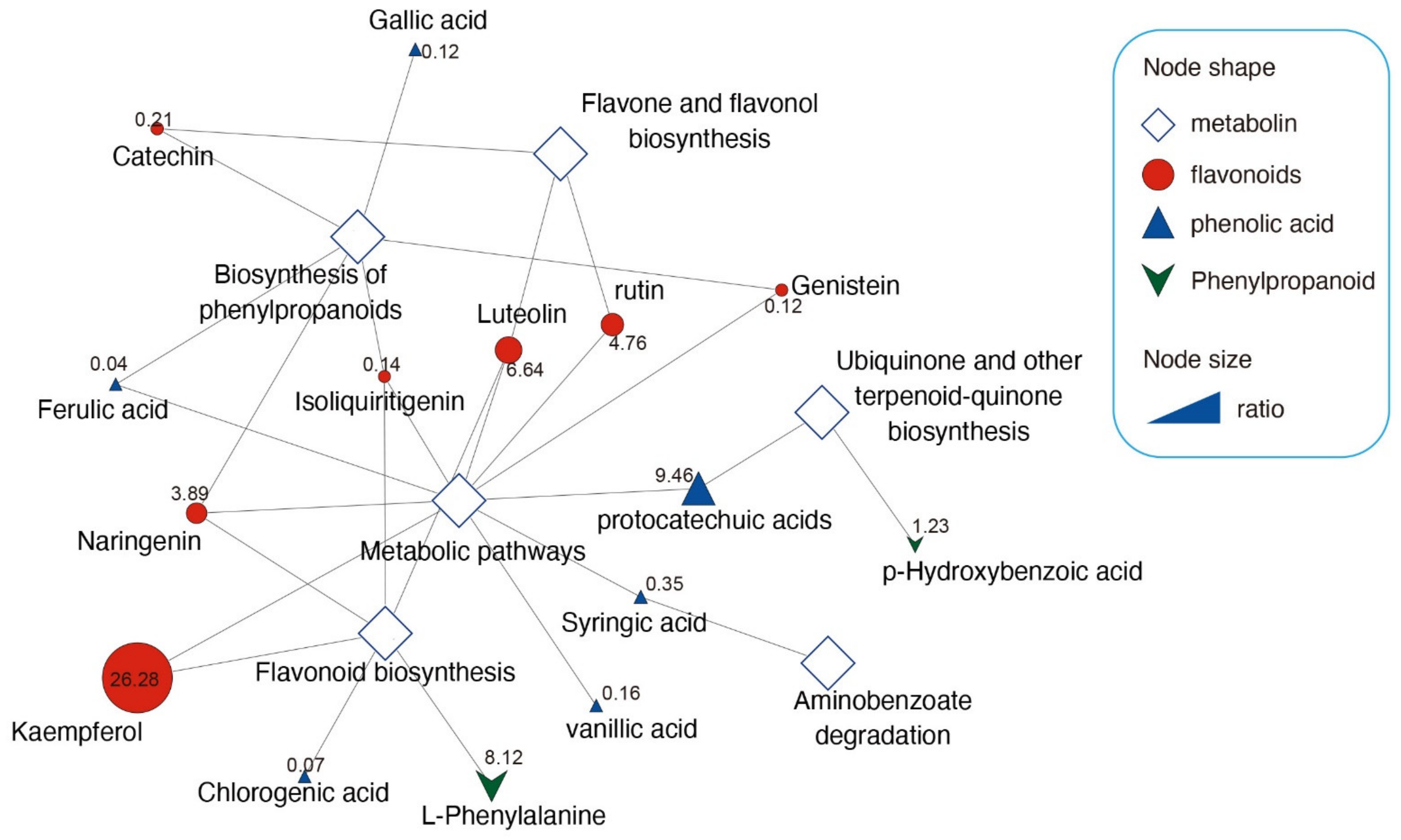
3.3. Secondary Metabolite Profiles of Leaves between S. glauca and S. salsa
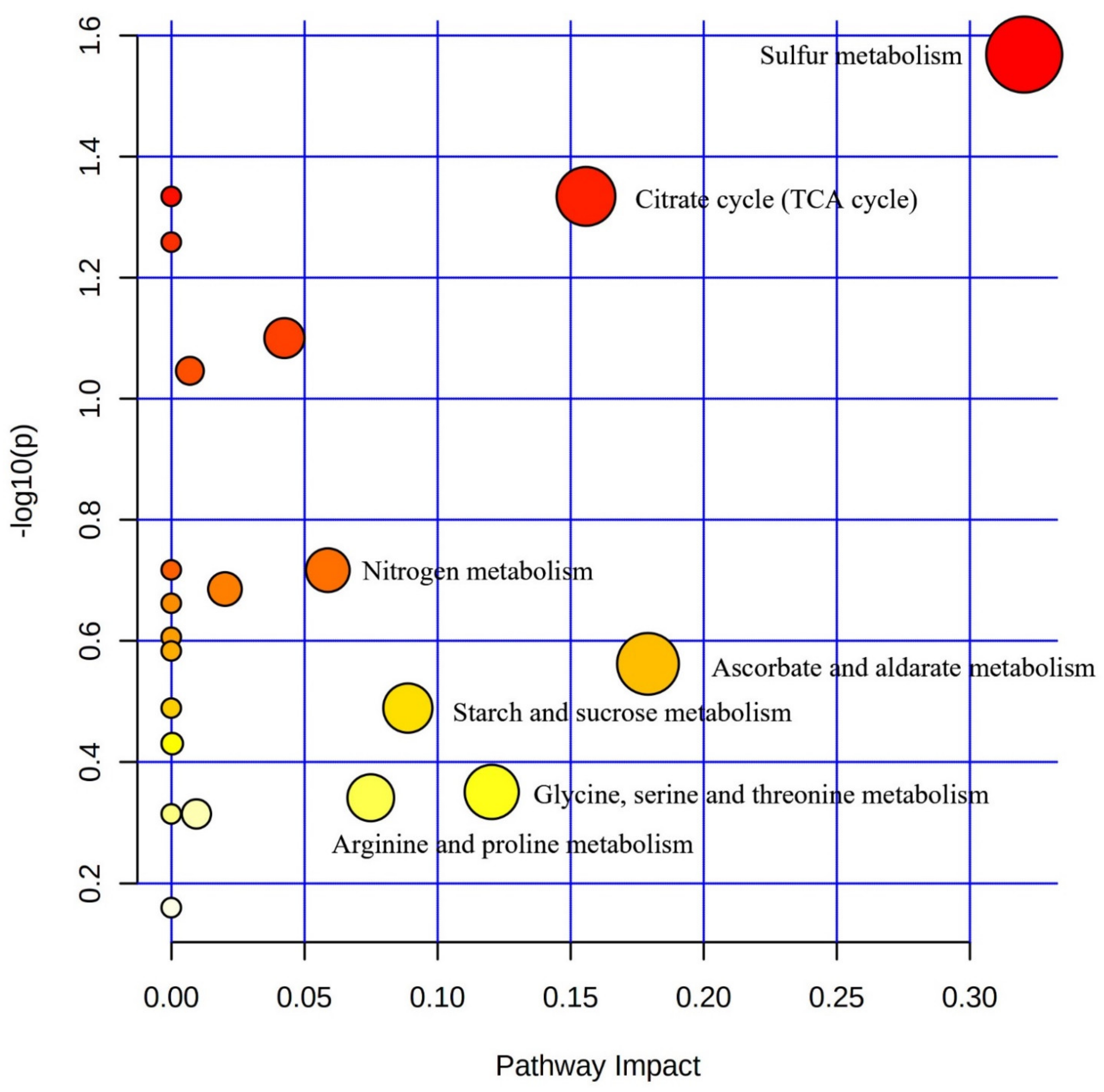
3.4. Correlation-Based Network Analysis for Uncovering Metabolite Variation in S. glauca and S. salsa under Saline–Alkali Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Total Compounds | Expected | Hits | Raw p | Log (p) | Holm Adjust | FDR | Impact | |
|---|---|---|---|---|---|---|---|---|
| a Sulfur metabolism | 15 | 0.26298 | 2 | 0.026984 | 1.5689 | 1 | 1 | 0.32044 |
| Propanoate metabolism | 20 | 0.35064 | 2 | 0.046282 | 1.3346 | 1 | 1 | 0 |
| a Citrate cycle (TCA cycle) | 20 | 0.35064 | 2 | 0.046282 | 1.3346 | 1 | 1 | 0.15581 |
| Valine, leucine and isoleucine biosynthesis | 22 | 0.3857 | 2 | 0.05508 | 1.259 | 1 | 1 | 0 |
| Galactose metabolism | 27 | 0.47336 | 2 | 0.079357 | 1.1004 | 1 | 1 | 0.04252 |
| Glyoxylate and dicarboxylate metabolism | 29 | 0.50843 | 2 | 0.089865 | 1.0464 | 1 | 1 | 0.00702 |
| Aminoacyl-tRNA biosynthesis | 46 | 0.80647 | 2 | 0.19166 | 0.71748 | 1 | 1 | 0 |
| a Nitrogen metabolism | 12 | 0.21038 | 1 | 0.19188 | 0.71696 | 1 | 1 | 0.05882 |
| Nicotinate and nicotinamide metabolism | 13 | 0.22792 | 1 | 0.20617 | 0.68578 | 1 | 1 | 0.0202 |
| Amino sugar and nucleotide sugar metabolism | 50 | 0.8766 | 2 | 0.21762 | 0.6623 | 1 | 1 | 0 |
| Pentose and glucuronate interconversions | 16 | 0.28051 | 1 | 0.24758 | 0.60629 | 1 | 1 | 0 |
| Butanoate metabolism | 17 | 0.29804 | 1 | 0.26091 | 0.58351 | 1 | 1 | 0 |
| a Ascorbate and ldarate metabolism | 18 | 0.31558 | 1 | 0.27402 | 0.56222 | 1 | 1 | 0.1791 |
| Alanine, aspartate and glutamate metabolism | 22 | 0.3857 | 1 | 0.32425 | 0.48912 | 1 | 1 | 0 |
| a Starch and sucrose metabolism | 22 | 0.3857 | 1 | 0.32425 | 0.48912 | 1 | 1 | 0.0889 |
| Glutathione metabolism | 26 | 0.45583 | 1 | 0.37113 | 0.43047 | 1 | 1 | 0 |
| Glycolysis/Gluconeogenesis | 26 | 0.45583 | 1 | 0.37113 | 0.43047 | 1 | 1 | 0.00038 |
| a Glycine, serine and threonine metabolism | 33 | 0.57856 | 1 | 0.44575 | 0.35091 | 1 | 1 | 0.1204 |
| a Arginine and proline metabolism | 34 | 0.59609 | 1 | 0.45569 | 0.34133 | 1 | 1 | 0.07496 |
| Valine, leucine and isoleucine degradation | 37 | 0.64869 | 1 | 0.48448 | 0.31472 | 1 | 1 | 0 |
| Glycerophospholipid metabolism | 37 | 0.64869 | 1 | 0.48448 | 0.31472 | 1 | 1 | 0.00947 |
| Glucosinolate biosynthesis | 65 | 1.1396 | 1 | 0.69133 | 0.16032 | 1 | 1 | 0 |
References
- Bencherif, K.; Boutekrabt, A.; Fontaine, J.; Laruelle, F.; Dalpè, Y.; Lounès-Hadj Sahraoui, A. Impact of soil salinity on arbuscular mycorrhizal fungi biodiversity and microflora biomass associated with Tamarix articulata Vahll rhizosphere in arid and semi-arid Algerian areas. Sci. Total Environ. 2015, 533, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Boughattas, S.; Hu, S.; Oh, S.-H.; Sa, T. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 2014, 24, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Cao, F.F.; Wu, D.; Fu, X.; Tian, Y.; Wu, G. Determining Soil Nutrients Reference Condition in Alpine Region Grassland, China: A Case Study of Hulun Buir Grassland. Sustainability 2018, 10, 4666. [Google Scholar] [CrossRef]
- El-Banna, M.F.; AL-Huqail, A.A.; Farouk, S.; Belal, B.E.A.; El-Kenawy, M.A.; Abd El-Khalek, A.F. Morpho-physiological and anatomical alterations of salt-affected thompson seedless grapevine (Vitis vinifera L.) to brassinolide spraying. Horticulturae 2022, 8, 568. [Google Scholar] [CrossRef]
- Farouk, S.; AL-Huqail, A.A. Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 2022, 11, 765. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, Q.; Wang, H.; Nie, S.M.; Liang, Z.W. Effects of soil salinity on the content, composition, and ion binding capacity of glomalin-related soil protein (GRSP). Sci. Total Environ. 2017, 581, 657–665. [Google Scholar] [CrossRef]
- Sofy, M.R.; Elhindi, K.M.; Farouk, S.; Alotaibi, M.A. Zinc and paclobutrazol mediated regulation of growth, upregulating antioxidant aptitude and plant productivity of pea plants under salinity. Plants 2020, 9, 1197. [Google Scholar] [CrossRef]
- Kumari, A.; Parida, A.K. Metabolomics and network analysis reveal the potential metabolites and biological pathways involved in salinity tolerance of the halophyte Salvadora persica. Environ. Exp. Bot. 2018, 148, 85–99. [Google Scholar] [CrossRef]
- Derakhshani, Z.; Bhave, M.; Shah, R.M. Metabolic contribution to salinity stress response in grains of two barley cultivars with contrasting salt tolerance. Environ. Exp. Bot. 2020, 179, 104229. [Google Scholar] [CrossRef]
- Llanes, A.; Arbona, V.; Gomez-Cadenas, A.; Luna, V. Metabolomic profiling of the halophyte Prosopis strombulifera shows sodium salt- specific response. Plant Physiol. Bioch. 2016, 108, 145–157. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Brosche, M.; Vinocur, B.; Alatalo, E.R.; Lamminmaki, A.; Teichmann, T.; Ottow, E.A.; Djilianov, D.; Afif, D.; Bogeat-Triboulot, M.B.; Altman, A.; et al. Gene expression and metabolite profiling of Populus euphratica growing in the Negev desert. Genome Biol. 2005, 6, R101. [Google Scholar] [CrossRef]
- Rambla, J.L.; Lopez-Gresa, M.P.; Belles, M.; Granell, A. Metabolomic Profiling of Plant Tissues. Methods Mol. Biol. 2015, 1284, 221–235. [Google Scholar]
- Yu, B.F.; Liu, Y.; Pan, Y.J.; Liu, J.; Wang, H.Z.; Tang, Z.H. Light enhanced the biosynthesis of terpenoid indole alkaloids to meet the opening of cotyledons in process of photomorphogenesis of Catharanthus roseus. Plant Growth Regul. 2018, 84, 617–626. [Google Scholar] [CrossRef]
- Abideen, Z.; Qasim, M.; Rasheed, A.; Adnan, M.Y.; Gul, B.; Khan, M.A. Antioxidant Activity and Polyphenolic Content of Phragmites Karka under Saline Conditions. Pak. J. Bot. 2015, 47, 813–818. [Google Scholar]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Bioch. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Rasmussen, S.; Parsons, A.J.; Jones, C.S. Metabolomics of forage plants: A review. Ann. Bot-Lond. 2012, 110, 1281–1290. [Google Scholar] [CrossRef]
- Song, X.Q.; Su, Y.H.; Zheng, J.W.; Zhang, Z.H.; Liang, Z.W.; Tang, Z.H. Study on the Effects of Salt Tolerance Type, Soil Salinity and Soil Characteristics on the Element Composition of Chenopodiaceae Halophytes. Plants 2022, 11, 1288. [Google Scholar] [CrossRef]
- Lake, J.A.; Field, K.J.; Davey, M.P.; Beerling, D.J.; Lomax, B.H. Metabolomic and physiological responses reveal multi-phasic acclimation of Arabidopsis thaliana to chronic UV radiation. Plant Cell Environ. 2009, 32, 1377–1389. [Google Scholar] [CrossRef]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Bioch. 2019, 135, 528–545. [Google Scholar] [CrossRef]
- Jia, X.M.; Zhu, Y.F.; Zhang, R.; Zhu, Z.L.; Zhao, T.; Cheng, L.; Gao, L.Y.; Liu, B.; Zhang, X.Y.; Wang, Y.X. Ionomic and metabolomic analyses reveal the resistance response mechanism to saline-alkali stress in Malus halliana seedlings. Plant Physiol. Bioch. 2020, 147, 77–90. [Google Scholar] [CrossRef]
- Yang, N.; Song, X.Q.; Lu, X.Y.; Chen, Q.; Liu, J.; Liu, Y.; Wang, H.Z.; Zhang, Z.H.; Tang, Z.H. Comparative study on metabolites and elements of two dominant plant communities in saline-alkali grassland. Environ. Exp. Bot. 2021, 190, 104587. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, Y.; Zhang, Z.H.; Cao, M.; Wei, G.Y.; Guo, X.R.; Zhang, J.; Lu, X.Y.; Tang, Z.H. Ionomic and Metabolomic Analyses Reveal Different Response Mechanisms to Saline-Alkali Stress Between Suaeda salsa Community and Puccinellia tenuiflora Community. Front. Plant Sci. 2021, 12, 774284. [Google Scholar] [CrossRef]
- Lu, X.Y.; Chen, Q.; Cui, X.Y.; Abozeid, A.; Liu, Y.; Liu, J.; Tang, Z.H. Comparative metabolomics of two saline-alkali tolerant plants Suaeda glauca and Puccinellia tenuiflora based on GC-MS platform. Nat. Prod. Res. 2021, 35, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Jiang, L.; Zhang, K.; Tanveer, M.; Tian, C.Y.; Zhao, Z.Y. Reclamation of saline soil by planting annual euhalophyte Suaeda salsa with drip irrigation: A three-year field experiment in arid northwestern China. Ecol. Eng. 2021, 159, 106090. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, H.; Song, X.O.A.; Liang, Z.W.; Tang, Z.H. Arbuscular mycorrhizal fungal diversity is affected by soil salinity and soil nutrients in typical saline-sodic grasslands dominated by Leymus chinensis. Arid. Land Res. Manag. 2020, 34, 68–82. [Google Scholar] [CrossRef]
- Wilke, B.-M. Determination of Chemical and Physical Soil Properties. In Monitoring and Assessing Soil Bioremediation; Margesin, R., Schinner, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 47–95. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Liu, J.; Liu, Y.; Wang, Y.; Abozeid, A.; Zu, Y.G.; Tang, Z.H. The integration of GC-MS and LC-MS to assay the metabolomics profiling in Panax ginseng and Panax quinquefolius reveals a tissue- and species-specific connectivity of primary metabolites and ginsenosides accumulation. J. Pharm. Biomed. 2017, 135, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Kiani-Pouya, A.; Roessner, U.; Jayasinghe, N.S.; Lutz, A.; Rupasinghe, T.; Bazihizina, N.; Bohm, J.; Alharbi, S.; Hedrich, R.; Shabala, S. Epidermal bladder cells confer salinity stress tolerance in the halophyte quinoa and Atriplex species. Plant Cell Environ. 2017, 40, 1900–1915. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Siahpoosh, M.R.; Roessner, U.; Udvardi, M.; Kopka, J. Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol. Plantarum. 2008, 132, 209–219. [Google Scholar] [CrossRef]
- Shabala, S.; Mackay, A. Ion Transport in Halophytes. Adv. Bot. Res. 2011, 57, 151–199. [Google Scholar]
- Bose, J.; Rodrigo-Moreno, A.; Lai, D.W.; Xie, Y.J.; Shen, W.B.; Shabala, S. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann. Bot-Lond. 2015, 115, 481–494. [Google Scholar] [CrossRef]
- Ma, H.Y.; Liang, Z.W.; Yang, H.Y.; Huang, L.H.; Zhao, M.L. Ion adaptive mechanisms of Leymus chinensis to saline-alkali stress. J. Food Agric. Environ. 2011, 9, 688–692. [Google Scholar]
- Venkatesalu, V.; Kumar, R.R.; Chellappan, K.P. Growth and Mineral Distribution of Sesuvium Portulacastrum L, a Salt Marsh Halophyte, under Sodium Chloride Stress. Commun. Soil Sci. Plan. 1994, 25, 2797–2805. [Google Scholar] [CrossRef]
- Yang, C.W.; Chong, J.N.; Li, C.Y.; Kim, C.M.; Shi, D.C.; Wang, D.L. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Yang, C.W.; Shi, D.C.; Wang, D.L. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Hamada, A.; Shono, M.; Xia, T.; Ohta, M.; Hayashi, Y.; Tanaka, A.; Hayakawa, T. Isolation and characterization of a Na+/H+ antiporter gene from the halophyte Atriplex gmelini. Plant Mol. Biol. 2001, 46, 35–42. [Google Scholar] [CrossRef]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotech. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Mansour, M. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plantarum. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Phys. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot.-Lond. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araujo, W.L.; Braun, H.P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Pieckenstain, F.L.; Escaray, F.; Erban, A.; Kraemer, U.; Udvardi, M.K.; Kopka, J. Comparative ionomics and metabolomics in extremophile and glycophytic Lotus species under salt stress challenge the metabolic pre-adaptation hypothesis. Plant Cell Environ. 2011, 34, 605–617. [Google Scholar] [CrossRef]
- Liu, B.S.; Kang, C.L.; Wang, X.; Bao, G.Z. Tolerance mechanisms of Leymus chinensis to salt-alkaline stress. Acta Agr. Scand. B-S P. 2015, 65, 723–734. [Google Scholar]
- Combs, J.G.F.; McClung, J.P. Chapter 12-Niacin. In The Vitamins, 6th ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 361–386. [Google Scholar]
- Mukherjee, S.; David, A.; Yadav, S.; Baluska, F.; Bhatla, S.C. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol. Plantarum. 2014, 152, 714–728. [Google Scholar] [CrossRef]
- Kaur, H.; Mukherjee, S.; Baluska, F.; Bhatla, S.C. Regulatory roles of serotonin and melatonin in abiotic stress tolerance in plants. Plant Signal Behav. 2015, 10, e1049788. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.Q.; Zhang, H.J.; Cao, Y.Y.; Weeda, S.; Ren, S.X.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef]
- Widodo; Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Lull, C.; Boscaiu, M.; Bautista, I.; Lidon, A.; Vicente, O. Soluble Carbohydrates as Osmolytes in Several Halophytes from a Mediterranean Salt Marsh. Not. Bot. Horti. Agrobo. 2011, 39, 9–17. [Google Scholar] [CrossRef]
- Loescher, W.H.; Everard, J.D. Sugar alcohol metabolism in sinks and sources. In Photoassimilate Distribution in Plants and Crops; Routledge: New York, NY, USA, 2017; pp. 185–207. [Google Scholar]
- Yu, J.J.; Du, H.M.; Xu, M.; Huang, B.R. Metabolic Responses to Heat Stress under Elevated Atmospheric CO2 Concentration in a Cool-season Grass Species. J. Am. Soc. Hortic. Sci. 2012, 137, 221–228. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
- Tattini, M.; Remorini, D.; Pinelli, P.; Agati, G.; Saracini, E.; Traversi, M.L.; Massai, R. Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytol. 2006, 170, 779–794. [Google Scholar] [CrossRef]
- Li, M.X.; Guo, R.; Jiao, Y.; Jin, X.F.; Zhang, H.Y.; Shi, L.X. Comparison of Salt Tolerance in Soja Based on Metabolomics of Seedling Roots. Front Plant Sci. 2017, 8, 01101. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, Y.L.; Wang, H.; Li, H.; Zhang, Z.W. Free-Radical Scavenging Properties and Reducing Power of Grape Cane Extracts from 11 Selected Grape Cultivars Widely Grown in China. Molecules 2011, 16, 10104–101022. [Google Scholar] [CrossRef]
- Fry, S.C. Cross-Linking of Matrix Polymers in the Growing Cell-Walls of Angiosperms. Annu. Rev. Plant Phys. 1986, 37, 165–186. [Google Scholar] [CrossRef]
- Haghighi, L.; Majd, A.; Nematzadeh, G.; Shokri, M.; Irian, S. Salt-induced changes in cell wall peroxidase (CWPRX) and phenolic content of Aeluropus littoralis (Willd) Parl. Aust. J. Crop Sci. 2014, 8, 296–300. [Google Scholar]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M.; Talebi, M.; Ghanati, F. Melatonin and calcium modulate the production of rosmarinic acid, luteolin, and apigenin in Dracocephalum kotschyi under salinity stress. Phytochemistry 2020, 177, 112422. [Google Scholar] [CrossRef]

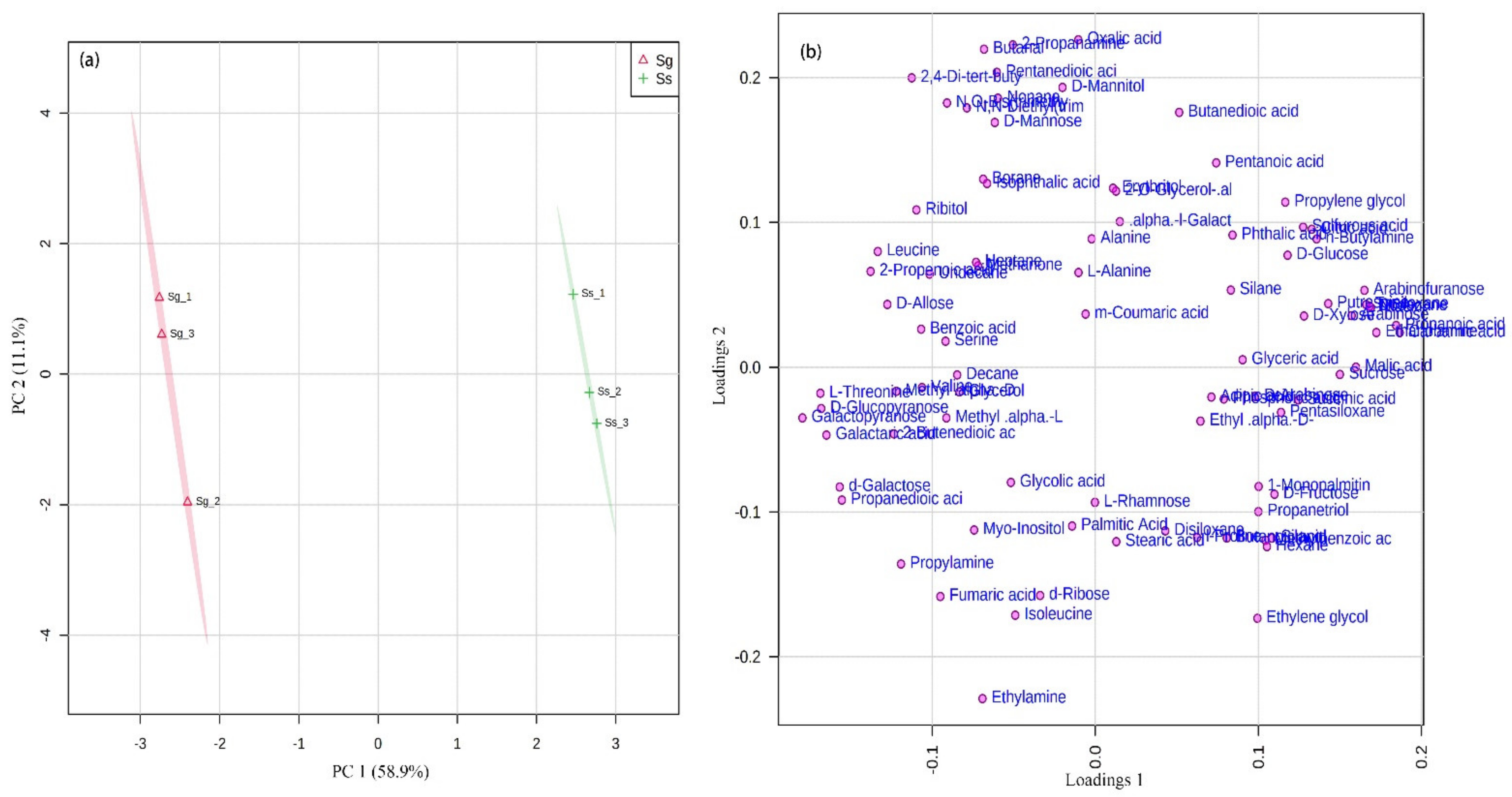
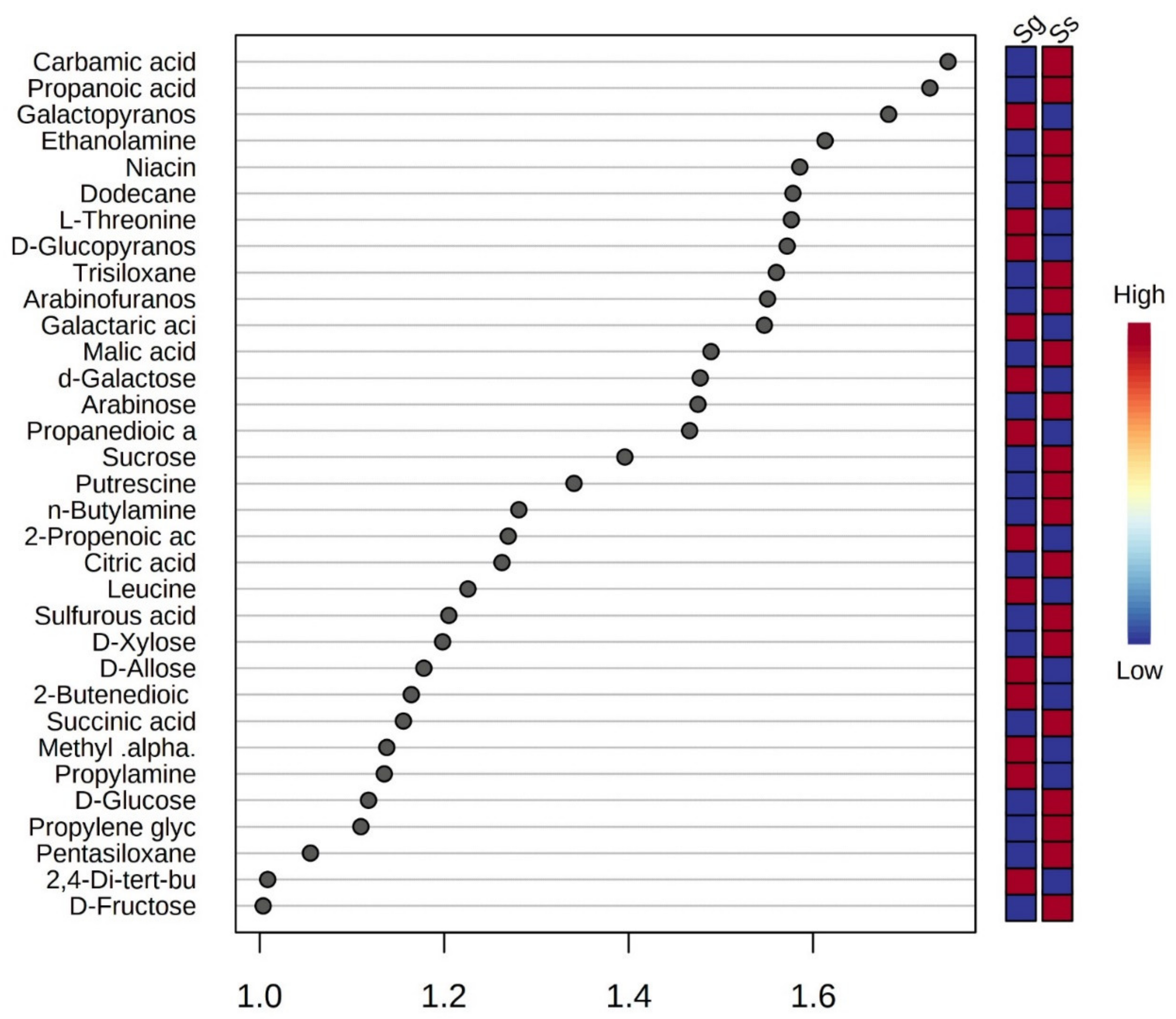
| Specie | Soil Properties | Ion Content (cmol·kg−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | EC mS/cm | K+ | Na+ | Ca2+ | Mg2+ | Cl− | CO32− | HCO3− | SO42− | ||
| S. glauca | AV | 9.75 | 1.00 | 0.10 | 1.49 | 1.17 | 0.84 | 0.37 | 0.02 | 0.04 | 0.12 |
| SE | 0.57 | 0.01 | 0.03 | 0.19 | 0.54 | 0.26 | 0.02 | 0.01 | 0.01 | 0.02 | |
| S. salsa | AV | 9.76 | 1.30 | 0.13 | 2.34 | 2.03 | 1.35 | 0.78 | 0.03 | 0.09 * | 0.15 |
| SE | 0.67 | 0.33 | 0.05 | 0.45 | 0.09 | 0.39 | 0.29 | 0.02 | 0 | 0.01 | |
| Specie | Essential Microelement (mg·g−1) | Essential Microelement (mg·g−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | TN | TOC | K | Ca | Mg | Na | Fe | Cu | Zn | Mn | Na/K | ||
| S. glauca | AV | 0.73 | 13.07 * | 320.62 * | 10.80 | 2.11 * | 6.81 | 90.28 * | 0.55 | 0.02 | 0.02 | 0.08 * | 4.47 * |
| SE | 0.1 | 1.29 | 5.57 | 1.36 | 0.15 | 1.05 | 6.5 | 0.1 | 0 | 0.01 | 0 | 0.84 | |
| S. salsa | AV | 1.05 | 19.79 | 385 | 12.92 | 3.41 | 8.85 | 119.21 | 0.39 | 0.02 | 0.01 | 0.10 | 8.37 |
| SE | 0.21 | 0.69 | 5.29 | 1.28 | 0.35 | 0.36 | 5.93 | 0.08 | 0 | 0 | 0 | 0.42 | |
| VIP | Change | p-Value | |
|---|---|---|---|
| Isoliquiritigenin | 1.13271 | Sg > Ss | ** |
| Kaempferol | 1.1305 | Ss > Sg | ** |
| Ferulic acid | 1.1293 | Ss > Sg | ** |
| Gallic acid | 1.12871 | Sg > Ss | ** |
| Chlorogenic acid | 1.12648 | Sg > Ss | ** |
| protocatechuic acids | 1.11508 | Ss > Sg | ** |
| vanillic acid | 1.10797 | Sg > Ss | ** |
| p-Hydroxybenzoic acid | 1.10255 | Ss > Sg | ** |
| Luteolin | 1.10158 | Sg > Ss | ** |
| L-Phenylalanine | 1.09893 | Ss > Sg | ** |
| rutin | 1.0904 | Ss > Sg | ** |
| Genistein | 1.07673 | Sg > Ss | ** |
| Naringenin | 1.06547 | Ss > Sg | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Yang, N.; Su, Y.; Lu, X.; Liu, J.; Liu, Y.; Zhang, Z.; Tang, Z. Suaeda glauca and Suaeda salsa Employ Different Adaptive Strategies to Cope with Saline–Alkali Environments. Agronomy 2022, 12, 2496. https://doi.org/10.3390/agronomy12102496
Song X, Yang N, Su Y, Lu X, Liu J, Liu Y, Zhang Z, Tang Z. Suaeda glauca and Suaeda salsa Employ Different Adaptive Strategies to Cope with Saline–Alkali Environments. Agronomy. 2022; 12(10):2496. https://doi.org/10.3390/agronomy12102496
Chicago/Turabian StyleSong, Xiaoqian, Nan Yang, Yuhang Su, Xueyan Lu, Jia Liu, Yang Liu, Zhonghua Zhang, and Zhonghua Tang. 2022. "Suaeda glauca and Suaeda salsa Employ Different Adaptive Strategies to Cope with Saline–Alkali Environments" Agronomy 12, no. 10: 2496. https://doi.org/10.3390/agronomy12102496
APA StyleSong, X., Yang, N., Su, Y., Lu, X., Liu, J., Liu, Y., Zhang, Z., & Tang, Z. (2022). Suaeda glauca and Suaeda salsa Employ Different Adaptive Strategies to Cope with Saline–Alkali Environments. Agronomy, 12(10), 2496. https://doi.org/10.3390/agronomy12102496







