Progress of Research on Phytohormone Interaction in Germination of Direct-Seeded Rice under Submergence
Abstract
1. Introduction
2. Phytohormone Interactions Regulate Seed Germination under Submergence Conditions
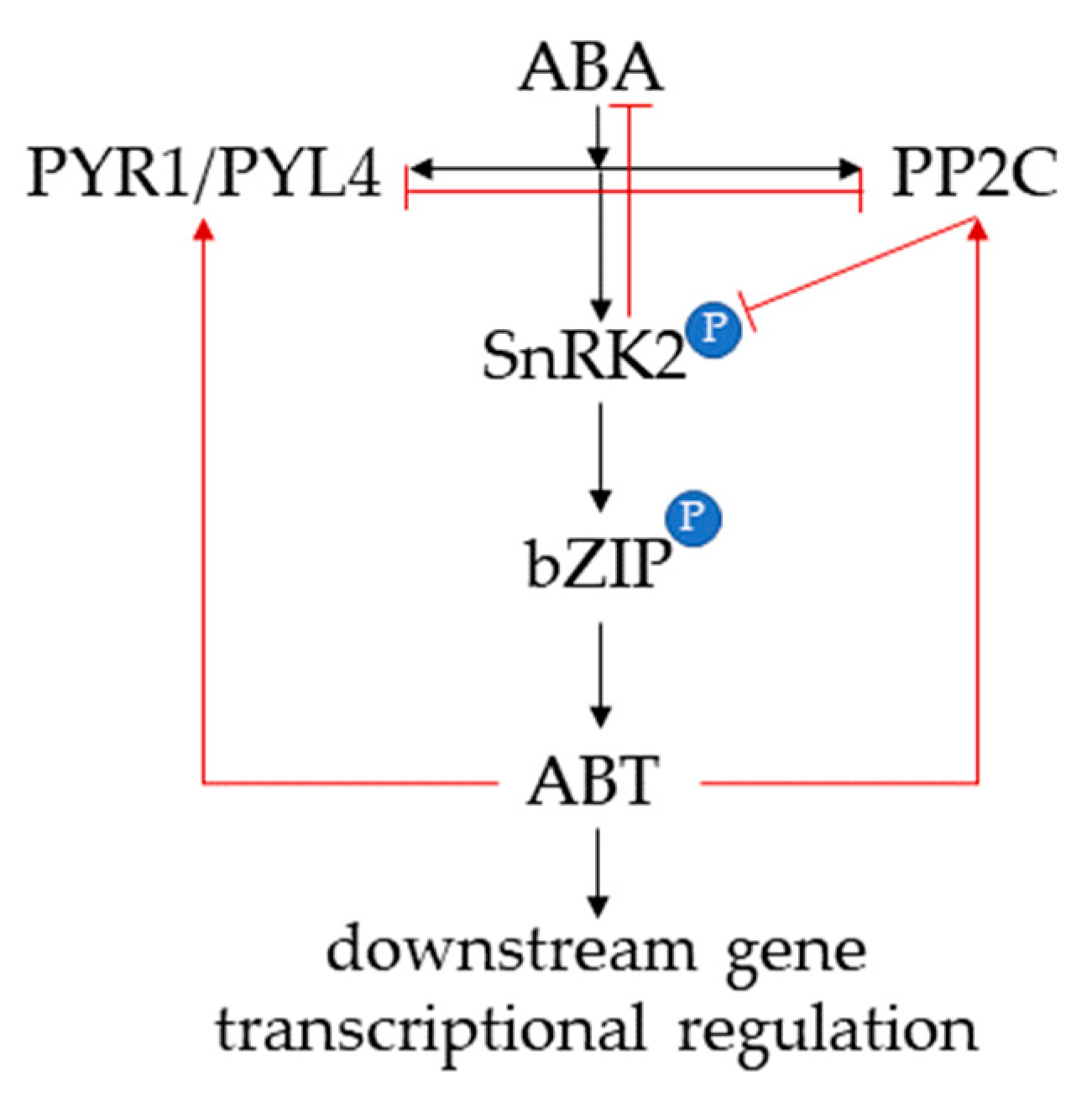
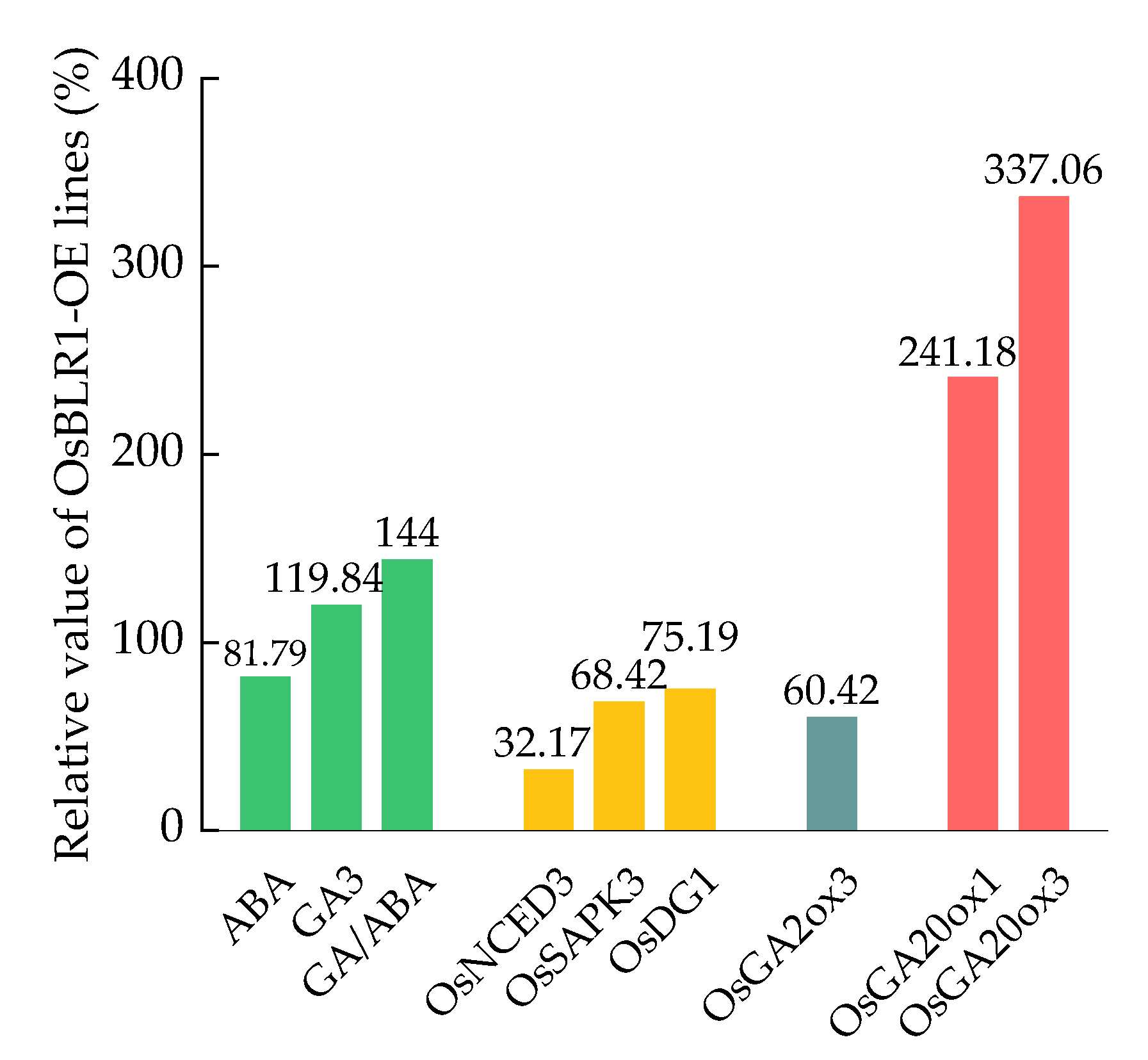
2.1. Antagonistic Actions of GA and ABA Regulate Germination and Growth of Rice Seeds
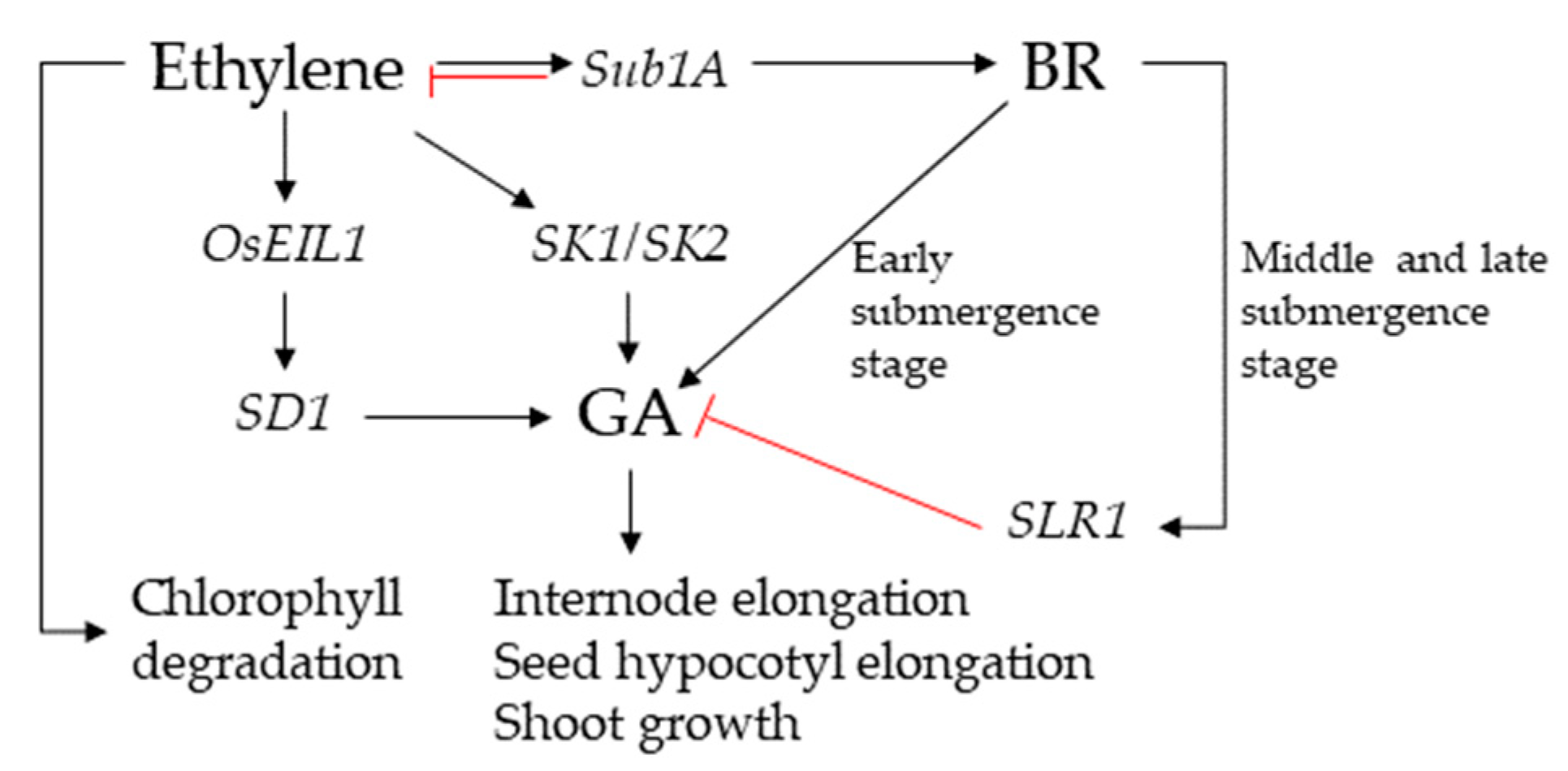
2.2. BR Affects GA/ABA during Germination of Rice Seeds
2.3. Effects of AUX on the ABA–BR Interaction Regulation Pathway
3. Phytohormone Crosstalk Influences the Morphological Development of Submerged Rice Seeds
3.1. Submergence Tolerance Strategy of Rice Seeds and Seedlings
3.2. Germination and Seedling Morphogenesis Are Regulated by Phytohormone Interaction
4. Phytohormone Interactions Regulate Storage Material Metabolism and Energy Supply
4.1. Phytohormones Regulate Nutrient Metabolism in Rice Seeds under Submergence
4.2. Phytohormone Interactions Regulate Anaerobic Respiration and Energy Metabolism
5. Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, D.; Zhang, Y.; Chen, H.; Xiang, J.; Zhang, Y. Innovation and practice of high-yield rice cultivation technology in China. Sci. Agric. Sin. 2015, 48, 3404–3414. [Google Scholar]
- National Bureau of Statistics of China. National Data. Available online: https://data.stats.gov.cn/english/easyquery.htm?cn=C01 (accessed on 8 August 2022).
- National Bureau of Statistics of China. Bulletin of the Seventh National Census (No. 5). Available online: http://www.stats.gov.cn/tjsj/tjgb/rkpcgb/qgrkpcgb/202106/t20210628_1818824.html (accessed on 8 August 2022).
- Xu, L.; Yuan, S.; Wang, X.; Chen, Z.; Li, X.; Cao, J.; Wang, F.; Huang, J.; Peng, S. Comparison of yield performance between direct-seeded and transplanted double-season rice using ultrashort-duration varieties in central China. Crop J. 2022, 10, 515–523. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Wang, X.; Xiong, D.; Wang, F. Comparing the grain yields of direct-seeded and transplanted rice: A meta-analysis. Agronomy 2019, 9, 767. [Google Scholar] [CrossRef]
- Liu, H.; Hussain, S.; Zheng, M.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Dry direct-seeded rice as an alternative to transplanted-flooded rice in Central China. Agron. Sustain. Dev. 2015, 35, 285–294. [Google Scholar] [CrossRef]
- Sun, J.; Yin, C.; Wang, S.; Liu, H.; Hu, X.; Wang, S.; Tian, F.; Ma, C.; Zhang, X.; Zhang, R. Discussion on the key techniques of direct seeding rice in North China. China Rice 2019, 25, 125–128. [Google Scholar]
- Chen, X.; Tang, Y.; Xie, Y.; Li, S.; Chu, J.B.; Ao, F.Y.; Peng, W.; Li, H.S.; Wan, Y.H. Research advances of rice mechanical direct-seeding technology in China. China Rice 2018, 24, 9–15. [Google Scholar]
- Sun, K.; Li, D.; Yuan, J.; Dong, J.; Yan, X.; Luo, L.; Liu, Y.; Xiao, W.; Wang, H.; Chen, Z.; et al. Genome-wide association analysis for rice submergence seedling rate. Sci. Agric. Sin. 2019, 52, 385–398. [Google Scholar]
- Septiningsih, E.M.; Ignacio, J.; Sendon, P.M.D.; Sanchez, D.L.; Ismail, A.M.; Mackill, D.J. QTL mapping and confirmation for tolerance of anaerobic conditions during germination derived from the rice landrace Ma-Zhan Red. Theor. Appl. Genet. 2013, 126, 1357–1366. [Google Scholar] [CrossRef]
- Jin, Q.; Ouyang, Y.; Lu, Y.; Xu, Y. Some problems and technical countermeasures of direct seeding rice in South China. Chin. Agric. Sci. Bull. 2001, 17, 44–48. [Google Scholar]
- Ismail, A.; Ella, E.; Vergara, G.; Vergara, G.; Mackill, D. Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Ann. Bot. 2009, 103, 197–209. [Google Scholar] [CrossRef]
- Magneschi, L.; Kudahettige, R.; Alpi, A.; Perata, P. Comparative analysis of anoxic coleoptile elongation in rice varieties: Relationship between coleoptile length and carbohydrate levels, fermentative metabolism and anaerobic gene expression. Plant Biol. 2009, 11, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Sauter, M. Role of ethylene and other plant hormones in orchestrating the responses to low oxygen conditions. In Low-Oxygen Stress in Plants; van Dongen, J.T., Licausi, F., Eds.; Springer: Vienna, Austria, 2014; pp. 117–132. [Google Scholar]
- Jackson, M. Ethylene-promoted elongation: An adaptation to submergence stress. Ann. Bot. 2008, 101, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Bao, Y.; Wu, Y.; Zhang, H. Quantitative trait loci controlling rice seed germination under salt stress. Euphytica 2011, 178, 297–307. [Google Scholar] [CrossRef]
- Dametto, A.; Sperotto, R.; Adamski, J.; Blasi, É.; Cargnelutti, D.; Oliveira, L.; Ricachenevsky, F.; Fregonezi, J.; Mariath, J.; Cruz, D.; et al. Cold tolerance in rice germinating seeds revealed by deep RNAseq analysis of contrasting indica genotypes. Plant Sci. 2015, 238, 1–12. [Google Scholar] [CrossRef]
- Yu, M.; Xu, H.; Zhang, H.; Zhu, Y. Regulation of plant hormones on seed dormancy and germination. Plant Physiol. 2016, 52, 599–606. [Google Scholar]
- Fukao, T.; Bailey-Serres, J. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 16814–16819. [Google Scholar] [CrossRef]
- Das, K.; Sarkar, R.; Ismail, A. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Sci. 2005, 168, 131–136. [Google Scholar] [CrossRef]
- Sun, T.; Gubler, F. Molecular mechanism of gibberellin signaling in plants. Annu. Rev. Plant Biol. 2004, 55, 197–223. [Google Scholar] [CrossRef]
- Cheng, H.; Qin, L.; Lee, S.; Fu, X.; Richards, D.; Cao, D.; Luo, D.; Harberd, N.; Peng, J. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 2004, 131, 1055–1064. [Google Scholar] [CrossRef]
- Jasinski, S.; Tattersall, A.; Piazza, P.; Hay, A.; Martinez Garcia, J.; Schmitz, G.; Tsiantis, M. PROCERA encodes a DELLA protein that mediates control of dissected leaf form in tomato. Plant J. 2008, 56, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, Z.; Cheng, C.; Wang, T.; Ji, H.; Zhao, Y.; Deng, Z.; Zhi, L.; Lu, J.; Wu, X.; et al. Counteraction of ABA-mediated inhibition of seed germination and seedling establishment by ABA signaling terminator in Arabidopsis. Mol. Plant 2020, 13, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Wang, Y.; Li, X.; Wu, S.; Wang, Y.; Luo, J.; Mattson, N.; Xu, Y. Interactions between ethylene, gibberellin and abscisic acid in regulating submergence induced petiole elongation in Nelumbo nucifera. Aquat. Bot. 2017, 137, 9–15. [Google Scholar] [CrossRef]
- Yaish, M.; El-kereamy, A.; Zhu, T.; Beatty, P.; Good, A.; Bi, Y.; Rothstein, S. The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet. 2010, 6, e1001098. [Google Scholar] [CrossRef]
- Zentella, R.; Zhang, Z.; Park, M.; Thomas, S.; Endo, A.; Murase, K.; Fleet, C.; Jikumaru, Y.; Nambara, E.; Kamiya, Y.; et al. Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 2007, 19, 3037–3057. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, C.; Mo, Y.; Luo, Z.; Zhu, F.; Xiao, L.T.; Wang, R. Bioinformatics analysis of PLA3 gene and its involvement in gibberellin regulation of rice seed germination. Plant Physiol. Commun. 2020, 56, 1475–1483. [Google Scholar]
- Chang, Y.; Geng, Z.; Wang, K.; Li, M.; Zhang, B.; Zhao, W. Mechanism study of OsBLR1 in promoting rice germination under hormones induction. J. Henan Agric. Univ. 2021, 55, 1058–1064. [Google Scholar]
- Gui, J.; Zheng, S.; Liu, C.; Shen, J.; Li, J.; Li, L. OsREM4. 1 interacts with OsSERK1 to coordinate the interlinking between abscisic acid and brassinosteroid signaling in rice. Dev. Cell 2016, 38, 201–213. [Google Scholar] [CrossRef]
- Clouse, S. Brassinosteroid/abscisic acid antagonism in balancing growth and stress. Dev. Cell 2016, 38, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Ryu, H.; Cho, H. Brassinosteroid signaling pathways interplaying with diverse signaling cues for crop enhancement. Agronomy 2021, 11, 556. [Google Scholar] [CrossRef]
- Chung, Y.; Maharjan, P.; Lee, O.; Fujioka, S.; Jang, S.; Kim, B.; Takatsuto, S.; Tsujimoto, M.; Kim, H.; Cho, S.; et al. Auxin stimulates DWARF4 expression and brassinosteroid biosynthesis in Arabidopsis. Plant J. 2011, 66, 564–578. [Google Scholar] [CrossRef]
- Li, T.; Lei, W.; He, R.; Tang, X.; Han, J.; Zou, L.; Zou, L.; Yin, Y.; Lin, H.; Zhang, D. Brassinosteroids regulate root meristem development by mediating BIN2-UPB1 module in Arabidopsis. PLoS Genet. 2020, 16, e1008883. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Walcher, C.; Chory, J.; Nemhauser, J. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc. Natl. Acad. Sci. USA 2008, 105, 9829–9834. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.; Meng, Y.; Luo, X.; Chen, F.; Zhou, W.; Dai, Y.; Qi, Y.; Du, J.; Yang, F.; Liu, J.; et al. Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Sci. Rep. 2017, 7, 12620. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Gao, Q.; Luo, L.; Yang, J.; Chen, C.; Guo, T.; Liu, Y.; Huang, Y.; Wang, H.; Chen, Z.; et al. Transcriptome analysis of hormone signal transduction and glutathione metabolic pathway in rice seeds at germination stage. Chin. J. Rice Sci. 2021, 35, 554–564. [Google Scholar]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.; Luan, S.; Li, J.; He, Z. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef]
- Gimeno, C.; Lelièvre, E.; Viau, L.; Malik-Ghulam, M.; Ricoult, C.; Niebel, A.; Leduc, N.; Limami, A. ABA-mediated inhibition of germination is related to the inhibition of genes encoding cell-wall biosynthetic and architecture: Modifying enzymes and structural proteins in Medicago Truncatula embryo axis. Mol. Plant 2009, 2, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Jong, M.; Wolters, M.; Garcia, J.L.; Mariani, C.; Vriezen, W. The Solanum Lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J. Exp. Bot. 2010, 62, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, Y.; Qiu, J.; Wang, H.; Wang, S.; Tang, L.; Tong, X.; Zhang, J. Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’ pathway to synergistically inhibit seed germination in rice (Oryza sativa). N. Phytol. 2020, 228, 1336–1353. [Google Scholar] [CrossRef]
- Alpi, A.; Beevers, H. Effects of O2 concentration on rice seedlings. Plant Physiol. 1983, 71, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Turner, F.; Chen, C.; McCauley, G. Morphological development of rice seedlings in water at controlled oxygen levels. Agron. J. 1981, 73, 566–570. [Google Scholar] [CrossRef]
- Setter, T.; Ella, E.; Valdez, A. Relationship between coleoptile elongation and alcoholic fermentation in rice exposed to anoxia. II. Cultivar differences. Ann. Bot. 1994, 74, 3. [Google Scholar] [CrossRef]
- Lee, K.; Chen, P.; Lu, C.; Chen, S.; Ho, T.; Yu, S. Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci. Signal. 2009, 2, ra61. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang, R.; Heuer, S.; Ismail, A.; Serres, J.; Ronald, C.; Mackill, D. Sub1A is an Ethylene-Response-Factor-Like Gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Ram, P.; Singh, B.; Singh, A.; Ram, P.; Singh, P.; Singh, H.; Boamfa, I.; Harren, F.; Santosa, E.; Jackson, M.; et al. Submergence tolerance in rainfed lowland rice: Physiological basis and prospects for cultivar improvement through marker-aided breeding. Field Crops Res. 2002, 76, 131–152. [Google Scholar] [CrossRef]
- Bailey, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence tolerant rice: SUB1’s journey from landrace to modern cultivar. Rice 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Ke, Z. Variation of Major Storage Materials and Expression Analysis of Gibberellins Metabolic Enzyme Genes during Seed Germination in Foxtail Millet; Shanxi Agricultural University: Taigu, China, 2015. [Google Scholar]
- Yu, Y.; Deng, L.; Zhou, L.; Chen, G.; Wang, Y. Exogenous melatonin activates antioxidant systems to increase the ability of Rice seeds to germinate under high temperature conditions. Plants 2022, 11, 886. [Google Scholar] [CrossRef]
- Lv, Y. Cloning and Functional Analysis of the Rice Mesocotyl Elongation Gene QME1; Huazhong Agricultural University: Hubei, China, 2020. [Google Scholar]
- Fukao, T.; Yeung, E.; Bailey, J. The submergence tolerance regulator Sub1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 2011, 23, 412–427. [Google Scholar] [CrossRef]
- Wu, H.; Chen, H.; Zhang, Y.; Zhang, Y.; Zhu, D.; Xiang, J. Effects of 1-aminocyclopropane-1-carboxylate and paclobutrazol on the endogenous hormones of two contrasting rice varieties under submergence stress. Plant Growth Regul. 2019, 87, 109–121. [Google Scholar] [CrossRef]
- Zhao, T.; Li, Q.; Pan, X.; Hua, X.; Zhang, W. Adaptive mechanism of terrestrial plants to waterlogging stress. Plant Physiol. Commun. 2021, 57, 2091–2103. [Google Scholar]
- Yang, Y.; Tong, H. Functional mechanism of brassinosteroid and utilization for molecular design in rice. Chem. Life 2021, 41, 1171–1180. [Google Scholar]
- Jantapo, K.; Wimonchaijit, W.; Wang, W.; Chaiwanon, J. Supraoptimal brassinosteroid levels inhibit root growth by reducing root meristem and cell elongation in rice. Plants 2021, 10, 1962. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nakamura, Y.; Asami, T.; Yoshida, S.; Matsuo, T.; Okamoto, S. Physiological roles of brassinosteroids in early growth of Arabidopsis: Brassinosteroids have a synergistic relationship with gibberellin as well as auxin in light-grown hypocotyl elongation. J. Plant Growth Regul. 2003, 22, 259–271. [Google Scholar] [CrossRef]
- Wu, H.; Xiang, J.; Chen, H.; Zhang, Y.; Zhang, Y.; Zhu, F. Effects of exogenous growth regulators on plant elongation and carbohydrate consumption of rice seedlings under submergence. J. Appl. Ecol. 2018, 29, 149–157. [Google Scholar]
- Xiang, J.; Wu, H.; Zhang, Y.; Zhang, Y.; Wang, Y.; Li, Z.; Lin, H.; Chen, H.; Zhang, J.; Zhu, D. Transcriptomic analysis of gibberellin- and paclobutrazol-treated rice seedlings under submergence. Int. J. Mol. Sci. 2017, 18, 2225. [Google Scholar] [CrossRef]
- Manoj, M.; Sreeshma, N.; Pawan, S.; Balwant, S.; Amitha, S.; Nagendra, K. Variation in aerobic recovery and vigor of cultivated and wild rice genotypes after anaerobic germination stress in rice (Oryza sativa). Indian J. Agric. Sci. 2020, 90, 2351–2356. [Google Scholar]
- Panda, D.; Sharma, S.; Sarkar, R. Chlorophyll fluorescence parameters, CO2 photosynthetic rate and regeneration capacity as a result of complete submergence and subsequent re-emergence in rice (Oryza sativa L.). Aquat. Bot. 2008, 88, 127–133. [Google Scholar] [CrossRef]
- Panda, D.; Sarkar, R. Mechanism associated with nonstructural carbohydrate accumulation in submergence tolerant rice (Oryza sativa L.) cultivars. J. Plant Interact. 2014, 9, 62–68. [Google Scholar] [CrossRef]
- Fukao, T.; Xu, K.; Ronald, P.; Bailey-Serres, J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef]
- Hoffmann, S.; Kende, H. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol. 1992, 99, 1156–1161. [Google Scholar] [CrossRef]
- Neeraja, C.; Maghirang, R.; Pamplona, A.; Heuer, S.; Collard, B.; Septiningsih, E.; Vergara, G.; Aanchez, D.; Xu, K.; Iamail, A.; et al. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor. Appl. Genet. 2007, 115, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, J.; Lu, J.; Xiong, M.; Zhang, C.; Liu, Q. Effect of oleuropein lactone on starch metabolism during rice germination and its mechanism. Jiangsu Agric. Sci. 2018, 46, 60–64. [Google Scholar]
- Zhang, G.; Liu, Z.; Liu, Y.; Kuya, N.; Hua, Y.; Shi, H.; Zhao, W.; Han, Y.; Yamamoto, T.; Chen, W.; et al. iTRAQ-based proteomics investigation of critical response proteins in embryo and coleoptile during rice anaerobic germination. Rice Sci. 2021, 28, 391–401. [Google Scholar]
- Zhao, Y.; Wang, T. Analysis of the relationship between α-amylase and germinating rate of rice seeds during the process of seed germination. Chin. Bull. Bot. 2001, 18, 226–230. [Google Scholar]
- Chen, S.; Wang, J.; Pan, Y.; Ma, J.; Zhang, J.; Zhang, H.; Teng, S. Genetic analysis of seed germinability under submergence in rice. Chin. Bull. Bot. 2012, 47, 28–35. [Google Scholar]
- Huang, S.; Greenway, H.; Colmer, T. Anoxia tolerance in rice seedlings: Exogenous glucose improves growth of an anoxia-’intolerant’, but not of a ’tolerant’ genotype. J. Exp. Bot. 2003, 54, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Alpi, A.; Perata, P. α-amylase expression under anoxia in rice seedlings: An update. Russ. J. Plant Physiol. 2003, 50, 737–743. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Sun, H.; Zhang, J.; Peng, T.; Sun, H.; Xin, Z.; Zhao, Q. Comparative morphological and transcriptomic responses of lowland and upland rice to root-zone hypoxia. Environ. Exp. Bot. 2020, 169, 103916. [Google Scholar] [CrossRef]
- Gibbs, J.; Greenway, H. Review: Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct. Plant Biol. 2003, 30, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Takahashi, H.; Greenway, H.; Matsumura, H.; Tsutsumi, N.; Nakazono, M. Rice alcohol dehydrogenase 1 promotes survival and has a major impact on carbohydrate metabolism in the embryo and endosperm when seeds are germinated in partially oxygenated water. Ann. Bot. 2014, 113, 851–859. [Google Scholar] [CrossRef]
- Fukao, T.; Bailey-Serres, J. Plant responses to hypoxia–is survival a balancing act? Trends Plant Sci. 2004, 9, 449–456. [Google Scholar] [CrossRef]
- Rahman, M.; Grover, A.; Peacock, W.; Dennis, E.; Ellis, M. Effects of manipulation of pyruvate decarboxylase and alcohol dehydrogenase levels on the submergence tolerance of rice. Australian J. Plant Physiol. 2001, 28, 1231–1241. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Li, F.; Qian, B.; Liu, Z.; Gao, F.; Yang, D.; Li, X. Effects of exogenous 6-BA on root growth and pod yield of flooded peanut at different growth stages. Sci. Agric. Sin. 2020, 53, 3665–3678. [Google Scholar]
- Peng, R. Hydrogen Sulfide Enhances Nitric Oxide-Induced Tolerance of Hypoxia in Maize (Zea mays L.) Tip Cells; Lanzhou University: Lanzhou, China, 2016. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Huang, H.; Wang, X.; Dai, H.; Zhang, Y.; Wang, Y.; Zhang, Y.; Zhu, D.; Chen, H.; Xiang, J. Progress of Research on Phytohormone Interaction in Germination of Direct-Seeded Rice under Submergence. Agronomy 2022, 12, 2454. https://doi.org/10.3390/agronomy12102454
Wu H, Huang H, Wang X, Dai H, Zhang Y, Wang Y, Zhang Y, Zhu D, Chen H, Xiang J. Progress of Research on Phytohormone Interaction in Germination of Direct-Seeded Rice under Submergence. Agronomy. 2022; 12(10):2454. https://doi.org/10.3390/agronomy12102454
Chicago/Turabian StyleWu, Hui, Hua Huang, Xuhui Wang, Haifang Dai, Yikai Zhang, Yaliang Wang, Yuping Zhang, Defeng Zhu, Huizhe Chen, and Jing Xiang. 2022. "Progress of Research on Phytohormone Interaction in Germination of Direct-Seeded Rice under Submergence" Agronomy 12, no. 10: 2454. https://doi.org/10.3390/agronomy12102454
APA StyleWu, H., Huang, H., Wang, X., Dai, H., Zhang, Y., Wang, Y., Zhang, Y., Zhu, D., Chen, H., & Xiang, J. (2022). Progress of Research on Phytohormone Interaction in Germination of Direct-Seeded Rice under Submergence. Agronomy, 12(10), 2454. https://doi.org/10.3390/agronomy12102454




