Methyl Jasmonate Alleviates the Deleterious Effects of Salinity Stress by Augmenting Antioxidant Enzyme Activity and Ion Homeostasis in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Design, and Experiment
2.2. Preparation of MeJA Foliar Spray
2.3. Measurements of Plant Growth and Yield Parameters
2.3.1. Plant Growth Parameters
2.3.2. Yield Components
2.4. Physiological Parameters
2.4.1. Chlorophyll Determination
2.4.2. Leaf Relative Water Contents (RWC)
2.4.3. Membrane Stability Index (MSI)
2.4.4. Leaf Chlorophyll Fluorescence (LCf)
2.4.5. Gas Exchange Attributes
2.5. Measurements of MDA, H2O2, APX, POD, and SOD Activities
2.6. Analysis of Different Genes
2.7. Measurements of Na+, K+, and Na+/K+ Ratio
2.8. Statistical Analysis
3. Results
3.1. Influence of Salinity and MeJA Foliar Applications on Agronomical Attributes
3.2. Influence of Salinity and MeJA Foliar Applications on Rice Yield Components
3.3. Influence of Salinity and MeJA on Rice Chlorophyll Content, RWC, and MSI
3.4. Influence of Salinity and MeJA Foliar Application on Rice Leaf Chlorophyll Fluorescence (LCf)
3.5. Influence of Salinity and MeJA Foliar Application on Rice Gas Exchange Attributes
3.6. Influence of Salinity and MeJA Foliar Applications on MDA, H2O2, APX, POD, and SOD
3.7. Influence of Salinity and MeJA Foliar Applications on Expression Analysis of Stress Genes
3.8. Na+, K+, and Na+/K+ Homeostasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Wang, B. Protection of Halophytes and Their Uses for Cultivation of Saline-Alkali Soil in China. Biology 2021, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid Land Res. Manag. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- FAO. Land and Plant Nutrition Management Service. 2008. Available online: http://www.fao.org/agb/agl/agll/spush/ (accessed on 18 August 2022).
- Zhao, K.F.; Li, F.Z. Chinese Halophyte; Science Press: Beijing, China, 1999. [Google Scholar]
- Wang, B.S. Plant Biology under Stress; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Li, Q.; Yang, A.; Zhang, W. Efficient acquisition of iron confers greater tolerance to saline-alkaline stress in rice (Oryza sativa L.). J. Exp. Bot. 2016, 67, 6431–6444. [Google Scholar] [CrossRef]
- Gregorio, G.B. Progress in breeding for trace minerals in staple crops. J. Nutr. 2002, 132, 500S–502S. [Google Scholar] [CrossRef]
- Largier, J.L. Tidal intrusion fronts. Estuaries 1992, 15, 26–39. [Google Scholar] [CrossRef]
- Graham, R.D.; Welch, R.M.; Bouis, H.E. Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: Principles, perspectives and knowledge gaps. Adv. Agron. 2001, 70, 77–142. [Google Scholar]
- Khan, S.; Javed, M.A.; Jahan, N.; Manan, F.A. A short review on the development of salt tolerant cultivars in rice. Int. J. Public Health Sci. 2016, 5, 201–212. [Google Scholar]
- Long, X.; Liu, L.; Shao, T.; Shao, H.; Liu, Z. Developing and sustainably utilize the coastal mudflat areas in China. Sci. Total Environ. 2016, 56, 1077–1086. [Google Scholar] [CrossRef]
- Yu, R.P.; Chen, D.M. Saline soil resources in China and their exploitation. Chin. J. Soil Sci. 1999, 30, 158–159. [Google Scholar]
- Li, B.; Wang, Z.C.; Sun, Z.G.; Chen, Y.; Yang, F. Resources and sustainable resource exploitation of salinized land in China. Agric. Res. Arid Areas 2005, 23, 154–158. [Google Scholar]
- Quan, R.D.; Wang, J.; Jian, H.; Bai, H.B.; Lyu, X.L.; Zhu, Y.X.; Zhang, H.W.; Zhang, Z.J.; Li, S.H.; Huang, R.F. Improvement of salt tolerance using wild rice genes. Front. Plant Sci. 2018, 8, 2269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hussain, S.; Wang, Y.; Liu, Y.; Li, Q.; Chen, Y.; Wei, H.; Gao, P.; Dai, Q. Comprehensive Evaluation of Salt Tolerance in Rice (Oryza sativa L.) Germplasm at the Germination Stage. Agronomy 2021, 11, 1569. [Google Scholar] [CrossRef]
- Hussain, S.; Zhu, C.; Bai, Z.; Huang, J.; Zhu, L.; Cao, X.; Nanda, S.; Hussain, S.; Riaz, A.; Liang, Q.; et al. iTRAQ-Based Protein Profiling and Biochemical Analysis of Two Contrasting Rice Genotypes Revealed Their Differential Responses to Salt Stress. Int. J. Mol. Sci. 2019, 20, 547. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Zhang, J.H.; Zhong, C.; Zhu, L.F.; Cao, X.C.; Yu, S.M.; Bohr, J.A.; Hu, J.J.; Jin, Q.Y. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.J.; Zhu, L.F.; Yu, S.M.; Sanjoy, K.K.; Jin, Q.Y. Effects of 1-methylcyclopropene on function of flag leaf and development of superior and inferior spikelets in rice cultivars differing in panicle types. Field Crops Res. 2015, 177, 64–74. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Faizan, S.; Gulzar, B.; Mushtaq, H.; Bushra, S.; Hussain, A.; Hakeem, K.R. Changes in Growth, Photosynthetic Pigments, Cell Viability, Lipid Peroxidation and Antioxidant Defense System in Two Varieties of Chickpea (Cicer arietinum L.) Subjected to Salinity Stress. Phyton Int. J. Exp. Bot. 2022, 91, 149. [Google Scholar] [CrossRef]
- Raza, A.; Tabassum, J.; Fakhar, A.Z.; Sharif, R.; Chen, H.; Zhang, C.; Ju, L.; Fotopoulos, V.; Siddique, K.H.M.; Singh, R.K.; et al. Smart reprograming of plants against salinity stress using modern biotechnological tools. Crit. Rev. Biotechnol. 2022, 15, 1–28. [Google Scholar] [CrossRef]
- Mujtaba, M.; Wang, D.; Carvalho, L.B.; Oliveira, J.L.; Espirito Santo Pereira, A.D.; Sharif, R.; Jogaiah, S.; Paidi, M.K.; Wang, L.; Ali, Q.; et al. Nanocarrier-mediated delivery of miRNA, RNAi, and CRISPR-Cas for plant protection: Current trends and future directions. ACS Agric. Sci. Technol. 2021, 1, 417–435. [Google Scholar] [CrossRef]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Hormonal interactions underlying parthenocarpic fruit formation in horticultural crops. Hortic. Res. 2022, 9, uhab024. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Aref, I.M.; Khan, P.R.; Khan, S.; El-Atta, H.; Ahmed, A.I.; Iqbal, M. Modulation of antioxidant enzymes in Juniperus procera needles in relation to habitat environment and dieback incidence. Trees Struct. Funct. 2016, 30, 1669–1681. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins-major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Hamayun, M.; Lee, S.K.; Lee, I.J. Methyl jasmonate alleviated salinity stress in soybean. J. Crop Sci. Biotechnol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Manan, A.; Ayyub, C.M.; Pervez, M.A.; Ahmad, R. Methyl jasmonate brings about resistance against salinity stressed tomato plants by altering biochemical and physiological processes. Pak. J. Agric. Sci. 2016, 53, 35–41. [Google Scholar]
- Anjum, S.A.; Farooq, M.; Wang, L.C.; Xue, L.L.; Wang, S.G.; Wang, L.; Chen, M. Gas exchange and chlorophyll synthesis of maize cultivars are enhanced by exogenously applied glycine betaine under drought conditions. Plant Soil Environ. 2011, 57, 326–331. [Google Scholar] [CrossRef]
- Poonam, S.; Kaur, H.; Geetika, S. Effect of jasmonic acid on photosynthetic pigments and stress markers in [Cajanus cajan (L.) Millsp]. Seedlings under copper stress. Am. J. Plant Sci. 2013, 4, 817–823. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef]
- Tsonev, T.D.; Lazova, G.N.; Stoinova, Z.G.; Popova, L.P. A possible role for jasmonic acid in adaptation of barley seedlings to salinity stress. Plant Growth Regul. 1998, 17, 153–159. [Google Scholar] [CrossRef]
- Hristova, V.A.; Popova, L.P. Treatment with methyl jasmonate alleviates the effects of paraquat on photosynthesis in barley plants. Photosynthetica 2002, 40, 567–574. [Google Scholar] [CrossRef]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Modarres, S.S.; Allahdadi, I. The role of phytohormones in alleviating salt stress in crop plants. Aust. J. Crop Sci. 2011, 5, 726–734. [Google Scholar]
- Jin, S.; Chen, C.C.S.; Plant, A.L. Regulation by Aba of osmotic-stress-induced changes in protein synthesis in tomato roots. Plant Cell Environ. 2000, 23, 51–60. [Google Scholar] [CrossRef]
- Poor, P.; Gemes, K.; Horvath, F.; Szepesi, A.; Simon, M.L.; Tari, I. Salicylic acid treatment via the rooting medium interferes with stomatal response, CO2 fixation rate and carbohydrate metabolism in tomato, and decreases harmful effects of subsequent salt stress. Plant Biol. 2011, 13, 105–114. [Google Scholar] [CrossRef]
- Bruce, T.J.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Wang, S.Y. Methyl jasmonate reduces water stress in strawberry. J. Plant Growth Regul. 1999, 18, 127–134. [Google Scholar] [CrossRef]
- Velitchkova, M.; Fedina, I. Response of photosynthesis of Pisum sativum to salt stress as affected by methyl jasmonate. Photosynthetica 1998, 35, 89–97. [Google Scholar] [CrossRef]
- Del-Amor, F.M.; Cuadra-Crespo, P. Alleviation of salinity stress in broccoli using foliar urea or methyl jasmonate: Analysis of growth, gas exchange, and isotope composition. Plant Growth Regul. 2011, 63, 55–62. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mulpuri, R. The oxylipin pathway in Arabidopsis. Arab. Book/Am. Soc. Plant Biol. 2002, 1, e0012. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates and octadecanoids: Signals in plant stress responses and development. Prog. Nucleic Acid Res. 2002, 72, 165–221. [Google Scholar]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Walia, H.; Wilson, C.; Wahid, A.; Condamine, P.; Cui, X.; Close, T.J. Expression analysis of barley (Hordeumvulgare L.) during salinity stress. Funct. Integr. Genom. 2006, 6, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Zeng, F.; Wang, J.; Zhang, G. Identification and characterization of HAK/KUP/KT potassium transporter gene family in barley and their expression under abiotic stress. BMC Genom. 2021, 22, 317. [Google Scholar] [CrossRef]
- Riedelsberger, J.; Miller, J.K.; Valdebenito-Maturana, B.; Piñeros, M.A.; González, W.; Dreyer, I. Plant HKT Channels: An Updated View on Structure, Function and Gene Regulation. Int. J. Mol. Sci. 2021, 22, 1892. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, R.; Liu, S.; Li, R.; Zhou, Y.; Chen, Y.; Hou, H.; Dai, Q. Transcriptome-Wide Analysis Revealed the Potential of the High-Affinity Potassium Transporter (HKT) Gene Family in Rice Salinity Tolerance via Ion Homeostasis. Bioengineering 2022, 9, 410. [Google Scholar] [CrossRef]
- Chen, Y.; Li, R.; Ge, J.; Liu, J.; Wang, W.; Xu, M.; Zhang, R.; Hussain, S.; Wei, H.; Dai, Q. Exogenous melatonin confers enhanced salinity tolerance in rice by blocking the ROS burst and improving Na+/K+ homeostasis. Environ. Exp. Bot. 2021, 189, 104530. [Google Scholar] [CrossRef]
- Lafitte, R. Relationship between leaf relative water content during reproductive stage water deficit and grain formation in rice. Field Crop Res. 2002, 76, 165–174. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Sayed, M.A.; Islam, M.M.; Siddiqui, M.N.; Begum, S.N.; Hossain, M.A. Screening of rice landraces (Oryza sativa L.) for seedling stage salinity tolerance using morpho-physiological and molecular markers. Acta Physiol. Plant 2018, 40, 70. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Shukla, D.S. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 1997, 178, 171–178. [Google Scholar] [CrossRef]
- Maishanu, H.; Rabe, A. Cell Membrane Stability and Relative Water Content of Cymbopogoncitratus (Lemon Grass). Annu. Res. Rev. Biol. 2019, 33, 1–7. [Google Scholar] [CrossRef][Green Version]
- Hamani, A.K.M.; Wang, G.; Soothar, M.K.; Shen, X.; Gao, Y.; Qiu, R.; Mehmood, F. Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol. 2020, 20, 434. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W.; Baker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Jiang, D.; Hou, J.; Gao, W.; Tong, X.; Li, M.; Chu, X.; Chen, G. Exogenous spermidine alleviates the adverse effects of aluminum toxicity on photosystem II through improved antioxidant system and endogenous polyamine contents. Ecotox. Environ. Safe 2021, 207, 111265. [Google Scholar] [CrossRef]
- Molina, A.; Bueno, P.; Marín, M.C.; Rodríguez-Rosales, M.P.; Belver, A.; Venema, K.; Donaire, J.P. Involvement of endogenous salicylic acid content, lipoxygenase and antioxidant enzyme activities in the response of tomato cell suspension cultures to NaCl. New Phytol. 2002, 156, 409–415. [Google Scholar] [CrossRef]
- Jin, X.; Yang, X.; Islam, E.; Dan, L.; Mahmood, Q. Effects of cadmium on ultrastructure and antioxidative defense system in hyperaccumulator and non-hyperaccumulator ecotypes of Sedum alfredii Hance. J. Hazard. Mater. 2008, 156, 387–397. [Google Scholar] [CrossRef]
- Bartha, C.; Fodorpataki, L.; Martinez-Ballesta, M.C.; Popescu, O.; Carvajal, M. Sodium accumulation contributes to salt stress tolerance in lettuce cultivars. J. Appl. Bot. Food Qual. 2015, 88, 42–48. [Google Scholar]
- Khan, K.; Choi, J.Y.; Nho, E.Y.; Jamila, N.; Habte, G.; Hong, J.H.; Hwang, M.; Kim, K.S. Determination of minor and trace elements in aromatic spices by micro-wave assisted digestion and inductively coupled plasma-mass spectrometry. Food Chem. 2014, 158, 200–206. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Iqbal, S.; Amjad, M.; Naveed, M.; Zahir, Z.A.; Jacobsen, S.E. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 2016, 43, 632–642. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 2012, 17, 22–31. [Google Scholar] [CrossRef]
- Singh, A. Soil salinization and waterlogging: A threat to environment and agricultural sustainability. Ecol. Indic. 2015, 57, 128–130. [Google Scholar] [CrossRef]
- Ranjbarfordoei, A.R.; Samson, P.; Van, D. Chlorophyll fluorescence performance of sweet almond [Prunus dulcis (Miller) D. Webb] in response to salinity stress induced by NaCl. Photosynthetica 2006, 44, 513–522. [Google Scholar] [CrossRef]
- Rohmawat, T.; Kumala, D. Effect of Methyl Jasmonate on Vegetative Growth and Formation of Potato Tuber (Solanum tuberosum L.) var. Granola. Biog. J. Ilm. Biol. 2019, 7, 25–29. [Google Scholar] [CrossRef]
- Kumari, J.G.; Sudhakar, C. Effects of jasmonic acid on groundnut during early seedling growth. Biol. Plant 2003, 47, 453–456. [Google Scholar] [CrossRef]
- Amirjani, M.R. Salinity and photochemical efficiency of wheat. Int. J. Bot. 2010, 6, 273–279. [Google Scholar] [CrossRef]
- Jamil, M.; Rehman, S.; Rha, E.S. Salinity effect on plant growth, PSII photochemistry and chlorophyll content in sugar beet (Beta vulgaris L.) and cabbage (Brassica oleracea capitata L.). Pak. J. Bot. 2007, 39, 753–760. [Google Scholar]
- Sheteiwy, M.S.; Gong, D.; Gao, Y.; Pan, R.-H.; Hu, J.; Guan, Y. Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ. Exp. Bot. 2018, 15, 236–248. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Z.; Jiang, X.; Qin, Y. Effects of Salt Stress on Photosynthetic Fluorescence Characteristics, Antioxidant System, and Osmoregulation of Coreopsis tinctoria Nutt. HortScience 2021, 56, 1066–1072. [Google Scholar] [CrossRef]
- Jung, S. Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. Plant Physiol. Biochem. 2004, 42, 225–231. [Google Scholar] [CrossRef]
- Seif, S.N.; Tafazzoli, E.; Talaii, A.R.; Aboutalebi, A.; Abdosi, V. Evaluation of two grape cultivars (Vitisvinifera L.) against salinity stress and surveying the effect of methyl jasmonate and epibrassinolide on alleviation the salinity stress. Int. J. Biosci. 2014, 5, 116–125. [Google Scholar]
- Chen, K.; Yang, F.; Zhou, Z.; Fu, C. Effects of soil water stress on photosynthetic characteristics of betel nut seedlings. Chin. J. Trop. Agric. 2010, 30, 8–12. [Google Scholar]
- Diao, M.; Ma, L.; Wang, J.W.; Cui, J.X.; Fu, A.F.; Liu, H.-Y. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Sun, Z.W.; Ren, L.K.; Fan, J.W.; Li, Q.; Wang, K.J.; Guo, M.M.; Wang, L.; Li, J.; Zhang, G.X.; Yang, Z.Y.; et al. Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant Soil Environ. 2016, 62, 515–521. [Google Scholar]
- Amiri, A.; Baninasab, B.; Ghobadi, C.; Khoshgoftarmanesh, C. Zinc soil application enhance photosynthetic capacity and antioxidant enzyme activities in almond seedlings affected by salinity stress. Photosynthetica 2016, 54, 267–274. [Google Scholar] [CrossRef]
- Aftab, T.; Khan, M.M.A.K.; da-Silva, J.A.T.; Idrees, M.; Naeem, M. Role of salicylic acid in promoting salt stress tolerance and enhanced artemisinin production in Artemisia annua L. J. Plant Growth Regul. 2011, 30, 425–435. [Google Scholar] [CrossRef]
- Lekshmy, S.; Sairam, R.K.; Kushwaha, S.R. Effect of long-term salinity stress on growth and nutrient uptake in contrasting wheat genotypes. Ind. J. Plant Physiol. 2013, 18, 344–353. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef]
- Li, J.T.; Qiu, Z.B.; Zhang, X.W.; Wang, L.S. Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol. Plant. 2011, 33, 835–842. [Google Scholar] [CrossRef]
- Faghih, S.; Ghobadi, C.; Zarei, A. Response of strawberry plant cv. ‘camarosa’ to salicylic acid and methyl jasmonate application under salt stress condition. Plant Growth Regul. 2017, 36, 651–659. [Google Scholar] [CrossRef]
- Noriega, G.; Cruz, D.S.; Batlle, A.; Tomaro, M.; Balestrasse, K. Heme oxygenase is involved in the protection exerted by jasmonic acid against cadmium stress in soybean roots. J. Agron. Crop Sci. 2011, 197, 296–301. [Google Scholar] [CrossRef]
- Li, G.; Tang, J.; Zheng, J.; Chu, C. Exploration of rice yield potential: Decoding agronomic and physiological traits. Crop J. 2021, 9, 577–589. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2018, 46, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.R.; Niknejad, Y.; Fallah, H.; Tari, D.B. Methyl jasmonate alleviates arsenic toxicity in rice. Plant Cell Rep. 2020, 39, 1041–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, X.; Wang, L.; Cao, Y.; Song, W.; Shi, J.; Lai, J.; Jiang, C.A. HAK family Na+ transporter confers natural variation of salt tolerance in maize. Nat. Plants 2019, 5, 1297–1308. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Involvement of auxin in growth and stress response of cucumber. Veg. Res. 2022, 2, 13. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, A.; Kumar, N.; Lata, C.; Mann, A. Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi J. Biol. Sci. 2021, 28, 2510–2517. [Google Scholar] [CrossRef]
- Ahmad, S.; Chen, Y.; Shah, A.Z.; Wang, H.; Xi, C.; Zhu, H.; Ge, L. The Homeodomain-Leucine Zipper Genes Family Regulates the Jinggangmycin Mediated Immune Response of Oryza sativa to Nilaparvata lugens, and Laodelphax striatellus. Bioengineering 2022, 9, 398. [Google Scholar] [CrossRef]
- Sohail, H.; Noor, I.; Nawaz, M.A.; Ma, M.; Shireen, F.; Huang, Y.; Yang, L.; Bie, Z. Genome-wide identification of plasma-membrane intrinsic proteins in pumpkin and functional characterization of CmoPIP1-4 under salinity stress. Environ. Exp. Bot. 2022, 202, 104995. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A.; Datta, A.; Yadav, R.K. Evaluation of guava (Psidium guajava) and bael (Aegle marmelos) under shallow saline watertable conditions. Ind. J. Agric. Sci. 2018, 88, 720–725. [Google Scholar]
- Kumar, A.; Kumar, A.; Lata, C.; Kumar, S. Eco-physiological responses of Aeluropus lagopoides (grass halophyte) and Suaeda nudiflora (non-grass halophyte) under individual and interactive sodic and salt stress. S. Afr. J. Bot. 2016, 105, 36–44. [Google Scholar] [CrossRef]
- Lata, C.; Soni, S.; Kumar, N.; Kumar, A.; Pooja, M.A.; Rani, S. Adaptive mechanism of stress tolerance in Urochondra (grass halophyte) using roots study. Ind. J. Agric. Sci. 2019, 89, 1050–1053. [Google Scholar]
- Ahmad, P.; Azooz, M.M.; Prasad, M.N.V. Ecophysiology and Responses of Plants under Salt Stress. Effects of Salinity on Ion Transport, Water Relations and Oxidative Damage; Springer: New York, NY, USA, 2013; pp. 89–114. [Google Scholar]
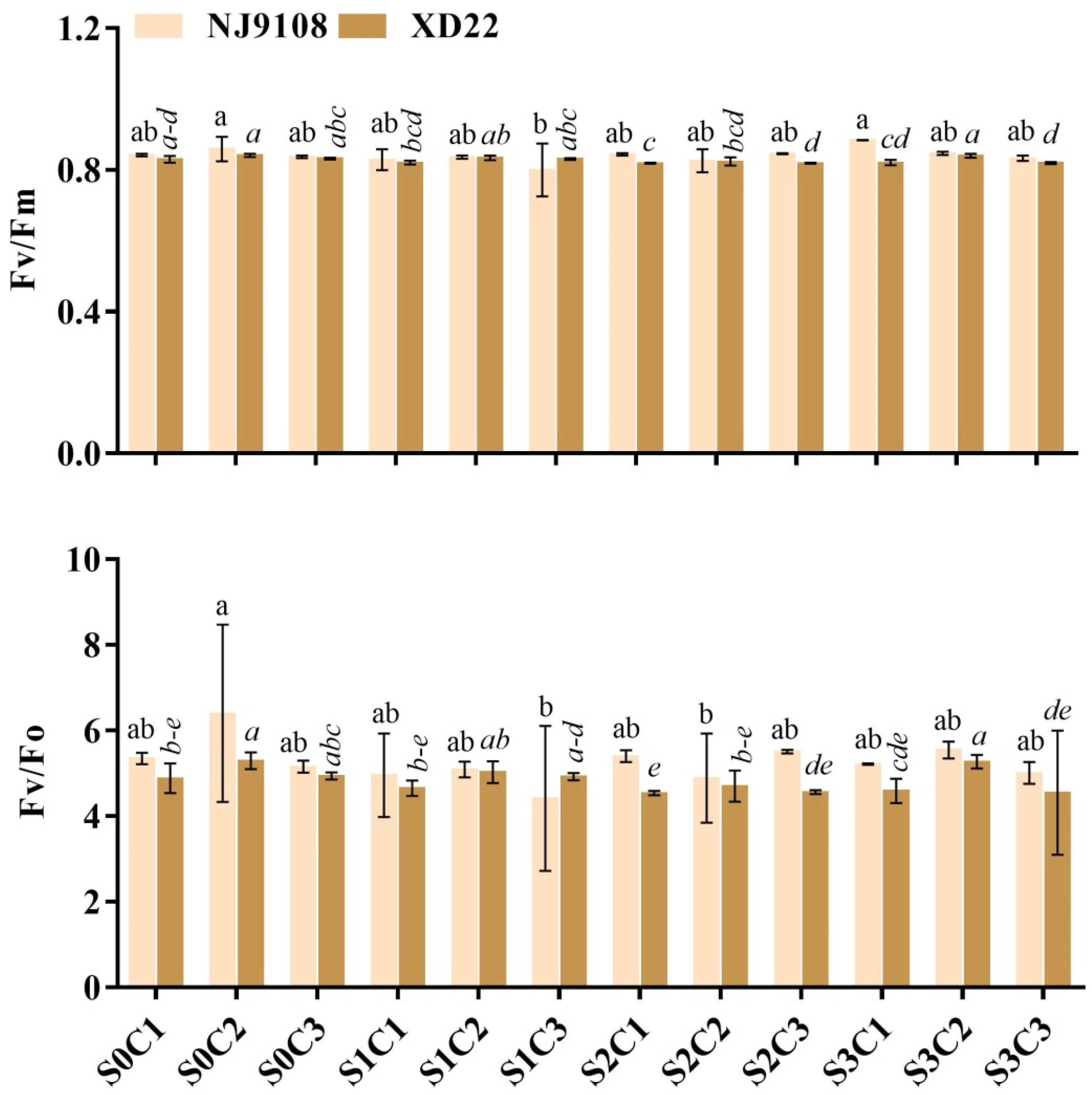
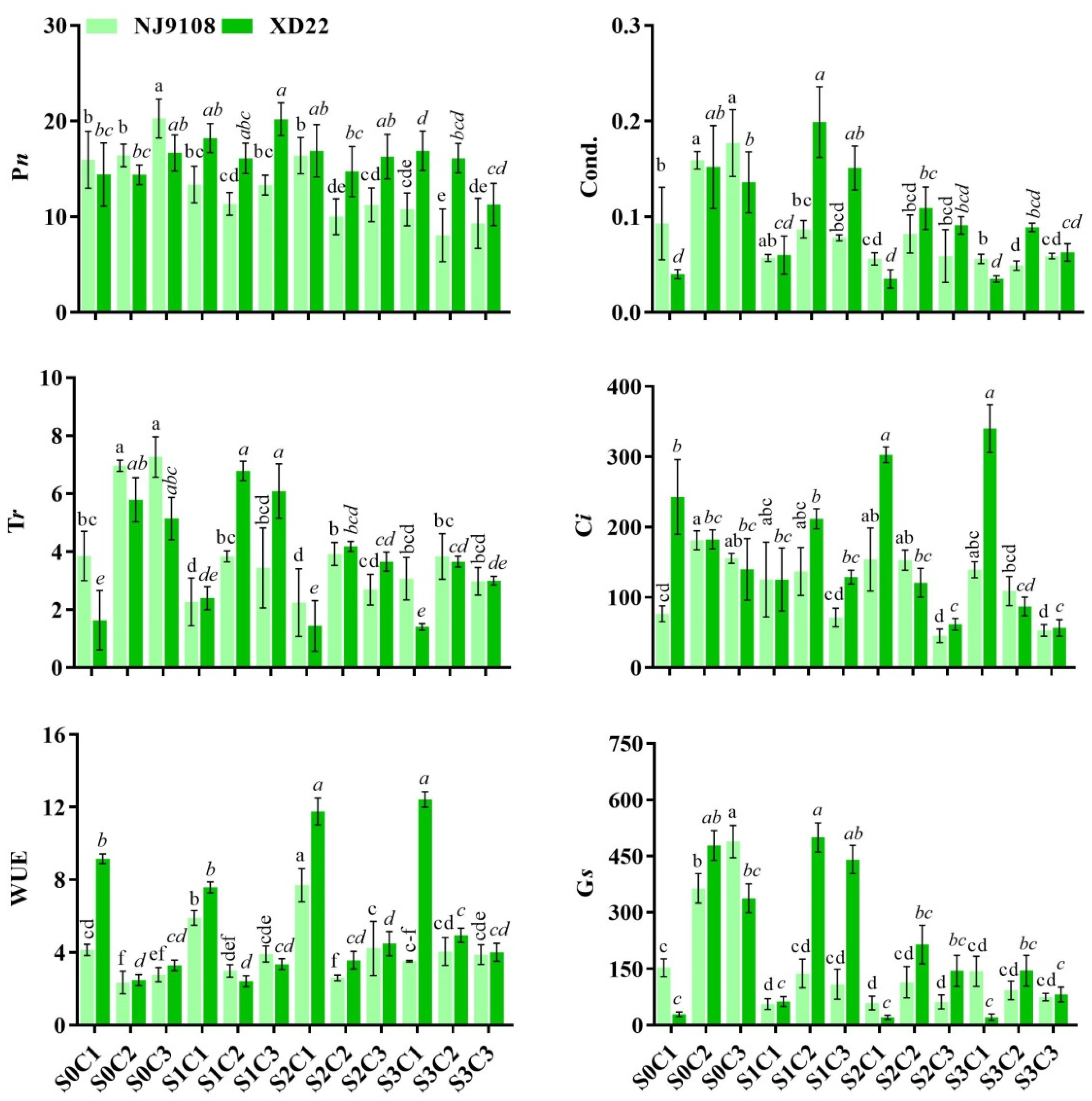
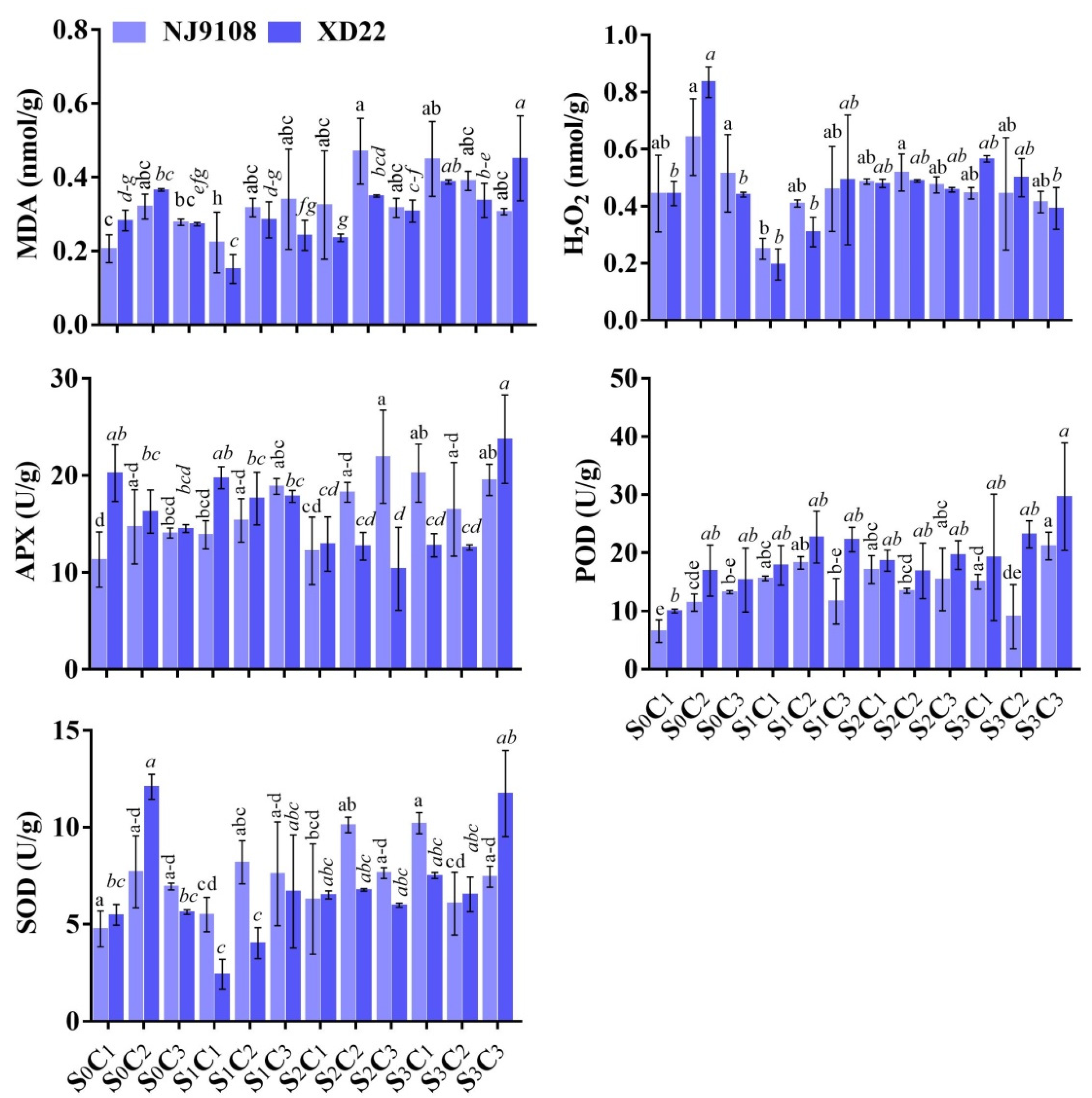
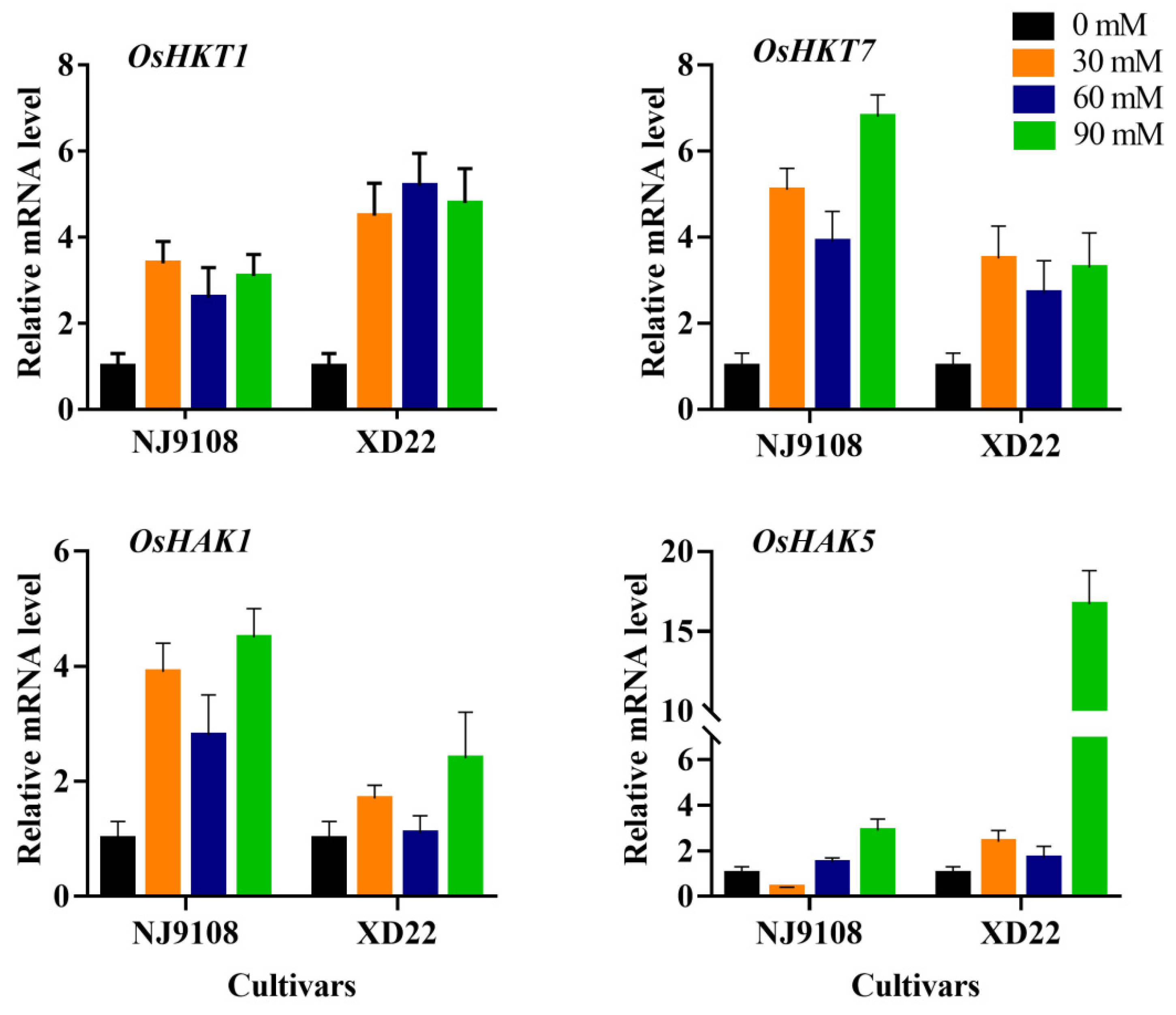

| Salinity | MeJA | PH (cm) | FLA | RL (cm) | FRW (g) | FLW (g) | StFW (g) | StTh (mm) | |
|---|---|---|---|---|---|---|---|---|---|
| NJ9108 | 0 | 0 | 85.01 a | 52.40 a | 35.2 a | 15.6 bc | 13.34 b | 37.96 b | 9.07 a |
| 125 | 74.01 bc | 52.94 a | 38.53 a | 26.25 a | 18.76 a | 54.83 a | 8.53 ab | ||
| 250 | 68.79 cd | 40.78 b | 37.53 a | 13.02 cd | 12.23 bc | 30.57 bcd | 8.03 abc | ||
| 30 | 0 | 77.78 b | 36.62 bc | 23.5 bc | 14.23 bc | 7.98 de | 21.66 efg | 7.95 a–d | |
| 125 | 72.24 c | 38.59 b | 23.53 bc | 17.7 b | 9.67 d | 36.53 bc | 7.58 b–e | ||
| 250 | 64.52 d | 27.81 d | 23.2 bc | 7.93 ef | 9.55 d | 16.72 ghi | 6.64 de | ||
| 60 | 0 | 72.35 c | 39.92 b | 31.00 ab | 12.11 cde | 9.99 d | 29.46 cde | 7.27 b–e | |
| 125 | 66.81d | 30.84 cd | 23.20 bc | 14.83 bc | 7.88 de | 25.70 def | 7.06 cde | ||
| 250 | 65.32 d | 24.40 de | 24.00 bc | 7.93 ef | 5.04 fg | 12.99 hi | 6.29 e | ||
| 90 | 0 | 37.89 e | 38.47 b | 21.83 c | 8.93 def | 6.71 ef | 17.80 fgh | 7.63 b–e | |
| 125 | 30.66 e | 15.27 f | 13.53 d | 2.07 g | 1.52 h | 11.86 hi | 8.53 ab | ||
| 250 | 38.63 f | 20.26 ef | 23.50 bc | 5.07 fg | 3.96 g | 9.04 i | 6.56 e | ||
| CV | 4.24 | 12.7 | 17.73 | 21.73 | 15.40 | 18.57 | 10.70 | ||
| XD22 | 0 | 0 | 82.02 a | 41.77 c | 35.10 bc | 28.04 a | 16.23 c | 53.74 ab | 8.73 ab |
| 125 | 79.73 a | 48.00 b | 46.00 a | 31.74 a | 20.37 a | 65.84 a | 8.99 a | ||
| 250 | 79.48 a | 52.86 a | 35.17 b | 17.35 b | 19.65 abc | 55.823 ab | 8.79 a | ||
| 30 | 0 | 71.94 b | 36.51 d | 31.57 bcd | 29.10 a | 9.66 d | 35.14 cd | 7.68 abc | |
| 125 | 72.27 b | 38.94 cd | 26.97 def | 18.73 b | 19.75 ab | 47.23 bc | 8.90 a | ||
| 250 | 68.56 b | 34.96 de | 27.70 cde | 17.94 b | 16.17 c | 45.23 bc | 7.85 abc | ||
| 60 | 0 | 60.52 cd | 30.49 fg | 19.17 gh | 6.16 cd | 5.06 e | 21.55 ef | 6.67 cd | |
| 125 | 63.22 c | 31.65 ef | 21.63 efg | 7.64 cd | 9.36 d | 33.22 de | 7.38 bc | ||
| 250 | 57.13 d | 27.13 gh | 19.67 fgh | 6.46 cd | 6.83 de | 24.81 def | 5.97 d | ||
| 90 | 0 | 38.17 e | 21.71 i | 25.60 d-g | 13.49 bc | 4.02 e | 23.09 def | 7.26 cd | |
| 125 | 36.89 e | 23.78 hi | 13.87 h | 5.58 d | 10.39 d | 21.99 ef | 7.06 cd | ||
| 250 | 36.63 e | 20.59 i | 22.20 efg | 4.64 d | 3.54 e | 14.85 f | 6.67 cd | ||
| CV | 4.86 | 7.67 | 16.18 | 28.75 | 17.93 | 17.78 | 10.41 |
| Salinity | MeJA | GLN/hill | YLN/hill | IFSpl/pan | FSS/pan | TSpl/pan | TSdS/pan | GW/pot (g) | ThGW (g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| NJ9108 | 0 | 0 | 29.66 b | 6 bc | 0.00 e | 119.00 a | 13.67 ab | 150.67 ab | 93.997 a | 25.35 ab |
| 125 | 37.66 a | 3 de | 0.00 e | 97.00 b | 14.00 a | 158.67 a | 63.397 b | 26.1 ab | ||
| 250 | 27.66 bcd | 3 de | 0.33 de | 107.67 ab | 13.67 ab | 165.33 a | 60.407 bc | 28.1 a | ||
| 30 | 0 | 27.33 bcd | 2.67 de | 0.00 e | 104.67 b | 11.33 abc | 130.67 b | 49.86 cd | 24.2 ab | |
| 125 | 29.00 bc | 2.33 de | 2.33 cd | 20.33 de | 9.33 cde | 101.00 c | 32.01 e | 23.6 ab | ||
| 250 | 21.00 d | 4.67 cd | 0.00 e | 64.33 c | 11.00 bcd | 96.00 cd | 38.43 de | 24.47 ab | ||
| 60 | 0 | 31.33 ab | 4.33 cd | 0.00 e | 31..33 d | 8.33 de | 82.00 cde | 16.58 f | 22.58 ab | |
| 125 | 21.33 d | 7.00 bc | 8.00 a | 1.67 f | 9.33 cde | 77.00 def | 3.13 g | 20.35 b | ||
| 250 | 21.00 d | 8.33 b | 6.00 ab | 7.33 ef | 9.00 cde | 65.67 efg | 9.82 fg | 13.38 c | ||
| 90 | 0 | 22.00 cd | 8.33 b | 3.33 c | 8.33 ef | 8.33 de | 52.67 gh | 6.29 fg | 0 d | |
| 125 | 03.00 f | 1.66 e | 6.67 ab | 0.00 f | 6.67 e | 33.00 h | 0.00 g | 0 d | ||
| 250 | 11.00 e | 13.00 a | 5.67 b | 3.33 f | 8.33 de | 61.00 fg | 2.11 g | 0 d | ||
| CV | 19.03 | 31.09 | 47.66 | 18.08 | 15.54 | 12.58 | 21.79 | 20.8 | ||
| XD22 | 0 | 0 | 43.00 bc | 3.67 cde | 0.00 d | 118.67 a | 13.00 a | 141.00 a | 92.21 a | 29.17 a |
| 125 | 47.67 ab | 7.00 bcd | 0.00 d | 70.67 bc | 11.33 ab | 95.33 bc | 80.13 b | 29.43 a | ||
| 250 | 53.00 a | 6.67 cde | 0.00 d | 80.67 b | 10.67 ab | 110.67 b | 88.55 ab | 28.67 ab | ||
| 30 | 0 | 32.33 de | 3.00 de | 0.00 d | 58.33 cd | 9.33 bcd | 75.67 cde | 45.82 c | 26.75 bc | |
| 125 | 36.67 cd | 2.33 e | 0.00 d | 39.00 e | 10.33 bc | 89.67 bcd | 39.89 c | 25.68 c | ||
| 250 | 52.00 ab | 8.00 bc | 1.00 cd | 44.33 de | 10.00 bc | 76.33 cde | 43.11 c | 25.88 c | ||
| 60 | 0 | 14.33 gh | 11.33 ab | 1.33 cd | 19.67 f | 8.00 cde | 59.33 ef | 4.68 d | 22.47 d | |
| 125 | 25.00 ef | 13.67 a | 5.67 b | 12.00 fg | 9.67 bcd | 66.33 def | 7.57 d | 12.9 e | ||
| 250 | 22.33 fg | 15.67 a | 4.00 bc | 9.67 fg | 7.33 de | 68.00 c–f | 3.07 d | 21.25 d | ||
| 90 | 0 | 15.67 gh | 5.67 cde | 5.67 b | 3.00 g | 7.33 de | 66.33 def | 0.73 d | 0 f | |
| 125 | 7.67 h | 7.67 bc | 11.67 a | 3.67 g | 6.00 e | 47.33 f | 1.49 d | 0 f | ||
| 250 | 9.67 h | 15.00 a | 6.00 b | 0.00 g | 11.67 ab | 51.67 ef | 0.00 d | 0 f | ||
| CV | 17.96 | 32.04 | 72.36 | 23.01 | 12.32 | 20.96 | 18.77 | 6.64 |
| Cultivars | NJ9108 | XD22 | |||||
|---|---|---|---|---|---|---|---|
| NaCl | MeJA(µM) | Chl.C | RWC | MSI | Chl.C | RWC | MSI |
| S0 | 0 | 42.59 a | 81.08 d | 83.76 a | 40.91 bc | 88.44 bc | 81.58 abc |
| 125 | 41.68 ab | 92.60 a | 87.68 a | 39.61 c | 94.54 a | 80.95 abc | |
| 250 | 41.97 ab | 83.65 cd | 85.89 a | 39.53 c | 92.99 ab | 83.96 ab | |
| S1 | 0 | 42.12 ab | 90.47 ab | 76.48 bc | 44.39 a | 92.62 ab | 87.62 a |
| 125 | 40.96 ab | 85.07 bcd | 81.85 ab | 41.94 abc | 69.25 e | 78.21 abc | |
| 250 | 43.94 a | 85.25 bcd | 87.45 a | 41.56 abc | 91.11 ab | 84.71 ab | |
| S2 | 0 | 38.03 b | 84.47 cd | 80.92 ab | 41.50 abc | 87.94 bc | 82.47 abc |
| 125 | 40.97 ab | 94.43 a | 73.06 c | 43.36 ab | 83.72 cd | 87.07 ab | |
| 250 | 43.13 a | 89.14 abc | 81.44 ab | 43.50 ab | 91.31 ab | 77.22 bc | |
| S3 | 0 | 41.18 ab | 92.09 a | 80.85 ab | 39.60 c | 93.79 a | 79.31 abc |
| 125 | 42.46 a | 92.97 a | 83.35 ab | 41.52 abc | 95.51 a | 80.36 abc | |
| 250 | 44.39 a | 82.16 d | 73.17 c | 36.02 d | 82.17 d | 73.17 c | |
| Salinity | ** | ** | *** | ** | * | ns | |
| MeJA | ** | ns | ns | ns | * | ns | |
| Salinity * MeJA | ** | ns | * | ns | * | ns | |
| CV | 3.4 | 4.58 | 5.37 | 4.76 | 3.38 | 7.27 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Zhang, R.; Liu, S.; Li, R.; Wang, Y.; Chen, Y.; Hou, H.; Dai, Q. Methyl Jasmonate Alleviates the Deleterious Effects of Salinity Stress by Augmenting Antioxidant Enzyme Activity and Ion Homeostasis in Rice (Oryza sativa L.). Agronomy 2022, 12, 2343. https://doi.org/10.3390/agronomy12102343
Hussain S, Zhang R, Liu S, Li R, Wang Y, Chen Y, Hou H, Dai Q. Methyl Jasmonate Alleviates the Deleterious Effects of Salinity Stress by Augmenting Antioxidant Enzyme Activity and Ion Homeostasis in Rice (Oryza sativa L.). Agronomy. 2022; 12(10):2343. https://doi.org/10.3390/agronomy12102343
Chicago/Turabian StyleHussain, Shahid, Rui Zhang, Shuli Liu, Rongkai Li, Yang Wang, Yinglong Chen, Hongyan Hou, and Qigen Dai. 2022. "Methyl Jasmonate Alleviates the Deleterious Effects of Salinity Stress by Augmenting Antioxidant Enzyme Activity and Ion Homeostasis in Rice (Oryza sativa L.)" Agronomy 12, no. 10: 2343. https://doi.org/10.3390/agronomy12102343
APA StyleHussain, S., Zhang, R., Liu, S., Li, R., Wang, Y., Chen, Y., Hou, H., & Dai, Q. (2022). Methyl Jasmonate Alleviates the Deleterious Effects of Salinity Stress by Augmenting Antioxidant Enzyme Activity and Ion Homeostasis in Rice (Oryza sativa L.). Agronomy, 12(10), 2343. https://doi.org/10.3390/agronomy12102343






