Abstract
The prevalence of tetracycline (TC) in aquatic environments has raised increasing concern due to its high ecotoxicology risk. The application of microalgae in the removal of antibiotics is a competitive alternative technology. However, the removal mechanism of TC by microalgae and its correlation with the ecotoxic response of microalgae are still not clear. In this study, the ecotoxicity of TC (0.5–10 mg L−1) and its removal by the freshwater alga Chlorella pyrenoidosa were investigated. The results show that TC has significant inhibitory effects on microalgal growth, photosynthetic pigment, and photosynthetic efficiency, with maximum inhibition rates of 49.68%, 62.54%, and 48.08%, respectively. However, the growth inhibition and photosynthesis impairment caused by TC seems to be reversible, as reflected by the decreased inhibition rates with prolonged exposure time. The simultaneous increases in the activities of superoxide dismutase (9.69–23.53%) and peroxidase (15.15–110.92%) and the contents of glutathione (17.62–64.17%) and malondialdehyde (25.16–35.25%) suggest that TC causes moderate oxidative stress. C. pyrenoidosa exhibits high removal efficiency (91.44–95.14%) for TC after 48 h of exposure with short half-lives of 11.31–13.48 h. Biodegradation (56.86–64.62%) is the primary removal mechanism of TC, accompanied by the abiotic process (24.68–40.97%), bioaccumulation (1.95–10.97%), and bioadsorption (0.09–0.38%). These findings demonstrate the toxicity resistance and high removal capacity of C. pyrenoidosa to TC, highlighting its potential application in the remediation of TC-contaminated water.
1. Introduction
Antibiotics in aquatic environments attracted more and more attention in the last two decades due to their potential risks to humans and other organisms [1,2,3]. Tetracycline (TC) is one of the most frequently used antibiotics for therapeutic medicine and livestock husbandry, due to its low cost and high antimicrobial activity [4]. After administration, more than 75% of TC is excreted through urine and feces [5]. The unaltered part of TC flows into wastewater treatment plants (WWTPs). However, TC cannot be completely removed by WWTPs, leading to its prevalence in aquatic environments [6,7]. Exposure to TC is documented to exert adverse effects on aquatic organisms such as fish, crustacea, and insects [8,9,10]. In addition, TC can induce the emergence and dissemination of antibiotic-resistant bacteria and genes in aquatic environments [11,12,13]. Therefore, there is an urgent need to explore a highly efficient and sustainable technique for removing TC.
Various physicochemical methods, such as photocatalytic degradation, advanced oxidation, adsorption, and membrane technology, were developed for removing TC [14,15,16,17,18,19]. However, photocatalytic degradation and advanced oxidation are prone to secondary pollution, leading to the formation of more toxic by-products [20]. For physical treatments, there is a need for a post-treatment to reuse the absorbent and membrane [14]. Moreover, the high cost of these methods limited their utilization in large-scale applications [21]. Microalgae exhibited high potential as a competitive alternative technology for treating wastewater containing antibiotics, with various advantages such as low cost, eco-friendliness, nutrient recovery, greenhouse gas mitigation, and value-added biomass production [20,22,23,24]. The major mechanisms underpinning antibiotics removal by microalgae include abiotic processes (photolysis and hydrolysis) and biotic processes (bioadsorption, bioaccumulation, and biodegradation) [21]. Bioadsorption is considered the first step of biotic processes, followed by a comparatively slow transference of molecules via the cell wall, and finally either ending up as bioaccumulation, biodegradation, or both [23]. The high removal efficiency of TC by microalgal technology was reported in recent years [25,26,27], but the removal mechanisms of TC were highly controversial. It was found that photodegradation and biosorption were the primary mechanisms for TC removal in high-rate algal ponds [25,26]. However, Pan et al. (2021) report that biodegradation mainly contribute to TC removal by a harmful cyanobacterial species, Microcystis aeruginosa [28]. Therefore, further investigations of the removal mechanisms of TC by different microalgal species will provide a better understanding of the microalgae-based treatment of TC.
The biodegradation capacity of microalgae is associated with their tolerance to the toxicity of antibiotics [24]. Previous studies report that TC could affect the growth and physiological progress of microalgae. Xu et al. (2019) report the 96 h half-maximal effective concentration (EC50) of TC on Chlorella vulgaris to be 7.73 mg L−1 [29]. In addition, significant increases in the levels of reactive oxygen species (ROS) and lipid peroxidation product malonaldehyde (MDA) were observed at concentrations of TC higher than 5 mg L−1 [29]. Low concentrations of TC (0.5–5 mg L−1) significantly inhibit the growth, photosynthesis, and antioxidant function of Microcystis aeruginosa and Selenastrum capricornutum [30]. Although these findings demonstrate the toxicity of TC to microalgae, a comprehensive investigation of the toxicity and removal performance of TC by microalgae is limited.
The green microalga Chlorella pyrenoidosa is widely distributed in freshwater ecosystems worldwide [31]. It has the advantages of small size, uniform distribution, and a short growth cycle [32]. In addition, C. pyrenoidosa is demonstrated to have a high tolerance and removal capacity to various antibiotics [33,34,35]. For instance, C. pyrenoidosa is more tolerant to chloramphenicol, florfenicol, and thiamphenicol than Isochrysis galbana and Tetraselmis chui [36]. C. pyrenoidosa shows the highest growth rate and removal efficiency for sulfamethoxazole among five common freshwater green microalgae (Pseudokirchneriella subcapitata, Scenedesmus quadricauda, Scenedesmus obliquus, Scenedesmus acuminatus, and C. pyrenoidosa) [34]. Moreover, C. pyrenoidosa is commercially available, making it easy to obtain for treating wastewater containing TC. Thus, in the present study, C. pyrenoidosa was selected as the test microalgal species to explore the removal mechanism of TC and its toxicity to microalgae during the treatment process. The objective of this work was to evaluate the effects of TC on the growth, photosynthesis, and antioxidant responses of C. pyrenoidosa. In addition, the removal efficiency and mechanism of TC by C. pyrenoidosa were investigated.
2. Materials and Methods
2.1. Chemicals
TC (97.7% purity) was obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Methanol and acetonitrile (HPLC grade) were provided by Tedia Company (Fairfield, OH, USA). All other chemicals and reagents were of analytical grade.
2.2. Pre-Culture of Microalgae
Chlorella pyrenoidosa (FACHB-10) was purchased from the Institute of Hydrobiology, Chinese Academy of Sciences. Under sterile conditions, 10 mL of the origin microalgal suspension was inoculated into 100 mL flasks containing 50 mL BG-11 medium. After 15 days of cultivation, 1 mL of the supernatant in the culture was plated on BG-11 agar plates and then cultivated in an illumination chamber. After 15 days, the microalgal colonies formed on the plates were picked up and separately dissolved in 50 mL BG-11 medium in 100 mL flasks to cultivate. The above processes were repeated several times until the microalgal strain was purified. The isolated microalgal strain was pre-cultured in 250 mL flasks containing 150 mL BG-11 medium to achieve the exponential phase before the exposure experiment. The culture conditions in the illumination incubator were set as follows: temperature, 25 ± 1 °C; light intensity, 5000 lux; light–dark period, 14 h:10 h. The culture flasks were manually shaken several times daily to prevent precipitation or adhesion.
2.3. Toxicity Assay
2.3.1. Experimental Design
Microalgae were exposed to different concentrations of TC (0.5, 1.0, 2.5, 4.0, 6.0, and 10 mg L−1) for 96 h. The highest exposure concentration was approximately equal to the 96 h EC50 value determined in the pre-experiment. The initial cell density was 1.0 × 106 cells mL−1, and cultivation conditions were adopted as described above. Each treatment was performed in three replicates. Microalgal growth was measured daily. The contents of photosynthetic pigments and chlorophyll fluorescence were measured at 48 and 96 h. Oxidative stress biomarkers were assayed at 96 h.
2.3.2. Determination of Microalgal Growth
Microalgal growth was monitored at the optical density of 680 nm (OD680) [37]. The cell densities were then calculated as follows:
Cell density (106 cells/mL) = 31.857 OD680 + 0.0643 (R2 = 0.998)
The growth inhibition rate (IR) was calculated using Equation (2):
where N and N0 are cell densities in the exposure and control groups, respectively.
IR = (1 − N/N0) × 100%
2.3.3. Determination of Photosynthetic Pigments
The contents of chlorophyll a (Chl-a), chlorophyll b (Chl-b), and carotenoids (Cars) were measured using the hot ethanol extraction method [38]. After 48 and 96 h of exposure, 30 mL microalgal suspension was centrifuged at 5000 rpm for 5 min. The cell pellets were extracted with 5 mL hot ethanol (60 °C) for 2 min, and then sonicated in a water bath (50–60 °C) for 15 min. The mixture was centrifuged again, and the absorbances of the supernatant at 665, 649, and 470 nm were measured. The contents of Chl-a, Chl-b, and Cars were calculated as follows:
where Ca and Cb represent the contents of Chl-a and Chl-b, respectively.
Chl-a (mg L−1) = 16.82A665 − 9.28A652
Chl-b (mg L−1) = 36.92A652 − 16.54A665
Cars (mg L−1) = (1000A470 − 1.91Ca − 95.15Cb)/225
2.3.4. Determination of Chlorophyll Fluorescence
After 48 and 96 h of exposure, 20 mL microalgal suspension was centrifuged at 5000 rpm for 5 min. The microalgal pellets were re-suspended in 0.5 mL BG-11 medium, and 200 µL of the solution was then added to each well of the 96-well plate. After 30 min of dark adaption, the maximum quantum efficiency (Fv/Fm) was determined using a pulse-amplitude-modulated chlorophyll fluorometer (Imaging-PAM; Heinz Walz GmbH, Effeltrich, Germany) according to the method described by Fan et al. (2019) [39].
2.3.5. Assays of Oxidative Stress
After 96 h of exposure, 120 mL microalgal suspension was centrifuged at 5000 rpm for 5 min at 4 °C. The harvested microalgal pellets were re-suspended in 10 mM phosphate buffer (pH 7.4). The cells were disrupted by sonication at 200 w for 15 min (ultrasonic time 3 s, rest time 3 s) in an ice bath. Afterward, the supernatants were obtained by centrifugation for oxidative stress assays. Oxidative stress biomarkers, including superoxide dismutase (SOD), peroxidase (POD), glutathione (GSH), and MDA, were determined using commercial kits according to the manufacturer’s instructions.
2.4. Removal Kinetics and Mechanisms of TC by Microalgae
Removal kinetics and mechanisms of TC (0.5, 1.0, 2.5, 4.0, 6.0, and 10 mg L−1) by microalgae for 48 h were investigated. The initial cell density was 5.0 × 106 cells mL−1, and the cultivation conditions were the same as the toxicity test. Abiotic controls were also included with the same concentrations of TC, but without microalgae in the culture medium to monitor abiotic removal of TC. After 6, 12, 24, 36, and 48 h of exposure, 1 mL microalgal suspension in exposure groups was sampled and centrifuged at 10,000 rpm for 5 min. The supernatant was used to determine the residual concentrations of TC in the culture medium. The removal kinetics of TC by microalgae were fitted using the pseudo-zero order model (Equation (6)), pseudo-first order model (Equation (7)), and pseudo-second order model (Equation (8)) as follows:
where C0 is the initial concentration of TC at time zero, Ct is the residual concentration of TC at time t, and k is the removal rate constant (h−1).
Ct = −kt + C0
lnCt = −kt + lnC0
1/Ct = kt + 1/C0
To elucidate the contribution of bioadsorption, bioaccumulation, biodegradation, and abiotic processes to remove TC, 35 mL microalgal suspension was centrifuged at 5000 rpm for 5 min. The obtained microalgal pellets were mixed with 2 mL ultrapure water and vortexed for 1 min. This step was repeated three times to completely elute the TC adsorbed on the cell surface. The supernatants were obtained by centrifugation and used to determine the amount of TC adsorbed by microalgae. The rest of the microalgal pellets were re-suspended with 5 mL Na2EDTA–McIlvaine buffer solution and sonicated for 20 min. The extraction was repeated three times. After centrifugation, the supernatant was purified by a preconditioned Oasis HLB cartridge and used to determine the bioaccumulated amount of TC by microalgae. The removal amount by abiotic processes could be obtained by determining the residual concentrations of TC in the culture medium of abiotic controls. Then, the biodegradation amount of TC by microalgae was calculated using the following equation [35,40]:
where At is the initial amount of TC added in the culture medium; Ar is the residual amount of TC in the culture medium at the end of exposure; and Ad, Ac, and Aa are the removal amounts by bioadsorption, bioaccumulation, and abiotic processes, respectively.
Pb (%) = (At − Ar − Ad − Ac − Aa) × 100/At
2.5. Instrumental Analysis of TC
The instrumental analysis of TC was performed according to our previous study [41]. TC was detected by an ultra-performance liquid chromatography (UPLC) (Waters, Milford, MA, USA) with a PDA detector (λ = 358 nm) and an ACQUITY UPLC BEH C18 reversed-phase column (100 mm × 2.1 mm, 1.7 μm). The column temperature was 40 °C. The mobile phase was comprised of 0.1% formic acid and acetonitrile. The injection volume and flow rate were 10 μL and 0.15 mL min−1, respectively.
2.6. Statistical Analysis
Data were expressed as the mean values and standard deviations. Statistical analyses of the data from different treatments were conducted by analysis of variance (ANOVA), followed by Duncan’s test. The significant difference was considered at a level of p < 0.05. Statistical analyses were performed using IBM SPSS statistics 20.0.
3. Results and Discussion
3.1. Microalgal Growth
The effects of TC on the growth of C. pyrenoidosa and its inhibition rates are presented in Figure 1. No significant growth inhibition is observed in microalgae exposed to TC after 24 h of exposure, but microalgal growth is significantly inhibited by TC in an obvious dose–response manner with prolonged exposure time (48–96 h). The inhibition rates of low concentrations of TC (0.5–2.5 mg L−1) begin to decline after reaching the peak at 48 h or 72 h. This phenomenon may be due to the high detoxification ability of microalgae to low concentrations of TC and the continuous removal of TC by microalgae. However, the inhibition rates of high concentrations of TC (4–10 mg L−1) increase with increasing exposure time. At the end of exposure period (96 h), the inhibition rates of TC at concentrations of 0.5, 1, 2.5, 4, 6, and 10 mg L−1 are 12.28%, 26.3%, 26.2%, 37%, 40.32%, and 49.68%, respectively. An earlier study reports the 96 h EC50 of TC on C. vulgaris to be 7.73 mg L−1 [29]. In another work, Yang et al. (2013) report the maximum inhibition rate of TC on M. aeruginosa (0.05–1 mg L−1) and S. capricornutum (0.2–5 mg L−1) to be 35% and 70%, respectively [30]. These results show that C. pyrenoidosa possesses a higher tolerance to TC exposure than other algal species, suggesting its potential application in the bioremediation of TC-polluted water.
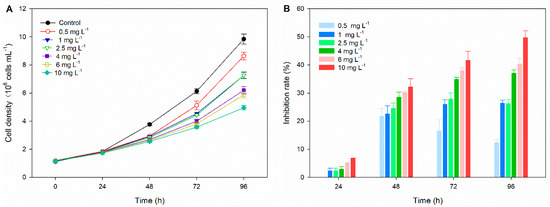
Figure 1.
Effects of TC on the growth (A) of C. pyrenoidosa and its inhibition rates (B) during 96 h exposure in different treatments.
3.2. Photosynthetic Performance
Photosynthesis is an essential metabolic activity in microalgae, and it contributes significantly to microalgal growth. Chlorophyll is the primary site for photosynthetic processes such as light collection, energy transduction, and light energy conversion [42]. Chl-a is the main photosynthetic pigment responsible for converting radiation into chemical energy [43]. As an accessory photosynthetic pigment, Chl-b can broaden the light absorption spectrum and transfer light energy to chlorophyll Chl-a [40]. As shown in Figure 2, the contents of Chl-a, Chl-b, and total chlorophyll are significantly decreased by TC at all exposure concentrations after 48 and 96 h of exposure, suggesting the impairment of photosynthesis in C. pyrenoidosa. The decrease in the content of chlorophyll is a common stress phenomenon in microalgae exposed to various pollutants, which may be due to the peroxidation of thylakoid membranes by ROS [44]. However, the inhibition rates of TC on the content of Chl-a, Chl-b, and total chlorophyll at 96 h (33.31–53.99%, 17.74–33.64%, and 31.52–51.75%, respectively) are lower than those at 48 h (47.53–62.54%, 52.99–58.15%, and 47.86–53.85%, respectively). The decreased degree of TC inhibition on chlorophyll content indicates that the photosynthesis capacity of C. pyrenoidosa recovers with prolonged exposure time.
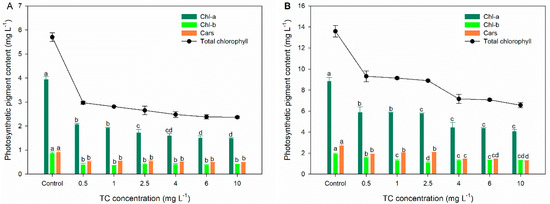
Figure 2.
Effects of TC on the Chl-a, Chl-b, Cars, and total chlorophyll of C. pyrenoidosa after 48 (A) and 96 h (B) of exposure in different treatments. The different letters indicate significant differences (p < 0.05) between the control and experimental treatments.
Cars play an important role in protecting the photosynthetic system of microalgae. They can react with lipid peroxidation products or neutralize excited chlorophyll [45]. After 48 and 96 h of exposure, the content of Cars significantly decreases in all TC treatment groups. This observation suggests that the protective function of Cars is weakened by TC. The decrease in the content of Cars may be due to the oxidation of Cars by superoxide anion radicals produced by triplet chlorophyll [46]. However, the inhibition rates of TC at low concentrations (0.5–2.5 mg L−1, 23.92–30.31%) on Cars at 96 h are much lower than those at 48 h (41.15–42.29%). For the high concentrations of TC, the inhibition rates increase slightly from 48 h (44.94–45.39%) to 96 h (47.62–53.61%). These results suggest that Cars could produce resistance to TC with a prolonged exposure period.
Fv/Fm is an important indicator of the effects of various pollutants on the photosynthetic activity of microalgae [39,47,48]. After 48 h of exposure, TC significantly decreases the Fv/Fm in all exposure groups, and the inhibition rates (6.56–48.18%) increase with increasing exposure concentration (Figure 3). The decrease in the values of Fv/Fm indicate PSII is damaged by TC [49]. However, the inhibition rates of TC on Fv/Fm are decreased at 96 h (4.09–23.10%) compared to those at 48 h, indicating that the damage of TC to PSII is reversible. The decreased inhibition rates of TC on Fv/Fm with prolonged exposure time are generally consistent with the photosynthetic pigments and growth described above. Thus, the repair of photosynthetic function contributes to the recovery of microalgae growth.
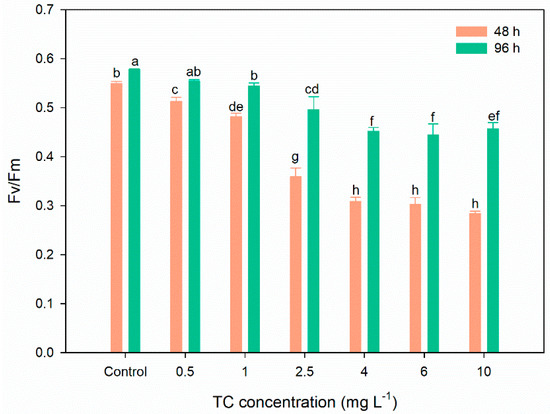
Figure 3.
Effects of TC on the Fv/Fm of C. pyrenoidosa after 48 and 96 h of exposure in different treatments. The different letters indicate significant differences (p < 0.05) between the control and experimental treatments.
3.3. Antioxidant Responses
Under normal growth conditions, the production and scavenging of ROS in microalgae are maintained in a state of dynamic equilibrium. However, exposure to exogenous pollutants can cause the overproduction of ROS, such as superoxide radicals (O2−), hydroxyl radicals (OH·), and hydrogen peroxide (H2O2) [50]. To alleviate oxidative damage, microalgae evolved enzymatic and non-enzymatic antioxidant systems to scavenge excessive ROS. SOD acts as the first line of defense against ROS toxicity, which can convert O2− into H2O2 and O2 [51,52]. As shown in Figure 4A, SOD activity is significantly increased by TC at concentrations of 2.5–10 mg L−1 (9.69–23.53%), suggesting the attempt of microalgae to remove the O2− induced by TC. The increase in SOD activity is expected to enhance the level of H2O2, which is still very toxic to microalgae. As a vital enzyme to decompose H2O2, POD activity usually increases simultaneously with SOD activity [31]. In the present study, POD activity is also significantly induced in C. pyrenoidosa exposed to 2.5–10 mg L−1 of TC by 15.15–110.92% (Figure 4B). GSH is a critical non-enzymatic antioxidant in resisting oxidative stress. It not only deactivates electrophiles via its sulfhydryl group, but also works as the substrate for glutathione peroxidase and glutathione S-transferase to disintegrate H2O2 [53]. A significant increase in the GSH content (17.62–64.17%) is observed in C. pyrenoidosa with exposure to TC at all concentrations (Figure 4C). The simultaneous increase in GSH, SOD, and POD suggest that the antioxidant system of C. pyrenoidosa could provide certain protection against oxidative damage. To evaluate the degree of oxidative damage caused by TC, the main lipid peroxidation product MDA was determined. As shown in Figure 4D, MDA content is significantly enhanced by TC at concentrations of 2.5–6 mg L−1 (25.16–35.25%), indicating the occurrence of oxidative damage. A possible explanation for this observation is that the antioxidant system cannot completely remove excessive ROS despite the activation of GSH, SOD, and POD. It is worth noticing that the increase in MDA content in this study is lower than those reported in previous studies [29,30]. For example, the MDA content in Chlorella vulgaris exposed to TC (1.25–10 mg L−1) increases by over 200% [45]. Thus, C. pyrenoidosa in the present work may suffer moderate oxidative damage when exposed to TC (2.5–6 mg L−1).
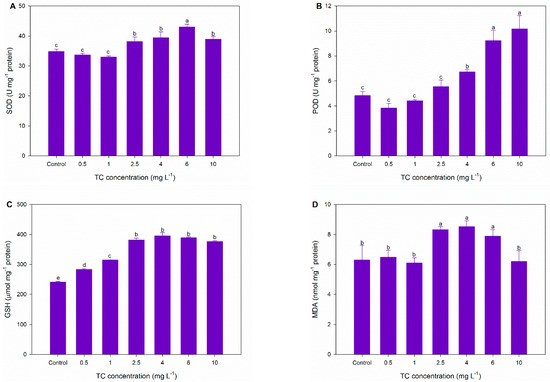
Figure 4.
Changes in the activities of SOD (A) and POD (B), and the levels of GSH (C) and MDA (D) of C. pyrenoidosa after 48 and 96 h of exposure in different treatments. The different letters indicate significant differences (p < 0.05) between the control and experimental treatments.
3.4. Removal Efficiency and Mechanism of TC
The removal rates of TC at all concentrations gradually increases with exposure time (Figure 5A). Interestingly, the removal rates of TC at high concentrations (4–10 mg L−1) are lower than those at low concentrations (0.5–2.5 mg L−1) after 12 h of exposure, but they are similar in a range of 91.44–95.14% at the end of exposure (48 h). This observation may be attributed to the recovery of C. pyrenoidosa from the toxicity of high concentrations of TC with prolonged exposure time. According to the kinetic parameters listed in Table 1, the pseudo-first order kinetic model could better describe the removal process of TC by C. pyrenoidosa than the pseudo-zero order kinetic model and pseudo-second order kinetic model based on the R2 values. The kinetic removal rate constants (k) and half-lives (t1/2) of the pseudo-first order kinetic model are 0.051–0.061 h−1 and 11.3–13.5 h, respectively. Previous studies demonstrate the feasible application of microalgae in removing TC. In high-rate algal ponds, a removal rate of 69 ± 1% is achieved for 2 mg L−1 TC [25], and 93% TC (0.1 mg L−1) is removed in 4 days [26]. TC (1 mg L−1) is completely removed from non-sterile swine wastewater treated by Chlorella vulgaris in 11 days with a k value of 0.367 ± 0.08 d−1. Compared to these earlier studies, our study shows that C. pyrenoidosa possesses an efficient and rapid removal capacity of TC. Previous studies demonstrate that the capability of microalgae to remove antibiotics is related to their physiological and biochemical status [33,54,55]. As discussed above, C. pyrenoidosa shows higher tolerance to TC than other algal species. In addition, it could recover from the poisoning of TC with prolonged exposure time. Thus, the better removal performance of TC by C. pyrenoidosa may be due to the high tolerance and resistance of this microalgal species to the toxicity of TC.
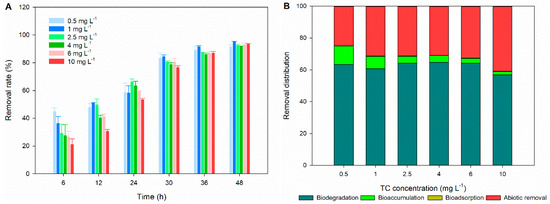
Figure 5.
Time course profiles of TC removal rates by C. pyrenoidosa (A) and mass balance of TC in terms of biodegradation, bioaccumulation, bioadsorption, and abiotic processes after 48 h of exposure (B).

Table 1.
Kinetic parameters of TC removal by C. pyrenoidosa after 48 h of exposure.
The contributions of abiotic processes, biodegradation, bioaccumulation, and bioadsorption to the removal of TC after 48 h of exposure are shown in Figure 5B. The concentrations of TC in the culture medium of abiotic control decrease by 22.74–38.62%. Correspondingly, the abiotic process contributes 24.68–40.97% to the total removal of TC. Previous studies demonstrate that TC is susceptible to photolysis and hydrolysis [25,26]. In addition to photolysis and hydrolysis, the formed low solubility complexes of TC and divalent cations in the culture medium may also involve the abiotic removal of TC [56]. This speculation may be supported by the increased abiotic removal rates with increasing TC concentration. The much higher removal rates (91.44–95.14%) in microalgae cultures suggest that the removal of TC is mainly mediated by the biotic processes of C. pyrenoidosa. Biodegradation is the dominant mechanism for TC removal, with a contribution of 56.86–64.62% to the total removal of TC. In contrast, low contributions are observed for bioadsorption (0.09–0.38%) and bioaccumulation (1.95–10.97%). The bioadsorption of antibiotics by microalgae depends on the lipophilicity of antibiotics [57]. The low hydrophobicity of TC (log Kow = −1.3) may be an important factor limiting biosorption and subsequent bioaccumulation. In addition, the high abiotic removal and biodegradation efficiency could also decrease the surface adsorption and intracellular accumulation of TC. A recent study also reports that biodegradation is the primary mechanism for TC removal by a harmful cyanobacterial species, Microcystis aeruginosa [28]. In contrast, photodegradation and biosorption mainly contribute to TC removal in high-rate algal ponds [25,26]. The differences among these studies might be attributable to differences in the microalgal species and experiment conditions. Compared to bioadsorption and bioaccumulation, biodegradation is regarded as a more effective mechanism for the microalgae-mediated removal of antibiotics [21]. In addition, as a green microalga, C. pyrenoidosa is more environmentally friendly than cyanobacterial species. Thus, our results suggest that C. pyrenoidosa can be a good candidate for the purification of TC from wastewater. Nevertheless, it should be noted that more toxic degradation products may be formed during the microalgae treatment. Further research is still required to identify the degradation products of TC by C. pyrenoidosa and assess the ecotoxicity of degradation products.
4. Conclusions
This study investigated the ecotoxicity and removal of TC in C. pyrenoidosa. TC displays a concentration-dependent inhibition on microalgal growth. The contents of photosynthetic pigments and Fv/Fm value are also significantly decreased by TC, indicating the damage of TC on microalgal photosynthesis. However, the decreased inhibition rates on growth, chlorophyll, and Fv/Fm with prolonged exposure time suggest that C. pyrenoidosa can produce certain resistance to the toxicity of TC. In addition, the antioxidant system is activated to scavenge the excessive ROS as indicated by synchronously increased activities of SOD and POD, and GSH level. The increase in MDA content indicates that TC causes moderate oxidative damage to C. pyrenoidosa. TC could be removed by C. pyrenoidosa with high efficiency. The removal of TC is mainly achieved by biodegradation, followed by abiotic processes, bioadsorption, and bioaccumulation. The results of this study show that C. pyrenoidosa can produce resistance against TC poisoning and efficiently remove TC. Therefore, C. pyrenoidosa could be a promising microalga for the remediation of TC-contaminated water.
Author Contributions
Conceptualization, J.T. and Z.X.; methodology, J.Y.; validation, J.T., J.Y. and X.W.; formal analysis, S.Z.; investigation, J.Y.; resources, Z.X.; data curation, S.Z.; writing—original draft preparation, J.T.; writing—review and editing, Z.X.; visualization, Z.X.; supervision, Z.X.; project administration, J.T.; funding acquisition, Z.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Provincial Foundation for Excellent Young Talents of Colleges and Universities of Anhui Province (Grant number, gxyqZD2022019), National Natural Science Foundation of China (Grant numbers, 51609001 and 51709002), and National Students’ Innovation and Entrepreneurship Training Program (Grant number, 202110364079).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment–a review–part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, P.; Xie, Z.; Wang, Z.; Hu, B. Effect of iron plaque on antibiotic uptake and metabolism in water spinach (Ipomoea aquatic Forsk.) grown in hydroponic culture. J. Hazard. Mater. 2021, 417, 125981. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Lundström, S.V.; Östman, M.; Bengtsson-Palme, J.; Rutgersson, C.; Thoudal, M.; Sircar, T.; Blanck, H.; Eriksson, K.M.; Tysklind, M.; Flach, C.-F.; et al. Minimal selective concentrations of tetracycline in complex aquatic bacterial biofilms. Sci. Total Environ. 2016, 553, 587–595. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, J.; Wu, X.; Li, X.; Hua, R. Bioconcentration, metabolism and the effects of tetracycline on multiple biomarkers in Chironomus riparius larvae. Sci. Total Environ. 2019, 649, 1590–1598. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, J.; Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 2015, 24, 707–719. [Google Scholar] [CrossRef]
- Kim, H.Y.; Asselman, J.; Jeong, T.Y.; Yu, S.; De Schamphelaere, K.A.C.; Kim, S.D. Multigenerational effects of the antibiotic tetracycline on transcriptional responses of Daphnia magna and its relationship to higher levels of biological organizations. Environ. Sci. Technol. 2017, 51, 12898–12907. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Research progress on distribution, migration, transformation of antibiotics and antibiotic resistance genes (ARGs) in aquatic environment. Crit. Rev. Biotechnol. 2018, 38, 1195–1208. [Google Scholar] [CrossRef]
- Shao, S.; Wu, X. Microbial degradation of tetracycline in the aquatic environment: A review. Crit. Rev. Biotechnol. 2020, 40, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Leichtweis, J.; Vieira, Y.; Welter, N.; Silvestri, S.; Dotto, G.L.; Carissimi, E. A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic. Process. Safe. Environ. 2022, 160, 25–40. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Xu, J.; Xie, Z.; Tang, J.; Li, X.; Fan, S. Simultaneous carbonization, activation, and magnetization for producing tea waste biochar and its application in tetracycline removal from the aquatic environment. J. Environ. Chem. Eng. 2021, 9, 105324. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Xu, H. Effect of Fe–N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Li, J.; Rong, S.; Jiang, J.; Liu, S. Photocatalytic Degradation of tetracycline by a novel (CMC)/MIL-101 (Fe)/β-CDP composite hydrogel. Front. Chem. 2021, 8, 593730. [Google Scholar] [CrossRef]
- Wang, J.S.; Yi, X.H.; Xu, X.; Ji, H.; Alanazi, A.M.; Wang, C.C.; Zhao, C.; Kaneti, Y.V.; Wang, P.; Liu, W. Eliminating tetracycline antibiotics matrix via photoactivated sulfate radical-based advanced oxidation process over the immobilized MIL-88A: Batch and continuous experiments. Chem. Eng. J. 2022, 431, 133213. [Google Scholar] [CrossRef]
- Pan, L.; Cao, Y.; Zang, J.; Huang, Q.; Wang, L.; Zhang, Y.; Fan, S.; Tang, J.; Xie, Z. Preparation of iron-loaded granular activated carbon catalyst and its application in tetracycline antibiotic removal from aqueous solution. Int. J. Environ. Res. Public Health 2019, 16, 2270. [Google Scholar] [CrossRef]
- Jingrui, X.; Asraful Alam, M.; Jing, W.; Wenchao, W.; Norhana Balia Yusof, Z.; Daroch, M.; Zhang, D.; Lifen, L.; Russel, M. Enhanced removal of tetracycline from synthetic wastewater using an optimal ratio of co-culture of Desmodesmus sp. and Klebsiella pneumoniae. Bioresour. Technol. 2022, 351, 127056. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, L.X.; Liu, Y.S.; Zhao, J.L.; He, L.Y.; Ying, G. Microalgae-based technology for antibiotics removal: From mechanisms to application of innovational hybrid systems. Environ. Int. 2021, 155, 106594. [Google Scholar] [CrossRef] [PubMed]
- Russel, M.; Meixue, Q.; Alam, M.A.; Lifen, L.; Daroch, M.; Blaszczak-Boxe, C.; Kumar Gupta, G. Investigating the potentiality of Scenedesmus obliquus and Acinetobacter pittii partnership system and their effects on nutrients removal from synthetic domestic wastewater. Bioresour. Technol. 2020, 299, 122571. [Google Scholar] [CrossRef] [PubMed]
- Hena, S.; Gutierrez, L.; Croué, J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef] [PubMed]
- De Godos, I.; Muñoz, R.; Guieysse, B. Tetracycline removal during wastewater treatment in high-rate algal ponds. J. Hazard. Mater. 2012, 229–230, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Norvill, Z.N.; Toledo-Cervantes, A.; Blanco, S.; Shilton, A.; Guieysse, B.; Muñoz, R. Photodegradation and sorption govern tetracycline removal during wastewater treatment in algal ponds. Bioresour. Technol. 2017, 232, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Michelon, W.; Matthiensen, A.; Viancelli, A.; Fongaro, G.; Gressler, V.; Soares, H.M. Removal of veterinary antibiotics in swine wastewater using microalgae-based process. Environ. Res. 2022, 207, 112192. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lyu, T.; Zhan, L.; Matamoros, V.; Angelidaki, I.; Cooper, M.; Pan, G. Mitigating antibiotic pollution using cyanobacteria: Removal efficiency, pathways and metabolism. Water Res. 2021, 190, 116735. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xiao, Y.; Pan, H.; Mei, Y. Toxic effects of tetracycline and its degradation products on freshwater green algae. Ecotoxicol. Environ. Saf. 2019, 174, 43–47. [Google Scholar] [CrossRef]
- Yang, W.; Tang, Z.; Zhou, F.; Zhang, W.; Song, L. Toxicity studies of tetracycline on Microcystis aeruginosa and Selenastrum capricornutum. Environ. Toxicol. Pharmacol. 2013, 35, 320–324. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, Y.; Ma, Z.; Li, H.; Yu, W.; Cao, L.; He, Q. Azithromycin induces dual effects on microalgae: Roles of photosynthetic damage and oxidative stress. Ecotoxicol. Environ. Saf. 2021, 222, 112496. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Tao, M.T.; Xu, C.M.; Chen, M. Quantitative characterization of toxicity interaction within antibiotic-heavy metal mixtures on Chlorella pyrenoidosa by a novel area-concentration ratio method. Sci. Total Environ. 2021, 762, 144180. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, K.; Li, W.; Zhang, M.; Li, P.; Han, J. Removal mechanisms of erythromycin by microalgae Chlorella pyrenoidosa and toxicity assessment during the treatment process. Sci. Total Environ. 2022, 848, 157777. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Liu, Y.S.; Hu, L.X.; Shi, Z.Q.; Cai, W.W.; He, L.Y.; Ying, G.G. Co-metabolism of sulfamethoxazole by a freshwater microalga Chlorella pyrenoidosa. Water Res. 2020, 175, 115656. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhang, X.; Zhou, T.; Lv, G.; Zhao, Q. Exploring kinetics, removal mechanism and possible transformation products of tigecycline by Chlorella pyrenoidosa. Sci. Total Environ. 2022, 817, 152988. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.T.; Hou, J.H.; Su, C.I.; Chen, C.L. Effects of chloramphenicol, florfenicol, and thiamphenicol on growth of algae Chlorella pyrenoidosa, Isochrysis galbana, and Tetraselmis chui. Ecotoxicol. Environ. Saf. 2009, 72, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhu, Y.; Wang, Y.; Zhao, Q.; Huang, H. Effects of three antibiotics on growth and antioxidant response of Chlorella pyrenoidosa and Anabaena cylindrica. Ecotoxicol. Environ. Saf. 2021, 211, 111954. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Fan, H.; Jin, M.; Wang, H.; Xu, Q.; Xu, L.; Wang, C.; Du, S.; Liu, H. Effect of differently methyl-substituted ionic liquids on Scenedesmus obliquus growth, photosynthesis, respiration, and ultrastructure. Environ. Pollut. 2019, 250, 155–165. [Google Scholar] [CrossRef]
- Lee, S.H.; Xiong, J.Q.; Ru, S.; Patil, S.M.; Kurade, M.B.; Govindwar, S.P.; Oh, S.E.; Jeon, B.H. Toxicity of benzophenone-3 and its biodegradation in a freshwater microalga Scenedesmus obliquus. J. Hazard. Mater. 2020, 389, 122149. [Google Scholar] [CrossRef]
- Zang, J.; Wu, T.; Song, H.; Zhou, N.; Fan, S.; Xie, Z.; Tang, J. Removal of tetracycline by hydrous ferric oxide: Adsorption kinetics, isotherms, and mechanism. Int. J. Environ. Res. Public Health 2019, 16, 4580. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Wu, Y.; Ding, H.; Zhang, W. Toxicity, biodegradation, and metabolic fate of organophosphorus pesticide trichlorfon on the freshwater algae Chlamydomonas reinhardtii. J. Agric. Food Chem. 2020, 68, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Lin, K.; Yang, B.; Yang, M.; Li, J. Toxic effects and metabolic fate of carbamazepine in diatom Navicula sp. as influenced by humic acid and nitrogen species. J. Hazard. Mater. 2019, 378, 120763. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, W.; Li, J.; Yuan, M.; Zhang, J.; Xu, F.; Xu, H.; Zheng, X.; Wang, L. Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum). Environ. Pollut. 2020, 263, 114554. [Google Scholar] [CrossRef] [PubMed]
- Cupellini, L.; Calvani, D.; Jacquemin, D.; Mennucci, B. Charge transfer from the carotenoid can quench chlorophyll excitation in antenna complexes of plants. Nat. Commun. 2020, 11, 662. [Google Scholar] [CrossRef]
- Wan, L.; Wu, Y.; Zhang, B.; Yang, W.; Ding, H.; Zhang, W. Effects of moxifloxacin and gatifloxacin stress on growth, photosynthesis, antioxidant responses, and microcystin release in Microcystis aeruginosa. J. Hazard. Mater. 2021, 409, 124518. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Gan, Y.; Cheng, H.; Fan, S.; Li, X.; Tang, J. Ecotoxicological effects of the antidepressant fluoxetine and its removal by the typical freshwater microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2022, 244, 114045. [Google Scholar] [CrossRef]
- Yang, M.; Fan, Z.; Xie, Y.; Fang, L.; Wang, X.; Yuan, Y.; Li, R. Transcriptome analysis of the effect of bisphenol A exposure on the growth, photosynthetic activity and risk of microcystin-LR release by Microcystis aeruginosa. J. Hazard. Mater. 2020, 397, 122746. [Google Scholar] [CrossRef]
- Wang, H.; Jin, M.; Mao, W.; Chen, C.; Fu, L.; Li, Z.; Du, S.; Liu, H. Photosynthetic toxicity of non-steroidal anti-inflammatory drugs (NSAIDs) on green algae Scenedesmus obliquus. Sci. Total Environ. 2020, 707, 136176. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Gao, X.; Yin, Z.; Bao, J.; Li, Z.; Chu, R.; Hu, D.; Zhang, J.; Zhu, L. Evaluation of growth and biochemical responses of freshwater microalgae Chlorella vulgaris due to exposure and uptake of sulfonamides and copper. Bioresour. Technol. 2021, 342, 126064. [Google Scholar] [CrossRef]
- Xie, Z.; Luan, H.; Zhang, Y.; Wang, M.; Cao, D.; Yang, J.; Tang, J.; Fan, S.; Wu, X.; Hua, R. Interactive effects of diclofenac and copper on bioconcentration and multiple biomarkers in crucian carp (Carassius auratus). Chemosphere 2020, 242, 125141. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gan, Y.; Tang, J.; Fan, S.; Wu, X.; Li, X.; Cheng, H.; Tang, J. Combined effects of environmentally relevant concentrations of diclofenac and cadmium on Chironomus riparius larvae. Ecotoxicol. Environ. Saf. 2020, 202, 110906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, J.; Li, Z.; Wu, Z.; Dang, C.; Fu, J. Enhanced removal efficiency of sulfamethoxazole by acclimated microalgae: Tolerant mechanism, and transformation products and pathways. Bioresour. Technol. 2022, 347, 126461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wei, Y.; Sun, J.; Wang, J.; Zhao, Z.; Liu, Y.; Li, X.; Cao, J. Degradation of cefradine in alga-containing water environment: A mechanism and kinetic study. Environ. Sci. Pollut. Res. 2019, 26, 9184–9192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dou, X.; Wu, J.; Meng, F. Attenuation pathways of erythromycin and biochemical responses related to algal growth and lipid synthesis in a microalga-effluent system. Environ. Res. 2021, 195, 110873. [Google Scholar] [CrossRef] [PubMed]
- Pala-Ozkok, I.; Ubay-Cokgor, E.; Jonas, D.; Orhon, D. Kinetic and microbial response of activated sludge community to acute and chronic exposure to tetracycline. J. Hazard. Mater. 2019, 367, 418–426. [Google Scholar] [CrossRef]
- Kiki, C.; Rashid, A.; Wang, Y.; Li, Y.; Zeng, Q.; Yu, C.P.; Sun, Q. Dissipation of antibiotics by microalgae: Kinetics, identification of transformation products and pathways. J. Hazard. Mater. 2020, 387, 121985. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).