Abstract
Cadmium (Cd) is highly toxic and not easily degradable. It damages plant growth and results in large-scale economic losses. The present study explored the feasibility of using melatonin to alleviate Cd toxicity, and to reduce Cd accumulation in tea seedlings cultivated in Cd-contaminated soil. Exogenous melatonin, especially at 150 μM, promoted tea seedling growth under Cd stress, and increased the photosynthetic pigment by 16% and soluble protein content by 5%. Furthermore, melatonin effectively increased the activities of superoxide dismutase (SOD), and peroxidase (POD) by 21 and 31%, respectively, contributed to a decrease of the malondialdehyde (MDA) by 2% and the Cd content in leaves by 52%. Furthermore, soil enzyme activities were enhanced, including acid phosphatase (ACP), urease (UE), soil sucrase (SC), and soil catalase (CAT), by 11, 70, 1, and 18%, respectively, along with a pH reduction and available Cd content increase, after melatonin application. Taken together, our results provide evidence that melatonin lessens the adverse Cd effects on tea seedlings’ physiology, mainly through enhancing the antioxidant capacity of the plants and soils to scavenge reactive oxygen species (ROS) upon Cd exposure. Therefore, melatonin may be used as a modulator to alleviate Cd-induced toxicity in tea seedlings, thereby resulting in healthier tea plant growth.
1. Introduction
The development of industrial and agricultural production, as well as accelerated urbanization [1,2], has resulted in serious heavy metal soil pollution [3], leading to tremendous economic loss [4]. Specifically, cadmium (Cd) is one of the most common heavy metal pollutants, and is extremely toxic and nonbiodegradable. Although Cd is not an essential element for plant growth and development, it is easily absorbed and accumulated by plant roots, and accumulates within the human body via the food chain, thereby poisoning human organs as well as reproductive and immune systems, and thus threatens human health [5,6]. Cd accumulation can induce peroxidation and hinders plant growth processes [7]. Some reports have shown that mild Cd stress leads to some visible symptoms in plants, e.g., dry and yellow leaves, shortened rhizomes, and a reduced number of lateral roots. High Cd dosages tend to disrupt metabolic processes, inhibit antioxidant enzyme activities, and promote reactive oxygen species accumulation. Furthermore, cell membrane permeability decreases, resulting in cell damage [8,9]. Ultimately, plant growth is tremendously inhibited, leading to low productivity [10,11]. Thus, exploring how to alleviate Cd stress in plants, reduce the Cd content in edible plant organs, and achieve effective Cd control in the food chain is vital for crop growth and human health.
Tea is well received by many consumers around the world, mainly because of its pleasant fragrance, unique flavor, and abundance of nutrients [12]. Furthermore, as a globally important industrial crop, tea has increasingly attracted attention regarding its safety for human consumption. Heavy metals have recently been found to pose a serious tea contamination risk owing to their high uptake by tea plants [13,14]. Consequently, heavy metal stress severely threatens its growth and yield. Cd pollution has therefore developed into an important sustainability issue. In some mining areas of the Hunan Province of China, Cd soil content can be up to 9.5 mg kg−1 [15], which greatly exceeds the risk screening value of the tea garden (0.2 mg kg−1). Previous studies on tea plants showed that Cd has a hazardous impact on tea plant growth and physiology [16,17,18,19]. Thus, enhancing tea plant resistance against Cd stress, and reducing its accumulation, are of great significance to tea yield, safety, and quality.
To our knowledge, melatonin (N-acetyl-5-methoxytryptamine) has been identified as a multi-beneficial hormone. It plays various roles in humans and animals. Moreover, melatonin is also involved in many aspects related to plant growth and development, particularly in plant resistance against biotic and abiotic stress. Melatonin is capable of strengthening plant tolerance towards drought [20], high temperatures [21], saline-alkali environments [22], nanoplastics [23], and other adverse conditions. Furthermore, melatonin exerts an ameliorative effect on heavy metal toxicity [24,25,26,27]. For example, melatonin’s effects on Cd toxicity has been extensively studied in Arabidopsis thaliana [28] and apple trees [29] via phytochelatin biosynthetic regulation, vacuolar isolation, and antioxidant capacity. These studies demonstrated that melatonin plays a regulatory role in plants in response to Cd stress. Therefore, can melatonin also be applied to tea seedlings?
Tea is one of the three most popular drinks in the world, with a huge market demand. Up to now, the potential threat of Cd has been mainly prevented by reducing chemical fertilizers, pesticides and other measures, but this will undoubtedly lead to a reduction in tea production and quality. Previous studies have confirmed that melatonin has the ability to enhance Cd tolerance in plants. However, little relevant information is currently available on melatonin’s alleviating effects in tea plants exposed to Cd. Therefore, the objective of this study was to investigate the effects of melatonin application on tea seedlings subjected to Cd, and to analyze the detoxifying mechanisms induced by melatonin. Here, we determined the changes in biomass and physiology, including photosynthetic pigments, membrane lipid peroxidation degree, antioxidant enzyme activity, etc. Specifically, we analyzed soil enzyme activity (ACP, UE, SC, and CAT) to provide additional evidence for melatonin to alleviate Cd-induced stress. This study was intended to provide cultivation strategies for tea plants, and other horticultural crops, growing in Cd-polluted soils, and to fill the current research gap.
2. Materials and Methods
2.1. Plant Materials and Soil Samples
One-year-old tea seedlings (Camellia sinensis, cultivar ‘Ziyan’) were obtained from the Yizhichun Tea Company, Leshan, Sichuan Province (E 103°53′16.3″, N 28°59′24.5″). A loam soil was used in the experiment, which was collected from the local environment. The soil properties were as follows: pH 5.62, organic matter 31.73 g kg−1, total nitrogen 1.05 g kg−1, total phosphorus 0.37 g kg−1, total potassium 25.71 g kg−1, available nitrogen 56.13 mg kg−1, available phosphorus 17.15 mg kg−1, available potassium 56.65 mg kg−1, and total Cd 0.025 mg kg−1.
2.2. Experimental Design
The experiment was carried out in a greenhouse at the Sichuan Agricultural University. Nine kilograms of soil was first dried, crushed, and screened, and then treated with 10 mg kg−1 Cd, thereafter it was used to fill plastic pots. All treated pots were kept in the shade at room temperature for two weeks. During this period, the soil was maintained at 80% field capacity. Our experiment utilized a completely randomized block design. Six tea plants were transplanted into each pot, thereby totaling 90 seedlings.
After one week, the leaves of the tea seedlings were sprayed every seven days either with distilled water (control; CK) or different melatonin concentrations (50, 100, 150, or 200 μM) for a total of four times. For each treatment, three pots with 18 plants were used. After 60 days of melatonin treatment, the roots, stems, and leaves of each plant, as well as their respective soils, were collected for further analysis. Three replicates were used to determine biomass, physiological index, and Cd concentration.
2.3. Determination of Biomass
Tea plants were harvested and divided into several parts, which consisted of main roots, lateral roots, stems, and leaves. These parts were all first washed with running water, followed by three rinses of deionized water. Each part’s fresh weight was then recorded after all surface water evaporated from the respective tissues.
2.4. Determination of Photosynthetic Parameters
The photosynthetic pigments of seedlings were measured after 60 days of treatment. Approximately 0.2 g of the third fresh leaf from each group was utilized and homogenized with 3 mL of 95% ethanol. Then, 10 mL of the 95% ethanol mixture was added and incubated for 5 min to whiten the tissues. The extracts were then filtered into a volumetric flask and diluted to a volume of 25 mL. An ultraviolet spectrophotometer (UV T5, Shanghai, China) was used to measure sample absorbances at 470, 649, and 665 nm, respectively, and calculated according to the methods of Maxwell et al. [30].
2.5. Activity Determination of Antioxidant Enzymes (SOD, POD, and CAT)
For enzyme analyses, a 0.1 g quantity of each fresh sample (i.e., the fourth leaf) was ground into a 10% homogenate in a pre-chilled mortar and pestle filled with a normal saline solution (1:9 mass volume ratio). The homogenates were centrifuged at 2500 rpm for 10 min at 4 °C, and the supernatants were tested for enzymatic activities following the manufacturer’s instructions for each commercial assay kit, which included superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), and peroxidase (POD, EC 1.11.1.7), all purchased from the Jiancheng Bio-engineering Institute (Jangsu, China). Specifically, SOD activity was determined by monitoring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) at 550 nm [31]. Furthermore, one unit of SOD activity represented the amount of enzyme corresponding to a fifty percent reduction of NBT. CAT activity estimation involved monitoring the rate of disappearance of H2O2 at 405 nm [32]. One unit of CAT activity was defined as the amount resulting in a 1 μmol H2O2 per min decrease. Finally, POD activity was assessed at 420 nm, and one unit was defined as the amount that catalyzed 1 μg H2O2 per min at 37 °C [33].
2.6. Determination of Soluble Protein Content
The Coomassie brilliant blue method was adapted and used for the determination of soluble protein content, as described by Zoufan et al. [34]. We weighed out 0.2 g of fresh sample (the fourth leaf) for each treatment, which was ground into a homogenate and extracted in 10 mL of distilled water. Homogenates were centrifuged at 4000 rpm min−1 for 10 min at 4 °C. Afterwards, 0.1 mL of supernatant was thoroughly mixed with 5 mL of 100 μg mL−1 Coomassie brilliant blue G-250 solution in each tube and allowed to react. The solution was left to react for 2 min, after which its optical density was measured at 595 nm.
2.7. Determination of Malondialdehyde
MDA content was evaluated as reported earlier [35], with minor modifications. Specifically, 0.1 g of fresh sample (the fourth leaf) was ground into a homogenate after the addition of 5 mL 0.1% (w/v) TCA (trichloroacetic acid) solution. Hereafter, the homogenate was centrifuged at 13,000 rpm for 10 min. A total of 1 mL of supernatant was vacuumed into 2 mL 0.6% TBA made in 10% TCA. The mixture was incubated in a water bath for 15 min, and then quickly cooled to terminate the reaction. Another centrifugation step was performed for 5 min at 13,000 rpm to obtain a supernatant, of which the absorbance was measured at 450, 532, and 600 nm, respectively.
2.8. Determination of Cd Content
To determine Cd content, tea seedling roots, stems, and leaves were sampled and dried at 70 °C until their weights remained constant according to GB 5009.15-2014. The dried samples of 0.3 g in weight were powdered and transferred into a polytetrafluoroethylene digestion tank. Thereafter a 10 mL mixture (a ratio of 4:1 HNO3:HClO4) was added to digest the samples overnight until they were clear. After filtering, the digested solution was diluted to 25 mL, and Cd content determined using an atomic absorption spectrometer (ICP 6300, MA, USA).
2.9. Determination of Soil pH and Available Cd Content
The soil of each treatment was collected, air dried, and screened. The soil pH values were assayed according to NY/T 1377-2007. Specifically, powdered soil, weight 10.0 g, was added to 25 mL of deionized water and stirred. After 30 min, an acidimeter (PHSJ-4F, Shanghai, China) was used to determine pH values.
The available Cd content was analyzed following the GB/T 23739-2009 approach. Specifically, 10 g of the soil samples were mixed with DTPH and digested at room temperature for 2 h. Hereafter, the mixtures were filtered to obtain extracts, which were assessed at 288.8 nm using an atomic absorption spectrophotometer (ICP 6300, MA, USA).
2.10. Determination of Soil Enzyme Activities (S-UE, S-SC, S-CAT)
Soil enzyme activities, namely acid phosphatase (ACP, EC 3.1.3.2), urease (UE, EC 3.5.1.5), sucrase (SC, EC 3.2.1.48), and catalase (CAT, EC 1.11.1.6), were measured using a Shimadzu spectrophotometer (UV-2500, Tokyo, Japan) following the instructions of the respective commercial assay kits purchased from the Solarbio Science & Technology Co., Ltd. (Beijing, China). Samples were weighed: 0.1 g air-dried soil for CAT, ACP and SC activities assay; 0.25 g for UE. ACP catalyzed the hydrolysis of disodium phenylphosphate to produce phenol and disodium hydrogen phosphate. At 37 °C, one enzyme activity unit was defined as 1 nmol phenol per gram of soil released per day. Soil UE activity was measured by detecting the concentration of the product of hydrolysis (NH3-N) using urea as the substrate. Furthermore, one unit of UE activity was defined as the amount of NH3-N produced by one gram of soil per day. For SC enzyme analysis, sucrose was catalyzed to produce a reducing sugar, which was further combined with 3,5-dinitrosalicylic acid to form a red compound with an absorption peak at 540 nm. The content of the reducing sugar produced by one gram of soil catalyzed sucrose per day was considered as one unit. Finally, CAT activity was determined by monitoring its ability to reduce H2O2 at 240 nm, and one unit was defined as the amount of H2O2 decrease from one gram of soil per day.
2.11. Statistical Analyses
Analysis of variance (ANOVA) was performed to determine the least significant difference (LSD) between the different treatments (p < 0.05) using the Statistica 25.0 software (SPSS, Chicago, IL, USA). Duncan’s multiple range test was used to compare the data. Mean expression and standard deviation (SD) values were calculated based on three biological replicates. Graphs were generated using OriginPro 2021 software (Origin Lab, Berkeley, CA, USA).
3. Results
3.1. Tea Seedling Biomass
Changes were observed in the tea seedlings’ roots, stems, and leaf biomasses under different treatments (Figure 1). Melatonin effectively alleviated Cd stress on tea seedlings’ growth, and also promoted their growth. With an increased melatonin concentration, the biomass of the main and lateral roots, as well as stem, leaf, and entire seedling biomass, increased and reached a maximum at 150 μM melatonin. Under this treatment, the main root, lateral root, stem, leaf, and entire biomass increased by 16, 44, 4, 18, and 10%, respectively. Melatonin application at a concentration of 200 μM inhibited tea seedling taproot and leaf growth, but this was not significant.
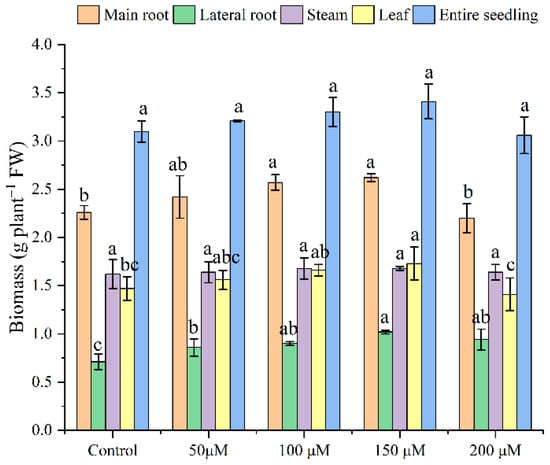
Figure 1.
Effects of exogenous melatonin on tea seedling biomass under Cd stress. The data represent means ± standard deviations for three replicates. Different letters indicate groups that are significantly different at p < 0.05.
3.2. Photosynthetic Pigment Content in Tea Seedlings
Only the 150 μM melatonin treatment significantly increased chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid content in Cd-stressed tea seedling leaves (Figure 2). When treated with 150 μM melatonin, the chlorophyll a, chlorophyll b, carotenoid, and total chlorophyll content increased by 10, 25, 5, and 16%, respectively (p < 0.05). However, the chlorophyll a, chlorophyll b, carotenoid, and total chlorophyll content was the lowest at an exogenous melatonin concentration of 200 μM, which decreased by 11, 18, 12, and 12%, respectively. Interestingly, only 200 μM melatonin increased chlorophyll a/b, while the other concentrations contrasted with this.
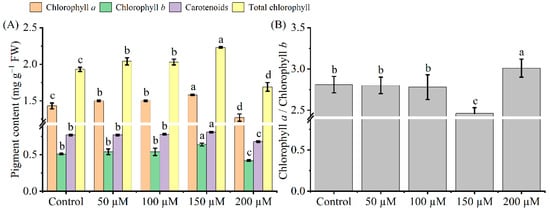
Figure 2.
Effects of exogenous melatonin on photosynthetic pigments (A), as well as the ratio of chlorophyll a and chlorophyll b (B) in Cd-stressed tea seedlings. The data represent means ± standard deviations for three replicates. Different letters indicate groups that are significantly different at p < 0.05.
3.3. Antioxidant Enzyme Activity, and Soluble Protein and MDA Content of Tea Seedlings
We evaluated the influence of melatonin by investigating changes in the antioxidant systems, soluble protein, and malondialdehyde (MDA) in melatonin-treated Cd-stressed tea seedling leaves. The activities of SOD (EC 1.15.1.1) and POD (EC 1.11.1.7) significantly increased due to different melatonin concentrations, both of which reached a maximum level at 200 μM melatonin, and increased by 22 and 61%, respectively (p < 0.05, Figure 3). Furthermore, the highest CAT (EC 1.11.1.6) value was recorded in the 50 μM melatonin group, which increased significantly by 31% (p < 0.05). Except for the 200 μM melatonin concentration, the soluble protein content of the other treatments increased (Figure 3A). Moreover, the highest soluble protein level occurred at 100 μM, and further increased by 16% (Figure 3B). Additionally, melatonin decreased the tea seedling leaf MDA content, and the lowest value was observed at 100 μM melatonin, which decreased by 12% (Figure 3C). These results imply that melatonin is effective in adjusting antioxidant enzyme activities and soluble protein content to alleviate Cd-induced toxicity.
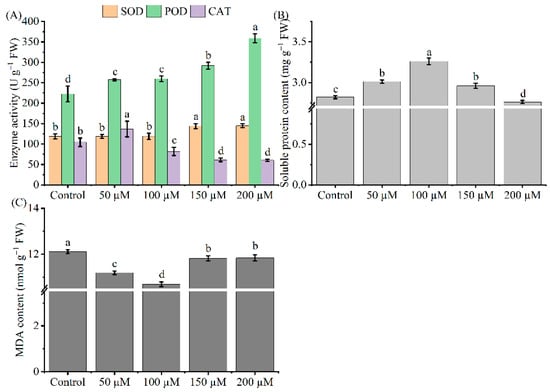
Figure 3.
Effects of exogenous melatonin application on antioxidant enzyme activity (A), soluble protein (B), and MDA (C) content of Cd-stressed tea seedlings. The data represent means ± standard deviations for three replicates. Different letters indicate groups that are significantly different at p < 0.05.
3.4. Cd Content in Tea Seedings
The tea seedling Cd accumulation trend can be described as follows: lateral roots > main roots > stems > leaves (Figure 4). Under Cd stress, the plants without melatonin application exhibited a maximum Cd amount in all parts of the tea seedlings. The Cd content in the main roots was 4.56 mg kg−1 DW, in lateral roots 92.88 mg kg−1 DW, in stems 2.22 mg kg−1 DW, and in leaves 1.50 mg kg−1 DW. However, the treatment with different melatonin levels resulted in a maximum Cd concentration decline of up to 39, 36, 32, and 52%, respectively, in the main roots, lateral roots, stems, and leaves. Therefore, based on the Cd content of all the treatments, the 150 μM melatonin group considerably reduced the tea seedlings’ Cd absorption.
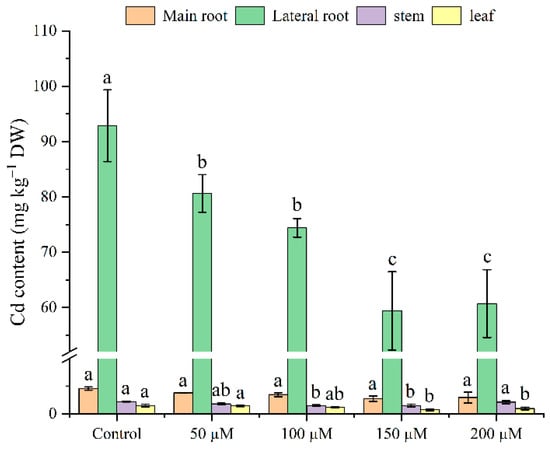
Figure 4.
Effects of exogenous melatonin application on the Cd contents of Cd-stressed tea seedlings. The data represent means ± standard deviations for three replicates. Different letters indicate groups that are significantly different at p < 0.05.
3.5. Soil Enzyme Activities
The effects of tea seedlings treated with different melatonin concentrations on soil enzyme activities were examined using soil acid phosphatase (S-ACP, EC 3.1.3.2), soil urease (S-UE, EC 3.5.1.5), soil sucrase (S-SC, EC 3.2.1.48), and soil catalase (S-CAT, EC 1.11.1.6). In this study, the 100 μM melatonin treatment prominently increased the activities of S-ACP and S-UE by 30 and 122%, respectively (Figure 5A,B). However, tea seedlings treated with melatonin had no significant effect on enhancing S-SC activity under Cd stress (Figure 5C). Moreover, the S-CAT activity significantly increased by 5, 18, and 37%, respectively, at the three higher melatonin levels (100, 150, and 200 μM; p < 0.05), but decreased at a lower melatonin concentration (Figure 5D). Overall, these results demonstrate that tea seedlings treated with melatonin can upregulate soil enzyme effectiveness in Cd-polluted soils to lessen Cd-induced toxicity.
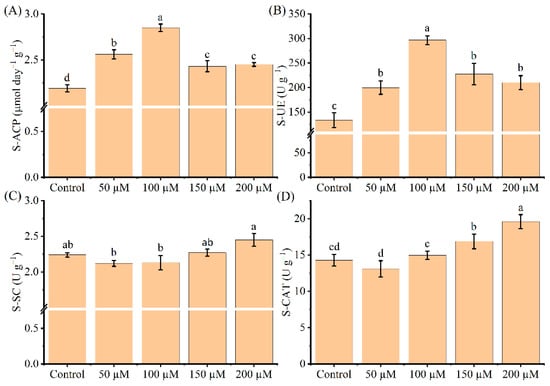
Figure 5.
Effects of exogenous melatonin application on soil enzyme activities of Cd-stressed tea seedlings, including S-ACP (A), S-UE (B), S-SC (C), and S-CAT(D). The data represent means ± standard deviations for three replicates. Different letters indicate groups that are significantly different at p < 0.05.
3.6. Effects of Exogenous Melatonin on Soil pH and Available Cd Content
To investigate whether melatonin application changes soil properties in Cd contaminated soils, we analyzed soil pH and available Cd content for all treatments. Soil pH continuously declined as the melatonin concentration increased (Figure 6). At 150 and 200 μM melatonin, the index was significantly lower compared to the control (p < 0.05), which predicted a severe soil acidification, while still being suitable for tea growth. In contrast, the amount of available soil Cd notably increased with increasing melatonin concentration. The available soil Cd content was higher (12 and 16%) when supplemented with melatonin at 150 and 200 μM, respectively (p < 0.05). These results provide evidence that exogenous melatonin reduces tea seedling Cd uptake, thereby leaving much of the available Cd in the soil.
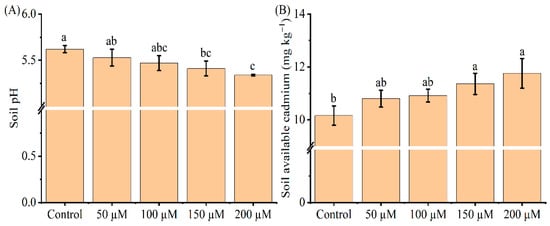
Figure 6.
Effect of exogenous melatonin application on the soil pH (A) and available Cd (B) content. The data represent means ± standard deviations for three replicates. Different letters indicate groups that are significantly different at p < 0.05.
4. Discussion
Cd is a non-essential plant element with a deleterious impact on plant growth and productivity, which occurs mainly through the irreversible inhibition of proton pumps; these elements are crucial for maintaining cell division and growth [36,37]. Previous studies demonstrated that exogenous melatonin relieved Cd stress and promoted the growth and biomass formation of wheat and tomato plants [38,39]. In this study, we observed that the biomass of each Cd-stressed tea seedling part increased after melatonin addition. The fresh weights of the main roots, lateral roots, and leaves significantly increased by 16, 44, and 18%, respectively, in the presence of 150 μM melatonin, but with a slight reduction seen with the 200 μM melatonin treatment. This agrees with previous studies which found that Cd-induced growth damage could be alleviated by exogenous melatonin application [28,29,40]. However, 150 μM melatonin performed well in tea seedlings exposed to Cd, which was different from that of most plants (less than 100 μM). We speculated the difference might be caused by different plant species, as well as the exposure duration or even other experimental conditions. Collectively, these findings imply that plant growth inhibition caused by Cd can be mitigated by exogenous melatonin at an appropriate concentration, but a dose-dependent manner exists. It was hypothesized that a high melatonin concentration might inhibit tea plant IAA synthesis, causing the depolymerization of tubulin through calmodulin, destroying the spindle structure of cells, thus affecting tea seedling growth [41].
Photosynthesis provides material and energy for plant growth and is sensitive to heavy metal stress. Sami et al. reported that Cd significantly decreased the rate of photosynthesis and hindered plant growth [42]. Simultaneously, Gil et al. reported that Cd toxicity altered photosynthetic efficiency by directly destroying chloroplast ultrastructures and inhibiting enzyme activities related to photosynthesis [43]. Numerous studies have confirmed that melatonin is one of the most potent endogenous free radical scavengers, and plays a crucial role in reducing the degradation of chlorophyll in adverse conditions [44,45]. The findings of Agami and Mohamed et al. support our results, namely that melatonin treatment enhanced Cd-stressed tea seedlings’ photosynthetic pigment content, which was consistent with biomass regulation [46]. The study conducted by Liang et al. showed that chlorophyll content was associated with ROS levels in the leaves of stressed plants [47]. Moreover, ROS could react with polyunsaturated fatty acids, thus leading to lipid peroxidation and triggering the generation of various lipid peroxidation products, such as MDA [48,49]. Our results showed that melatonin supplementation significantly decreased MDA accumulation in Cd-stressed tea leaves (Figure 3C). Furthermore, the photosynthetic pigment content in these treatments was higher compared to the control (Figure 2A). This indicates that melatonin relieved the plants from Cd-induced oxidative stress, which was consistent with the results from Sami et al. on Brassica napus L. [42]. Antioxidant enzymes, including SOD, POD, CAT, and antioxidants, are crucial in a plant’s defense system, and are responsible for scavenging active oxygen free radicals, thereby maintaining a delicate balance between their production and elimination. Our results demonstrated that melatonin caused changes in antioxidant enzyme activities in Cd-stressed tea seedlings (Figure 3A), which agreed well with previous studies on wheat and apple plants [50,51]. Interestingly, SOD and POD enzyme activity and MDA content of 150 and 200 μM melatonin treatment were higher than those of 50 and 100 μM groups, while CAT activity was lower. Combined with other physiological indicators, we suspected that melatonin probably enhanced the Cd tolerance of tea seedlings, so that the reaction time was later than other groups. Comparatively speaking, two treatment groups, 50 and 100 μM, were sensitive to cadmium, which caused a timely response to this threat, thus leading to a reduction in MDA levels. In addition, the results of the study only reflected the antioxidant level of Cd treated plants after 60 days, and could not represent the whole response process. Soluble protein, as an important osmoregulation substance, can improve the water-holding capacity of cells, protect the normal functions of various enzymes, and cell membrane structures. The 150 μM melatonin treatment promoted an accumulation of soluble protein content, which reflected a good growth state of tea seedlings exposed to Cd stress [16]. Overall, we hypothesized that melatonin mediated the Cd-induced damage by enhancing antioxidant enzyme activities and antioxidant levels, thereby reducing MDA levels and increasing photosynthetic pigments and growth.
Many studies have confirmed that Cd competes with Ca and Zn for the enzyme center, and binds to the residues on the constituent amino acids of enzymes, such as the hydrogen sulfur bond of cysteine, to inhibit enzyme activity, thus hindering plant growth and development [52]. Susa et al. explored the detoxification mechanism of melatonin on Cd, and found that alleviation was attributed to the chelation of melatonin with metal ions [53]. Furthermore, melatonin, as an antioxidant, provided protection for plants to avoid injury [54]. Generally, Cd accumulation in aboveground plant parts is lower compared to underground parts, except in some Cd hyperaccumulators [55]. Our results showed that Cd accumulation was distributed in tea plant lateral roots, main roots, stems, and leaves. Similar to most plants, a large amount of Cd was trapped within the lateral roots; this is because the xylem restricts Cd transport [14]. Furthermore, all tea seedling parts experienced a Cd content decrease after melatonin application, especially at a concentration of 150 μM (Figure 4). This agreed with previous studies showing that melatonin markedly alleviated Cd accumulation in mallow (Malva parviflora) and cucumber (Cucumis sativus) [56,57]. Based on this, we can conclude that melatonin plays a prominent role in reducing Cd intake, but elucidating a definite mechanism requires further study.
Soil enzymes are indicators for evaluating the degree of heavy metal pollution and potential ecological risk, and not only participate in the soil nutrient cycle, but also provide metabolites for maintaining soil health [58,59]. Previous studies focused on the effect of heavy metals on soil enzyme activities, and showed that heavy metal pollution inhibited such soil enzyme activities, as well as hindering the transformation of soil available nutrients [60,61,62]. In our study, application of the melatonin to tea seedlings grown in Cd-contaminated soils showed that soil enzyme activities were prominently enhanced compared to treatments without melatonin, especially S-ACP, S-UE, and S-CAT, thereby directly reflecting an improvement in soil conditions (Figure 5A,B,D). Combined with the other results in this paper, tea seedling biomass increased in such improved soils (Figure 1). Therefore, we hypothesize that tea seedlings treated with melatonin enhances enzyme activities in Cd-polluted soil, and accelerates the transformation of soil nutrients used for growth [59]. Additionally, soil enzyme activity is closely related to soil pH [63]. In this context, soil pH decreased as melatonin concentration increased. We assumed that melatonin promoted the secretion of acidic substances into the tea seedling rhizospheres, thus leading to soil acidification and changes in soil enzyme activities [64]. Wyszkowska et al. proposed that soil acidification exacerbated heavy metal pollution, thereby increasing the content of available heavy metals, which were then easily absorbed by plants [65]. In contrast, the results from Yang et al. showed that increased pH led to decreased heavy metal bioavailability in soil [66]. In our study, the available Cd content in the tea seedlings’ soil gradually increased with increasing melatonin concentration, which means that the Cd content absorbed and utilized by plants also increased, thus increasing the risk of Cd pollution in the tea seedlings. Thus, considering the tea seedlings’ Cd content, melatonin reduced tea seedling Cd accumulation, thereby retaining a large amount of Cd within the soil; however, the specific mechanism of melatonin alleviation requires further study.
In the past, the absorption of Cd by plants was mainly controlled through physical and chemical measures, consisting of soil amendments, surfactants, and metal antagonists, along with fertilizer and pesticide reduction or other technologies, which was not only at huge expense and difficulty, but also affected the yield and quality of plants, especially some horticultural crops. Subsequently, biological agents came into being. Melatonin showed potential to alleviate Cd-induced stress in tea seedlings, as well as other reported plants, contributing to an effective theoretical basis for crop cultivation and exhibiting the feasibility of the application. In this paper, the effect of melatonin on alleviating cadmium stress was studied through a pot experiment. However, the environmental conditions, e.g., climate, soil microbial communities, and Cd addition, etc., in the field were more complex, thus, leading to a limitation of our results. Therefore, it is necessary to conduct tests multiple times and in multiple areas, so as to achieve similar results in the field. Moreover, the characteristics of plants are different, and even the concentrations of melatonin that may have different detoxification effects on heavy metals for different tea varieties. Therefore, it is of great significance to determine an optimal concentration, or even a concentration range, so that it promotes healthier growth of different varieties, which is conducive to developing melatonin into a product for crop cultivation and management.
5. Conclusions
Overall, this study showed that different melatonin concentrations attenuated Cd toxicity in tea seedlings. The exogenous application of melatonin strengthened the antioxidant defense system and enhanced soluble protein content, which lessened MDA accumulation and increased tea plant biomass under Cd stress, as well as promoting the accumulation of photosynthetic pigments in leaves. Moreover, the spraying of melatonin regulated soil enzyme activities and inhibited Cd absorption by tea seedling roots, thereby reducing the Cd content in the tea seedling organs. Some ambiguity and confusion still remain regarding melatonin’s effect on the alleviation in tea seedlings growing in Cd-contaminated soil, especially the link with soil enzymes; therefore, this becomes the focus of future work. In conclusion, a treatment of 150 μM melatonin concentration is the most effective in reducing tea seedling Cd toxicity. This inspires us to increase the endogenous melatonin content of transgenic plants by genetic modification, so as to improve cadmium resistance. Therefore, our study provides a new strategy for improving the performance of tea seedlings or horticultural crops exposed to Cd by applying melatonin as a modulator to attenuate Cd-induced toxicity.
Author Contributions
Conceptualization, X.T., J.H. and Q.T.; formal analysis, X.T. and J.H.; investigation, X.T. and J.H.; resources, Q.T.; writing—original draft preparation, X.T. and J.H.; writing—review and editing, X.T. and L.L.; supervision, X.T., J.H. and Q.T.; project administration, Q.T.; funding acquisition, Q.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National key Research and Development Plan (grant numbers 2019YFC0840502) and the Department of Science and Technology of Sichuan Province (grant numbers 2021YFN0004).
Data Availability Statement
Not applicable.
Acknowledgments
We deeply appreciate the essential experimental platform from the Tea Refining and Innovation Key Laboratory of Sichuan Province, Sichuan Agricultural University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duruibe, J.O.; Ogwuegbu, M.; Egwurugwu, J. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Frišták, V.; Pipíška, M.; Lesný, J.; Soja, G.; Friesl-Hanl, W.; Packova, A. Utilization of biochar sorbents for Cd2+, Zn2+, and Cu2+ ions separation from aqueous solutions: Comparative study. Environ. Monit. Assess. 2015, 187, 4093. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, G.M.A.; Jasan, R.; Plá, R.; Pignata, M.L. Heavy metals and trace elements in atmospheric fall-out: Their relationship with topsoil and wheat element composition. J. Hazard. Mater. 2012, 213, 447–456. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, M.; Peterson, K.E.; Cantoral, A.; Song, P.X.K.; Jones, A.; Solano-Gonzales, M.; Meeker, J.D.; Basu, N.; Tellez-Rojo, M.M. Dietary predictors of urinary cadmium among pregnant women and children. Sci. Total Environ. 2017, 575, 1255–1262. [Google Scholar] [CrossRef]
- He, S.Y.; Yang, X.E.; He, Z.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Skórzyńska-Polit, E.; Drzkiewicz, M.; Krupa, Z. Lipid peroxidation and antioxidative response in Arabidopsis thaliana exposed to cadmium and copper. Acta Physiol. Plant. 2010, 32, 169–175. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.H.; Dai, H.X.; Cao, F.B.; Zhang, G.P.; Wu, F.B. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235, 343–351. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.R.; Anjum, N.A.; Khan, N.A. Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma 2015, 252, 399–413. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev. Environ. Contam. Toxicol. 2017, 241, 73–137. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Han, W.Y.; Shi, Y.Z.; Ma, L.F.; Ruan, J.Y. Arsenic, cadmium, chromium, cobalt, and copper in different types of Chinese tea. Bull. Environ. Contam. Toxicol. 2005, 75, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Z.; Ruan, J.Y.; Ma, L.F.; Han, W.Y.; Wang, F. Accumulation and distribution of arsenic and cadmium by tea plants. J. Zhejiang Univ.—Sci. B. 2008, 9, 265–270. [Google Scholar] [CrossRef]
- Lei, M.; Tie, B.Q.; Song, Z.G.; Liao, B.H.; Lepo, J.E.; Huang, Y.Z. Heavy metal pollution and potential health risk assessment of white rice around mine areas in Hunan Province, China. Food Secur. 2015, 7, 45–54. [Google Scholar] [CrossRef]
- Zhang, C.Y.; He, Q.; Wang, M.H.; Gao, X.Z.; Chen, J.J.; Shen, C.W. Exogenous indole acetic acid alleviates Cd toxicity in tea (Camellia sinensis). Ecotoxicol. Environ. Saf. 2020, 190, 110090. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress—A review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Raba, N.K.; Yadav, S.K. Cadmium induced oxidative stress influence on glutathione metabolic genes of Camellia sinensis (L.) O. Kuntze. Environ. Toxicol. 2007, 22, 368–374. [Google Scholar] [CrossRef]
- Cao, H.; Qiao, L.; Zhang, H. Exposure and risk assessment for aluminium and heavy metals in Puerh tea. Sci. Total Environ. 2010, 408, 2777–2784. [Google Scholar] [CrossRef]
- Liang, D.; Ni, Z.Y.; Xia, H.; Xie, Y.; Lv, X.L.; Wang, J.; Lin, L.L.; Deng, Q.X.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Qi, Z.Y.; Wang, K.X.; Yan, Y.M.; Kanwar, M.; Li, D.Y.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Zhou, J. Melatonin alleviates high temperature-induced pollen abortion in Solanum lycopersicum. Molecules 2021, 23, 386. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Yang, L.; Ahmad, S.; Mosaad, I.S.M.; Al-Ghamdi, A.A.; Abbasi, A.M.; Zhou, X.B. Melatonin Application Alleviates Stress-Induced Photosynthetic Inhibition and Oxidative Damage by Regulating Antioxidant Defense System of Maize: A Meta-Analysis. Antioxidants 2022, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Guo, J.H.; Wang, T.Y.; Gong, L.; Liu, F.L.; Brestic, M.; Liu, S.Q.; Song, F.B.; Li, X.N. Melatonin reduces nanoplastic uptake, translocation, and toxicity in wheat. J. Pineal Res. 2021, 71, e12761. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.P.; Wang, P.; Chen, X.L.; Peng, Y.F.; Cai, B.; Song, J.Y.; Yin, G.T.; Jia, S.W.; Zhang, H.Y. Melatonin alleviates cadmium toxicity and abiotic stress by promoting glandular trichome development and antioxidant capacity in Nicotiana tabacum. Ecotoxicol. Environ. Saf. 2022, 236, 113437. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.N.; Tahjib-Ul-Arif, M.; Hannan, A.; Sultana, N.; Akhter, S.; Hasanuzzaman, M.; Akter, F.; Hossain, M.S.; Sayed, M.A.; Hasan, M.T.; et al. Melatonin modulates plant tolerance to heavy metal stress: Morphological responses to molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 11445. [Google Scholar] [CrossRef] [PubMed]
- Kobylinska, A.; Reiter, R.J.; Posmyk, M.M. Melatonin protects cultured tobacco cells against lead-induced cell death via inhibition of cytochrome c translocation. Front. Plant. Sci. 2017, 8, 1560. [Google Scholar] [CrossRef]
- Wang, M.; Duan, S.H.; Zhou, Z.C.; Chen, S.B.; Wang, D. Foliar spraying of melatonin confers cadmium tolerance in Nicotiana tabacum L. Ecotoxicol. Environ. Saf. 2019, 170, 68–76. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.P.; Yu, X.L.; Cui, W.T.; Pan, J.C.; Zhao, G.; Xu, S.; Wang, R.; Shen, W.B. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant. Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef]
- He, J.L.; Zhuang, X.L.; Zhou, J.T.; Sun, L.Y.; Wan, H.X.; Li, H.F.; Lyu, D.G. Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N.; Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.; Zhong, Y.; Huang, J.; Fu, X.; Wang, L.; Teng, W. Growth and physiological response of an endangered tree, Horsfieldia hainanensis merr., to simulated sulfuric and nitric acid rain in southern China. Plant. Physiol. Biochem. 2019, 144, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhu, J.; Liu, H.; Xu, X.; Liang, C. Response of antioxidative system in rice (Oryza sativa) leaves to simulated acid rain stress. Ecotoxicol. Environ. Saf. 2018, 148, 851–856. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, B.; Zhang, R.; Gao, Y.; Zhang, X.; Li, Y.; Zuo, Z. Initial simulated acid rain impacts reactive oxygen species metabolism and photosynthetic abilities in Cinnamonum camphora undergoing high temperature. Ind. Crops Prod. 2019, 135, 352–361. [Google Scholar] [CrossRef]
- Zoufan, P.; Azad, Z.; Ghahfarokhie, A.R.; Kolahi, M. Modification of oxidative stress through changes in some indicators related to phenolic metabolism in Malva parviflora L. exposed to cadmium. Ecotoxicol. Environ. Saf. 2020, 187, 109811. [Google Scholar] [CrossRef]
- Xie, P.Q.; Deng, J.W.; Zhang, H.M.; Ma, Y.H.; Cao, D.J.; Ma, R.X.; Liu, R.J.; Liu, C.; Liang, Y.G. Effects of cadmium on bioaccumulation and biochemical stress response in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2015, 122, 392–398. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J.; Huang, X.Q.; Zheng, X.Z.; Tian, Y.C.; Shi, L.; Dong, P.; Li, Z.G. Melatonin: Biosynthesis, content, and function in horticultural plants and potential application. Sci. Hortic. 2021, 288, 110392. [Google Scholar] [CrossRef]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Q.J.; Shah, F.A.; Liu, W.B.; Wang, D.D.; Huang, S.W.; Fu, S.L.; Wu, L.F. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.C.; Li, M.Q.; Yin, H.Q.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Lal, M.K.; Tiwari, R.K.; Shakoor, A. Melatonin Mitigates Cadmium Toxicity by Promoting Root Architecture and Mineral Homeostasis of Tomato Genotypes. J. Soil Sci. Plant. Nutr. 2022, 22, 1112–1128. [Google Scholar] [CrossRef]
- Shah, A.A.; Ahmed, S.; Yasin, N.A. 2-Hydroxymelatonin induced nutritional orchestration in Cucumis sativus under cadmium toxicity: Modulation of non-enzymatic antioxidants and gene expression. Int. J. Phytoremediat. 2020, 22, 497–507. [Google Scholar] [CrossRef]
- Sami, A.; Shah, F.A.; Abdullah, M.; Zhou, X.; Yan, Y.; Zhu, Z.; Zhou, K. Melatonin mitigates cadmium and aluminium toxicity through modulation of antioxidant potential in Brassica napus L. Plant Biol. 2020, 22, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.G.; Apostolova, E.L.; Hanc, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, I.D.S.; Moustakas, M. Cadmium toxicity in Salvia sclarea L.: An integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicol. Environ. Saf. 2020, 209, 111851. [Google Scholar] [CrossRef] [PubMed]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef]
- Szafrańska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis. Front. Plant. Sci. 2017, 8, 878. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef]
- Liang, C.Z.; Li, A.F.; Yu, H.; Li, W.Z.; Liang, C.Z.; Guo, S.D.; Zhang, R.; Chu, C.C. Melatonin regulates root architecture by modulating auxin response in rice. Front. Plant. Sci. 2017, 8, 134. [Google Scholar] [CrossRef]
- Celekli, A.; Kapı, M.; Bozkurt, H. Effect of cadmium on biomass, pigmentation, malondialdehyde, and proline of Scenedesmus quadricauda var. longispina. Bull. Environ. Contam. Toxicol. 2013, 91, 571–576. [Google Scholar] [CrossRef]
- Qi, W.C.; Zhang, L.; Wang, L.; Xu, H.B.; Jin, Q.S.; Jiao, Z. Pretreatment with low-dose gamma irradiation enhances tolerance to the stress of cadmium and lead in Arabidopsis thaliana seedlings. Ecotoxicol. Environ. Saf. 2015, 115, 243–249. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.W.; Gao, T.T.; Liang, B.W.; Zhao, Q.; Ma, F.W.; Li, C. Effects of exogenous melatonin on methyl viologen-mediated oxidative stress in apple leaf. Int. J. Mol. Sci. 2018, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Li, M.; Li, H.; Yin, L.Y.; Li, W. Exposure to cadmium causes declines in growth and photosynthesis in the endangered aquatic fern (Ceratopteris pteridoides). Aquat. Bot. 2014, 112, 23–32. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.; Tang, M.J.; Wang, Y.; Dong, J.H.; Ying, J.L.; Chen, Y.L.; Hu, B.; Li, C.; Liu, L.W. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants. J. Pineal Res. 2020, 69, e12659. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Janas, K.M.; Yang, H.; Dai, L.J.; Wei, Y.X.; Deng, Z.; Li, D.J. Melatonin enhances salt stress tolerance in rubber tree (Hevea brasiliensis) seedlings. Ind. Crops Prod. 2020, 145, 111990. [Google Scholar] [CrossRef]
- Xv, L.L.; Ge, J.; Tian, S.K.; Wang, H.X.; Yu, H.Y.; Zhao, J.Q.; Lu, L.L. A Cd/Zn Co-hyperaccumulator and Pb accumulator, Sedum alfredii, is of high Cu tolerance. Environ. Pollut. 2020, 263, 114401. [Google Scholar] [CrossRef] [PubMed]
- Tousi, S.; Zoufan, P.; Ghahfarrokhie, A.R. Alleviation of cadmium-induced phytotoxicity and growth improvement by exogenous melatonin pretreatment in mallow (Malva parviflora) plants. Ecotoxicol. Environ. Saf. 2020, 206, 111403. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Ahmed, S.; Ali, A.; Yasin, N.A. 2-Hydroxymelatonin mitigates cadmium stress in cucumis sativus seedlings: Modulation of antioxidant enzymes and polyamines. Chemosphere 2020, 243, 125308. [Google Scholar] [CrossRef]
- Martinez, D.; Molina, M.J.; Sanchez, J.; Moscatelli, M.C.; Marinari, S. API ZYM assay to evaluate enzyme fingerprinting and microbial functional diversity in relation to soil processes. Biol. Fertil. Soils 2016, 52, 77–89. [Google Scholar] [CrossRef]
- Liu, P.; Chen, S.; Cui, Y.N.; Tan, W.B. Insights into the inhibition effects of Cd on soil enzyme activities: From spatial microscale to macroscale. J. Hazard. Mater. 2021, 418, 12674. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, E.Y.; Hyun, S.; Kim, J.G. Metal availability in heavy metal-contaminated open burning and open detonation soil: Assessment using soil enzymes, earthworms, and chemical extractions. J. Hazard. Mater. 2009, 170, 382–388. [Google Scholar] [CrossRef]
- Humberto, A.; Wence, H.; Clare, C.; Helaina, B.; Sebastian, M.; Jorge, P.; Yasna, T.; Pablo, C. Alteration of enzyme activities and functional diversity of a soil contaminated with copper and arsenic. Ecotoxicol. Environ. Saf. 2020, 192, 110264. [Google Scholar] [CrossRef]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef] [PubMed]
- Dick, W.A. Development of a Soil Enzyme Reaction Assay. In Methods of Soil Enzymology; Soil Science Society of America: Madison, WI, USA, 2011; pp. 71–84. [Google Scholar]
- Li, Y.L.; Li, Y.H.; Cui, Y.X.; Shi, Y.J.; Shang, Y.M.; Ma, F.G.; Zhang, J.; Li, C.Y. GABA-mediated inhibition of cadmium uptake and accumulation in apples. Environ. Pollut. 2022, 30, 118867. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Wieczorek, K.; Kucharski, J. Resistance of Arylsulfatase to Contamination of Soil by Heavy Metals. Polish J. Environ. Stud. 2016, 25, 365–375. [Google Scholar] [CrossRef]
- Yang, L.Q.; Liu, B.L.; Lu, Y.Y.; Lu, F.Y.; Wu, X.Y.; You, W.H.; Huang, B. Bioavailability of cadmium to celery (Apium graveolens L.) grown in acidic and Cd-contaminated greenhouse soil as affected by the application of hydroxyapatite with different particle sizes. Chemosphere 2020, 240, 124916. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).