Selenium Agronomic Biofortification of Durum Wheat Fertilized with Organic Products: Se Content and Speciation in Grain
Abstract
1. Introduction
2. Materials and Methods
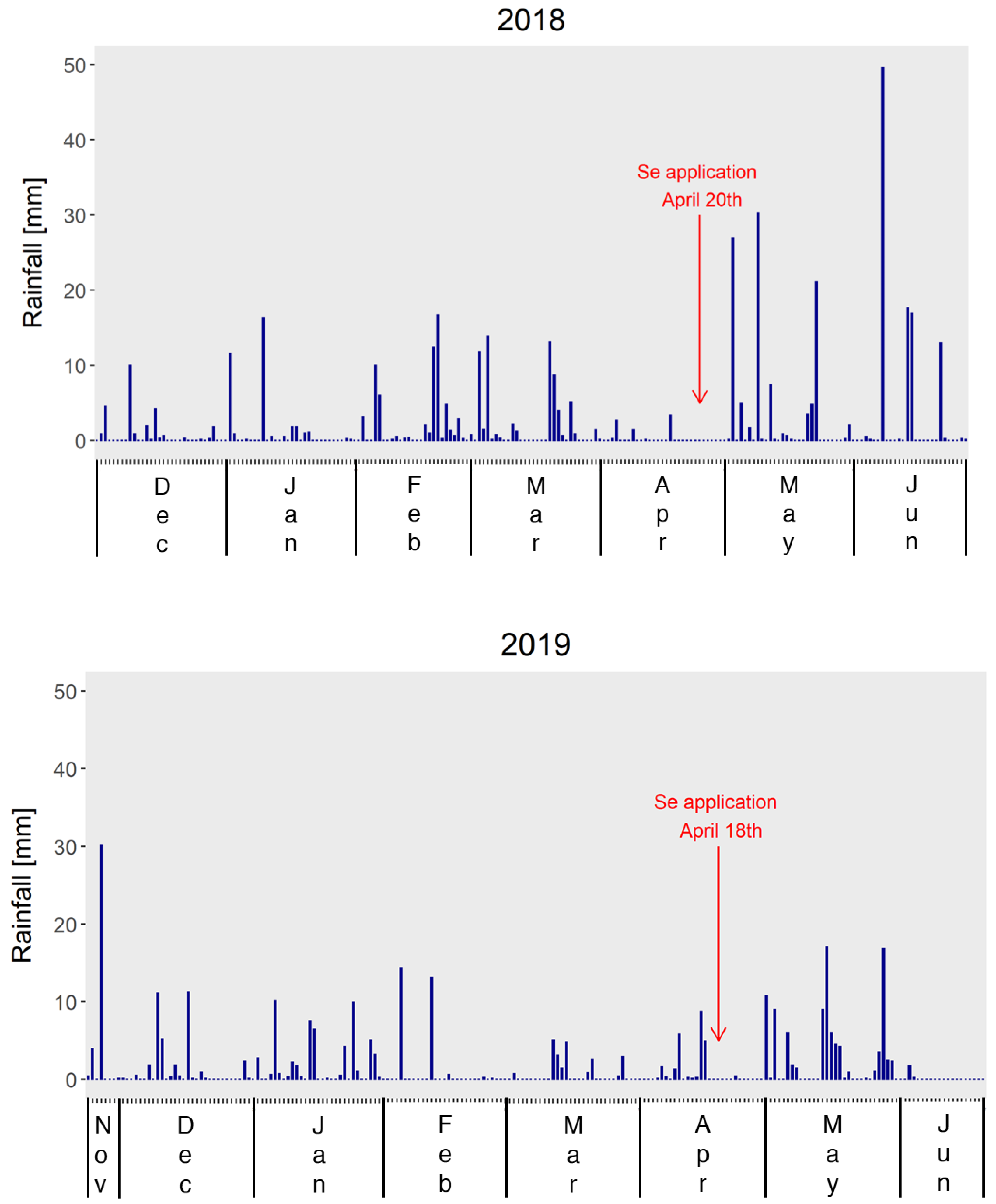
2.1. Field Experiment
2.2. Selenium Analyses
2.2.1. Determination of the Total Se Content by ICP-MS
2.2.2. Extraction Se-Species and HPLC-ICP-MS and HPLC-ESI-MS/MS Analysis
2.3. Statistical Analysis
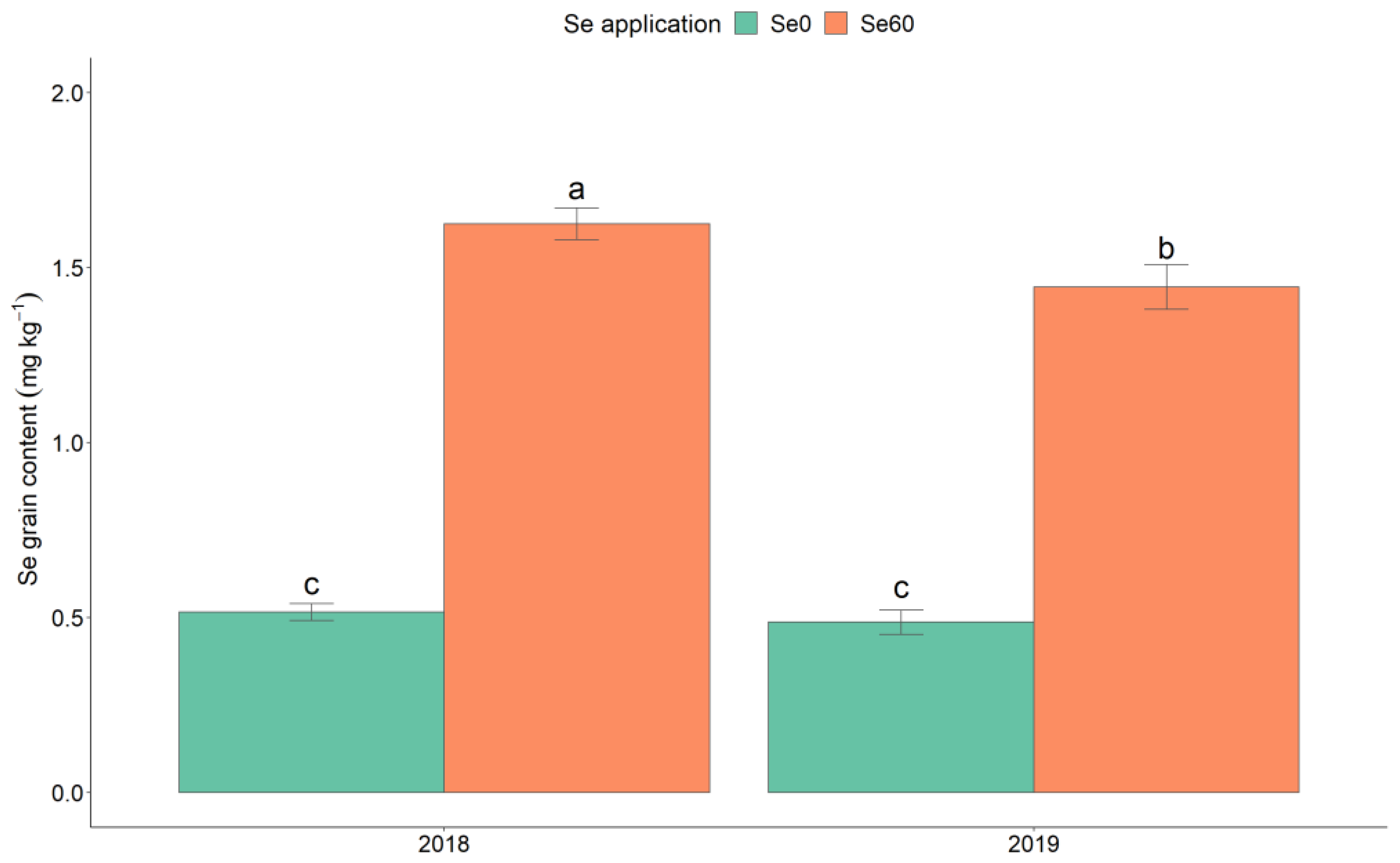
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAO Launches 2020 as the UN’s International Year of Plant Health. 2020. Available online: http://www.fao.org/news/story/pt/item/1253551/icode/#:~:text=Plants%20make%20up%2080%20percent%20of%20the%20food%20we%20eat,to%20plant%20pests%20and%20diseases (accessed on 5 September 2021).
- Martin, C.; Li, J. Medicine is not health care, food is health care: Plant metabolic engineering, diet and human health. New Phytol. 2017, 216, 699–719. [Google Scholar] [CrossRef]
- Lowe, N.M. The global challenge of hidden hunger: Perspectives from the field. Proc. Nutr. Soc. 2021, 80, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Nardi, S.; Dalla Vecchia, F.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Marek, K.; Stanisław, B. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Yang, L.; Kong, C.; Wang, J.; Li, Y. Selenium nutritional status of rural residents and its correlation with dietary intake patterns in a typical low-selenium area in China. Nutrients 2020, 12, 3816. [Google Scholar] [CrossRef]
- Petrović, M. Selenium: Widespread yet scarce, essential yet toxic. ChemTexts 2021, 7, 1–17. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Winkel, L.H. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Ligas, B.; Mikula, K.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biofortification of edible plants with selenium and iodine–A systematic literature review. Sci. Total Environ. 2021, 754, 141983. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.A.; Giuliani, M.M.; Giuzio, L.; De Vita, P.; Flagella, Z. Assessment of grain protein composition in old and modern Italian durum wheat genotypes. Ital. J. Agron. 2018, 13, 40–43. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Sacco, A.; Senatore, M.; Mori, M. Organic versus mineral fertilization: Assessing of yield and quality of durum wheat in marginal lands. Ital. J. Agron. 2021, 16, 117–126. [Google Scholar] [CrossRef]
- Giacco, R.; Vitale, M.; Riccardi, G. Pasta: Role in diet. Encycl. Food Health 2016, 242–245. [Google Scholar] [CrossRef]
- Galinha, C.; Freitas, M.D.C.; Pacheco, A.M.; Coutinho, J.; Maçãs, B.; Almeida, A.S. Selenium supplementation of Portuguese wheat cultivars through foliar treatment in actual field conditions. J. Radioanal. Nucl. Chem. 2013, 297, 227–231. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rodrigo, S.; Santamaría, O.; Chen, Y.; McGrath, S.P. Agronomic selenium biofortification in Triticum durum under Mediterranean conditions: From grain to cooked pasta. Food Chem. 2014, 146, 378–384. [Google Scholar] [CrossRef] [PubMed]
- De Vita, P.; Platani, C.; Fragasso, M.; Ficco, D.B.M.; Colecchia, S.A.; Del Nobile, M.A.; Petrozza, A. Selenium-enriched durum wheat improves the nutritional profile of pasta without altering its organoleptic properties. Food Chem. 2017, 214, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.G.; Maher, W.A.; Jagtap, R.; Krikowa, F.; Roper, M.M.; O’Sullivan, C.A. Selenium speciation in wheat grain varies in the presence of nitrogen and sulphur fertilisers. Environ. Geochem. Health 2017, 39, 955–966. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, Z.; Lv, C.; Zhang, Z.; Yuan, L.; Liu, X. Effects of sulfur application on selenium uptake and seed selenium speciation in soybean (Glycine max L.) grown in different soil types. Ecotoxicol. Environ. Saf. 2020, 209, 111790. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Deng, X.; Li, M.; Zhang, W.; Zhao, Z. Effects of sulfur and sulfate on selenium uptake and quality of seeds in rapeseed (Brassica napus L.) treated with selenite and selenate. Environ. Exp. Bot. 2017, 135, 13–20. [Google Scholar] [CrossRef]
- Silva, V.M.; Wilson, L.; Young, S.D.; Broadley, M.R.; White, P.J.; Reis, A.R.D. Interaction between sulfur and selenium in agronomic biofortification of cowpea plants under field conditions. Plant Soil 2022, 1–17. [Google Scholar] [CrossRef]
- Pompa, M.; Giuliani, M.M.; Giuzio, L.; Gagliardi, A.; Di Fonzo, N.; Flagella, Z. Effect of sulphur fertilization on grain quality and protein composition of durum wheat (Triticum durum Desf.). Ital. J. Agron. 2009, 4, 159–170. [Google Scholar] [CrossRef]
- Ercoli, L.; Lulli, L.; Arduini, I.; Mariotti, M.; Masoni, A. Durum wheat grain yield and quality as affected by S rate under Mediterranean conditions. Eur. J. Agron. 2011, 35, 63–70. [Google Scholar] [CrossRef]
- Gupta, U.C.; Gupta, S.C. Selenium in soils and crops, its deficiencies in livestock and humans: Implications for management. Commun. Soil Sci. Plant Anal. 2000, 31, 1791–1807. [Google Scholar] [CrossRef]
- Läuchli, A. Selenium in plants: Uptake, functions, and environmental toxicity. Bot. Acta 1993, 106, 455–468. [Google Scholar] [CrossRef]
- Buchner, P.; Stuiver, C.E.E.; Westerman, S.; Wirtz, M.; Hell, R.; Hawkesford, M.J.; De Kok, L.J. Regulation of sulfate uptake and expression of sulfate transporter genes in Brassica oleracea as affected by atmospheric H2S and pedospheric sulfate nutrition. Plant Physiol. 2004, 136, 3396–3408. [Google Scholar] [CrossRef]
- Smith, F.W.; Ealing, P.M.; Hawkesford, M.J.; Clarkson, D.T. Plant members of a family of sulfate transporters reveal functional subtypes. Proc. Natl. Acad. Sci. USA 1995, 92, 9373–9377. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.M.; De Souza, M.P.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Ellis, D.R.; Salt, D.E. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- Chan, Q.; Afton, S.E.; Caruso, J.A. Selenium speciation profiles in selenite-enriched soybean (Glycine Max) by HPLC-ICPMS and ESI-ITMS. Metallomics 2010, 2, 147–153. [Google Scholar] [CrossRef]
- Gagliardi, A.; Carucci, F.; Masci, S.; Flagella, Z.; Gatta, G.; Giuliani, M.M. Effects of genotype, growing season and nitrogen level on gluten protein assembly of durum wheat grown under mediterranean conditions. Agronomy 2020, 10, 755. [Google Scholar] [CrossRef]
- Ben Mariem, S.; González-Torralba, J.; Collar, C.; Aranjuelo, I.; Morales, F. Durum wheat grain yield and quality under low and high nitrogen conditions: Insights into natural variation in low-and high-yielding genotypes. Plants 2020, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Rehim, A.; Khan, M.; Imran, M.; Bashir, M.A.; Ul-Allah, S.; Khan, M.N.; Hussain, M. Integrated use of farm manure and synthetic nitrogen fertilizer improves nitrogen use efficiency, yield and grain quality in wheat. Ital. J. Agron. 2020, 15, 29–34. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, D.; Song, W.; Lei, L.; Yu, D.; Miao, S. Effects of nitrogen application on selenium accumulation, translocation and distribution of winter wheat at different growth periods. J. Plant Nutr. 2016, 22, 395–402. [Google Scholar] [CrossRef]
- Klikocka, H.; Szostak, B.; Barczak, B.; Kobiałka, A. Effect of sulphur and nitrogen fertilization on the selenium content and uptake by grain of spring wheat. J. Elem. 2017, 22, 985–994. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium biofortification and interaction with other elements in plants: A review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef] [PubMed]
- Benton, T.G.; Froggatt, A.; Wellesley, L.; Schröder, P. The Ukraine War and Threats to Food and Energy Security; Royal Institute of International Affairs: London, UK, 2022. [Google Scholar]
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Irshad, S.; Abbas, Q.; Ahmad, R. A comprehensive review on environmental transformation of selenium: Recent advances and research perspectives. Environ. Geochem. Health. 2019, 41, 1003–1035. [Google Scholar] [CrossRef]
- Carucci, F.; Gatta, G.; Gagliardi, A.; De Vita, P.; Bregaglio, S.; Giuliani, M.M. Agronomic strategies to improve N efficiency indices in organic durum wheat grown in Mediterranean area. Plants 2021, 10, 2444. [Google Scholar] [CrossRef]
- Moreno-Martín, G.; Sanz-Landaluze, J.; León-González, M.E.; Madrid, Y. Insights into the accumulation and transformation of Ch-SeNPs by Raphanus sativus and Brassica juncea: Effect on essential elements uptake. Sci. Total Environ. 2020, 725, 138453. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.R.-project.org (accessed on 9 May 2021).
- Wickham, H. Elegant Graphics for Data Analysis-ggplot2; Springer: New York, NY, USA, 2009; Available online: https://cran.r-project.org/web/packages/ggplot2/citation.html (accessed on 9 May 2021).
- Tan, J.A. The Atlas of Endemic Diseases and Their Environments in the People’s Republic of China; Science Press: Beijing, China, 1989. [Google Scholar]
- Gissel-Nielsen, G.; Gupta, U.C.; Lamand, M.; Westermarck, T. Selenium in soils and plants and its importance in livestock and human nutrition. Adv. Agron. 1984, 37, 397–460. [Google Scholar] [CrossRef]
- AL-Ghumaiz, N.S.; Motawei, M.I.; Abd-Elmoniem, E.M.; Al-Otayk, S.M. Selenium and zinc concentrations in spring wheat (Triticum aestivum) genotypes under organic and inorganic fertilization. J. Plant Nutr. 2020, 43, 1980–1987. [Google Scholar] [CrossRef]
- Laurent, C.; Bravin, M.N.; Crouzet, O.; Pelosi, C.; Tillard, E.; Lecomte, P.; Lamy, I. Increased soil pH and dissolved organic matter after a decade of organic fertilizer application mitigates copper and zinc availability despite contamination. Sci. Total Environ. 2020, 709, 135927. [Google Scholar] [CrossRef]
- Manojlović, M.S.; Lončarić, Z.; Cabilovski, R.R.; Popović, B.; Karalić, K.; Ivezić, V.; Singh, B.R. Biofortification of wheat cultivars with selenium. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 715–724. [Google Scholar] [CrossRef]
- Rodrigo, S.; Santamaria, O.; Chen, Y.; McGrath, S.P.; Poblaciones, M.J. Selenium speciation in malt, wort, and beer made from selenium-biofortified two-rowed barley grain. J. Agric. Food Chem. 2014, 62, 5948–5953. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fraga, H.; van Ieperen, W.; Santos, J.A. Assessing the impacts of recent-past climatic constraints on potential wheat yield and adaptation options under Mediterranean climate in southern Portugal. Agric. Syst. 2020, 182, 102844. [Google Scholar] [CrossRef]
- Soltanpour, P.N.; Olsen, S.R.; Goos, R.J. Effect of nitrogen fertilization of dryland wheat on grain selenium concentration. Soil Sci. Soc. Am. J. 1982, 46, 430–433. [Google Scholar] [CrossRef]
- Lyons, G.H.; Lewis, J.; Lorimer, M.F.; Holloway, R.E.; Brace, D.M.; Stangoulis, J.C.; Graham, R.D. High-selenium wheat: Agronomic biofortification strategies to improve human nutrition. Food Agric. Environ. 2004, 2, 171–178. [Google Scholar]
- Lyons, G.H.; Genc, Y.; Stangoulis, J.C.; Palmer, L.T.; Graham, R.D. Selenium distribution in wheat grain, and the effect of postharvest processing on wheat selenium content. Biol. Trace Elem. Res. 2005, 103, 155–168. [Google Scholar] [CrossRef]
- Zhao, F.J.; Su, Y.H.; Dunham, S.J.; Rakszegi, M.; Bedo, Z.; McGrath, S.P.; Shewry, P.R. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci. 2009, 49, 290–295. [Google Scholar] [CrossRef]
- De Vita, P.; Nicosia, O.L.D.; Nigro, F.; Platani, C.; Riefolo, C.; Di Fonzo, N.; Cattivelli, L. Breeding progress in morpho-physiological, agronomical and qualitative traits of durum wheat cultivars released in Italy during the 20th century. Eur. J. Agron. 2007, 26, 39–53. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Businelli, D. Current knowledge on selenium biofortification to improve the nutraceutical profile of food: A comprehensive review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Stadlober, M.; Sager, M.; Irgolic, K.J. Effects of selenate supplemented fertilisation on the selenium level of cereals—Identification and quantification of selenium compounds by HPLC–ICP–MS. Food Chem. 2001, 73, 357–366. [Google Scholar] [CrossRef]
- Cubadda, F.; Aureli, F.; Ciardullo, S.; D’Amato, M.; Raggi, A.; Acharya, R.; Prakash, N.T. Changes in selenium speciation associated with increasing tissue concentrations of selenium in wheat grain. J. Agric. Food Chem. 2010, 58, 2295–2301. [Google Scholar] [CrossRef]
- Ogra, Y.; Kitaguchi, T.; Ishiwata, K.; Suzuki, N.; Toida, T.; Suzuki, K.T. Speciation of selenomethioninemetabolites in wheat germ extract. Metallomics 2009, 1, 78–86. [Google Scholar] [CrossRef]
- Olson, O.E.; Novacek, E.J.; Whitehead, E.I.; Palmer, I.S. Investigations on selenium in wheat. Phytochemistry 1970, 9, 1181–1188. [Google Scholar] [CrossRef]
- Hart, D.J.; Fairweather-Tait, S.J.; Broadley, M.R.; Dickinson, S.J.; Foot, I.; Knott, P.; Hurst, R. Selenium concentration and speciation in biofortified flour and bread: Retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem. 2011, 126, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Mi, X.; Ouerdane, L.; Lobinski, R.; García-Reyes, J.F.; Molina-Díaz, A.; Dernovics, M. Quantification of Se-methylselenocysteine and its γ-glutamyl derivative from naturally Se-enriched green bean (Phaseolus vulgaris vulgaris) after HPLC-ESI-TOF-MS and orbitrap MS n-based identification. Food Anal. Methods 2014, 7, 1147–1157. [Google Scholar] [CrossRef]
- White, P.J. Selenium metabolism in plants. Biochim Biophys Acta 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]



| DF | Se Content in Grain | |
|---|---|---|
| Growing seasons | 1 | 12.0832 * |
| Variety | 3 | 42.9841 *** |
| Organic fertilization | 3 | 39.4836 *** |
| Selenium application | 1 | 3599.0316 *** |
| GS × V | 3 | 22.5878 *** |
| Gs × OF | 3 | 10.0963 *** |
| V × OF | 9 | 6.8474 *** |
| GS × Se | 1 | 19.5236 *** |
| V × Se | 3 | 18.7205 *** |
| OF × Se | 3 | 18.8835 *** |
| GS × V × OF | 9 | 4.5424 *** |
| GS × V × Se | 3 | 10.2129 *** |
| GS × OF × Se | 3 | 1.5649 n.s. |
| V × OF × Se | 9 | 0.7306 n.s. |
| GS × V × OF × Se | 9 | 1.0679 n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carucci, F.; Moreno-Martín, G.; Madrid-Albarrán, Y.; Gatta, G.; De Vita, P.; Giuliani, M.M. Selenium Agronomic Biofortification of Durum Wheat Fertilized with Organic Products: Se Content and Speciation in Grain. Agronomy 2022, 12, 2492. https://doi.org/10.3390/agronomy12102492
Carucci F, Moreno-Martín G, Madrid-Albarrán Y, Gatta G, De Vita P, Giuliani MM. Selenium Agronomic Biofortification of Durum Wheat Fertilized with Organic Products: Se Content and Speciation in Grain. Agronomy. 2022; 12(10):2492. https://doi.org/10.3390/agronomy12102492
Chicago/Turabian StyleCarucci, Federica, Gustavo Moreno-Martín, Yolanda Madrid-Albarrán, Giuseppe Gatta, Pasquale De Vita, and Marcella Michela Giuliani. 2022. "Selenium Agronomic Biofortification of Durum Wheat Fertilized with Organic Products: Se Content and Speciation in Grain" Agronomy 12, no. 10: 2492. https://doi.org/10.3390/agronomy12102492
APA StyleCarucci, F., Moreno-Martín, G., Madrid-Albarrán, Y., Gatta, G., De Vita, P., & Giuliani, M. M. (2022). Selenium Agronomic Biofortification of Durum Wheat Fertilized with Organic Products: Se Content and Speciation in Grain. Agronomy, 12(10), 2492. https://doi.org/10.3390/agronomy12102492








