Morphology and Nitrogen Uptake and Distribution of Wheat Plants as Influenced by Applying Remedial Urea Prior to or Post Low-Temperature Stress at Seedling Stage
Abstract
:1. Introduction
2. Materials and Methods
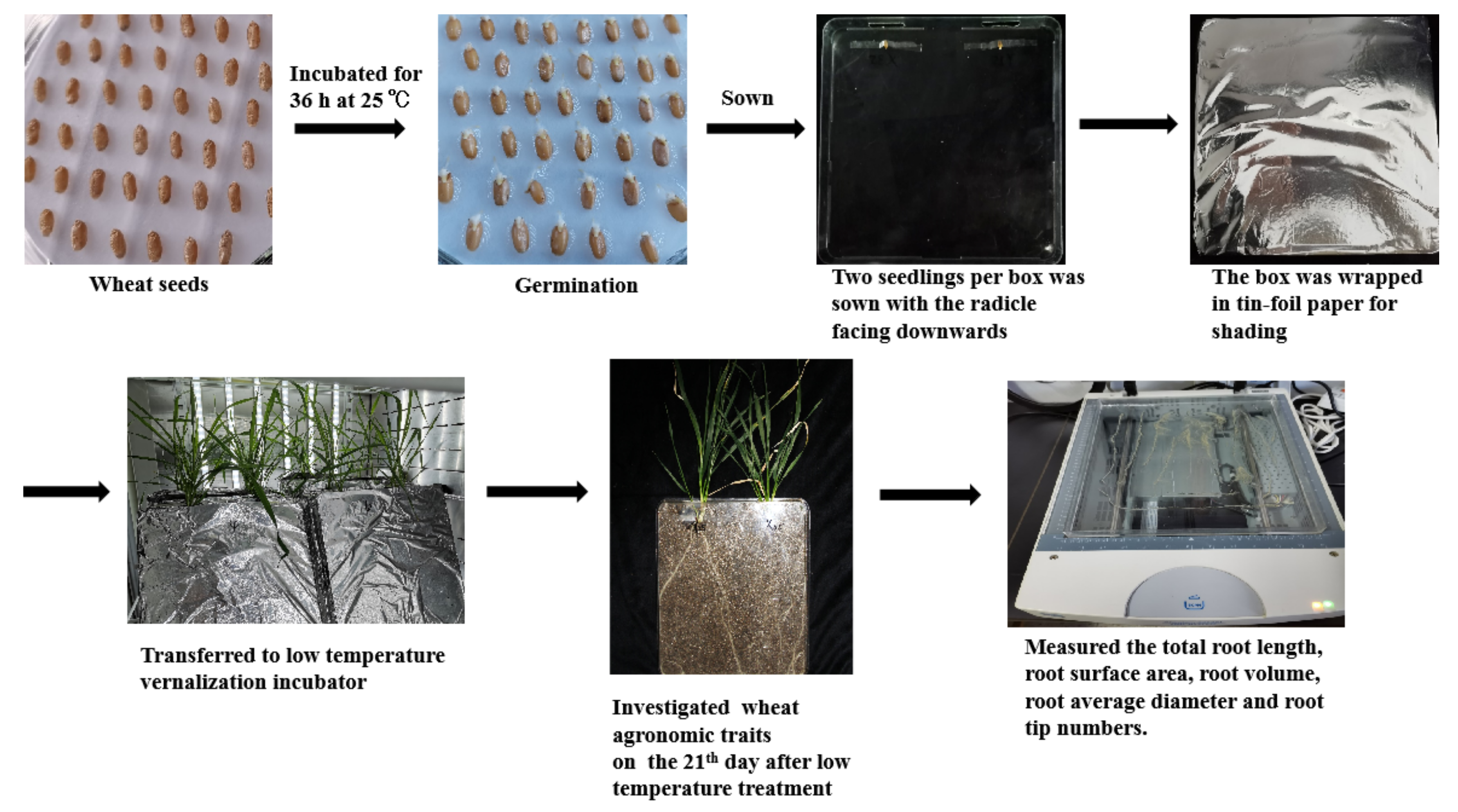
2.1. Materials and Growth Conditions
2.2. Experimental Design
2.3. Sampling and Measurement
2.3.1. SPAD Readings of Wheat Leaves
2.3.2. Agronomic Traits
2.3.3. Root Phenotype
2.3.4. Plant Nitrogen Content
2.4. Data Analysis
3. Results
3.1. Effects of Nitrogen Amendment Post or Prior to Low-Temperature Stress on SPAD Readings of Wheat Leaves
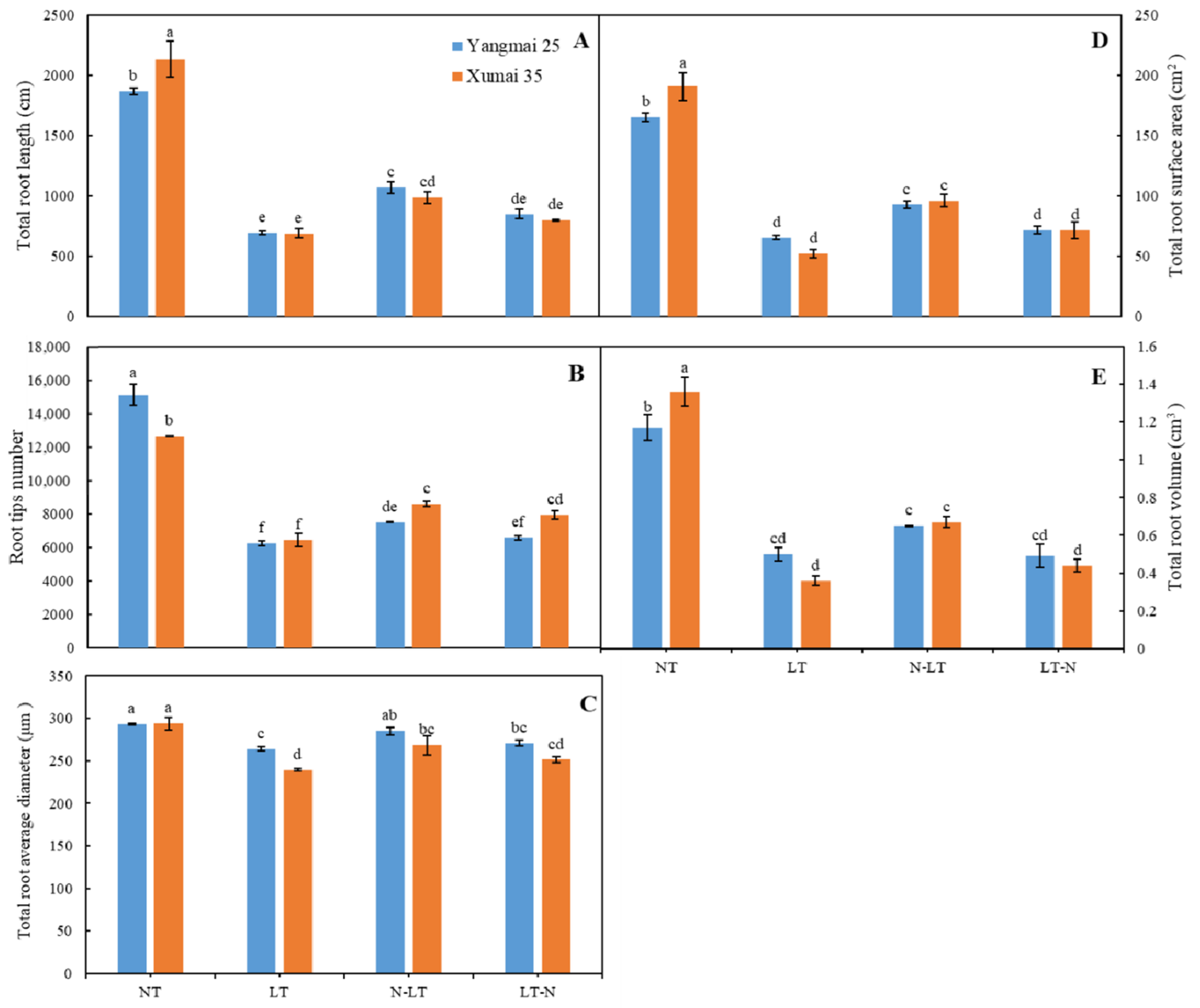
3.2. Effects of Nitrogen Amendment Post or Prior to Low-Temperature Stress on the Root Morphology of Wheat Plants
3.3. Effects of 15N Urea Amendment Post or Prior to Low-Temperature Stress on Nitrogen Accumulation and Distribution in Wheat Seedlings
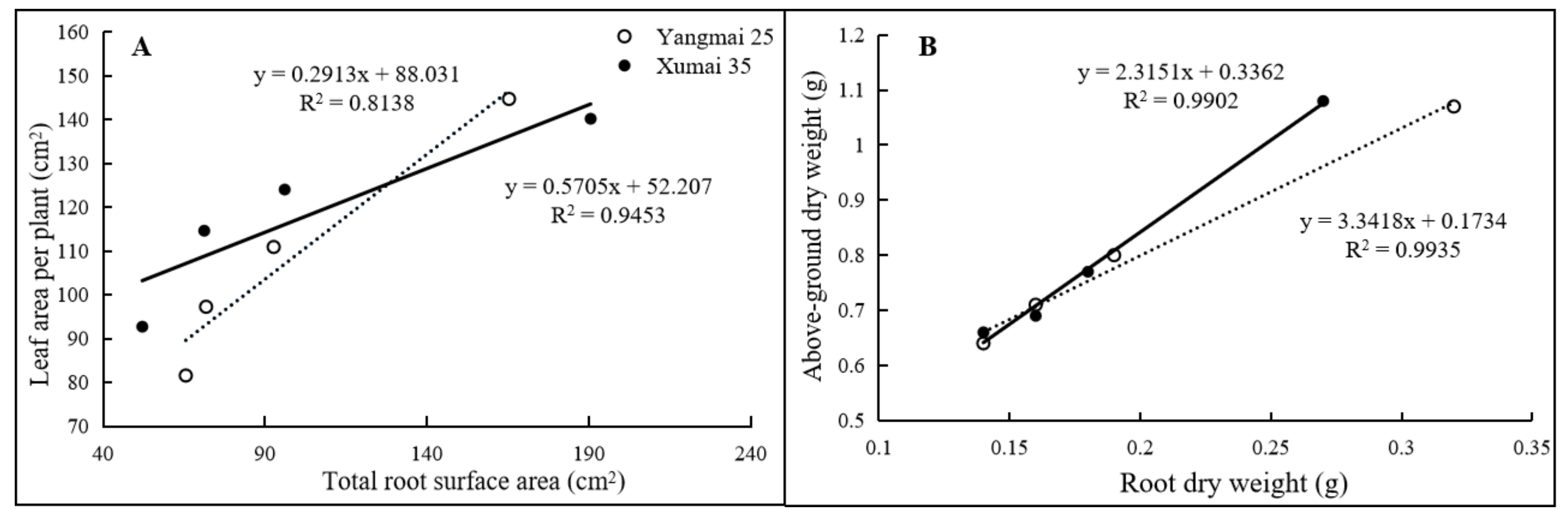
3.4. Effects of 15N Urea Amendment Prior to or Post Low-Temperature Stress on Agronomic Characteristics of Wheat Plant
4. Discussion
4.1. Response of Morphology to Remedial Nitrogen Prior to or Post Low-Temperature Stress at Seedling Stage
4.2. Changes in Nitrogen Uptake and Utilization with Remedial Fertilizer Prior to or Post Low-Temperature Stress at Wheat Seedling Stage
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asseng, S.; Foster, I.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Change Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAO Statistical Yearbook 2021; World Food and Agriculture: Rome, Italy, 2021. [Google Scholar]
- Zheng, B.; Chenu, K.; Dreccer, M.F.; Chapman, S.C. Breeding for the future: What are the potential impacts of future frost and heat events on sowing and flowering time requirements for Australian bread wheat (Triticum aestivium) varieties? Glob. Change Biol. 2012, 18, 2899–2914. [Google Scholar] [CrossRef] [PubMed]
- Licker, R.; Kucharik, C.J.; Dore, T.; Lindeman, M.J.; Makowski, D. Climatic impacts on winter wheat yields in Picardy, France and Rostov, Russia: 1973–2010. Agric. For. Meteorol. 2013, 176, 25–37. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Hakala, K.; Jauhiainen, L. Climate-induced overwintering challenges for wheat and rye in northern agriculture. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 75–83. [Google Scholar] [CrossRef]
- Zheng, D.; Yang, X.; Mínguez, M.I.; Mu, C.; He, Q.; Wu, X. Effect of freezing temperature and duration on winter survival and grain yield of winter wheat. Agric. For. Meteorol. 2018, 261, 1–8. [Google Scholar] [CrossRef]
- Yang, Q. Extended-range forecast for the low-frequency oscillation of temperature and low-temperature weather over the lower reaches of the Yangtze River in winter. Chin. J. Atmos. Sci. 2021, 45, 21–36. (In Chinese) [Google Scholar]
- Fuller, M.P.; Fuller, A.M.; Kaniouras, S.; Christophers, J.; Fredericks, T. The freezing characteristics of wheat at ear emergence. Eur. J. Agron. 2007, 26, 435–441. [Google Scholar] [CrossRef]
- Ji, H.T.; Xiao, L.J.; Xia, Y.M.; Song, H.; Liu, B.; Tang, L.; Cao, W.X.; Zhu, Y.; Liu, L.L. Effects of jointing and booting low temperature stresses on grain yield and yield components in wheat. Agric. For. Meteorol. 2017, 243, 33–42. [Google Scholar] [CrossRef]
- Yadav, S.K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef]
- Siddique1, M.R.B.; Hamid, A.; Islam, M.S. Drought stress effects on water relations of wheat. Bot. Bull. Acad. Sin. 2000, 41, 35–39. [Google Scholar]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Nagel, K.A.; Kastenholz, B.; Jahnke, S.; Dusschoten, V.D.; Aach, T.; Mühlich, M.; Truhn, D.; Scharr, H.; Terjung, S.; Walter, A.; et al. Temperature responses of roots: Impact on growth, root system architecture and implications for phenotyping. Funct. Plant Biol. 2009, 36, 947–959. [Google Scholar] [CrossRef]
- Hund, A.; Richner, W.; Soldati, A.; Fracheboud, Y.; Stamp, P. Root morphology and photosynthetic performance of maize inbred lines at low temperature. Eur. J. Agron. 2007, 27, 52–61. [Google Scholar] [CrossRef]
- Farooq, M.; Aziz, T.; Wahid, A.; Lee, D.; Siddique, K.H.M. Chilling tolerance in maize: Agronomic and physiological approaches. Crop Pasture Sci. 2009, 60, 501–516. [Google Scholar] [CrossRef]
- Jouyban, Z.; Hasanzade, R.; Sharafi, S. Chilling stress in plants. Int. J. Agric. Crop Sci. 2013, 5, 2961. [Google Scholar]
- Feng, X.; Xu, Y.Q.; Peng, L.; Yu, X.Y.; Zhao, Q.Q.; Feng, S.S.; Zhao, Z.Y.; Li, F.L.; Hu, B.Z. TaEXPB7-B, a β-expansin gene involved in low-temperature stress and abscisic acid responses, promotes growth and cold resistance in Arabidopsis thaliana. J. Plant Physiol. 2019, 240, 153004. [Google Scholar] [CrossRef]
- Hassan, M.A.; Chen, X.; Farooq, M.; Muhammad, N.; Zhang, Y.; Xu, H.; Ke, Y.Y.; Bruno, A.K.; Zhang, L.L.; Li, J.C. Cold stress in wheat: Plant acclimation responses and management strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef]
- Hou, M.Y. The QTL Mapping of Low Temperature Germinability and Anoxia Germination in Rice (Oryza aativa L.). Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2003; pp. 33–40. [Google Scholar]
- Kuk, Y.I.; Shin, J.S.; Burgos, N.R.; Hwang, T.E.; Han, O.; Cho, B.H.; Jung, S.; Guh, J.O. Antioxidative enzymes offer protection from chilling damage in rice plants. Crop Sci. 2003, 43, 2109–2117. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Li, C.Y.; Yang, J.; Zhang, Y.X.; Yao, M.H.; Zhu, X.K.; Guo, W.S. Retrieval effects of remedial fertilizer after freeze injury on wheat yield and its mechanism at tillering stage. Sci. Agric. Sin. 2017, 50, 1781–1791. (In Chinese) [Google Scholar]
- Li, C.Y.; Yang, J.; Zhu, M.; Ding, J.F.; Zhu, X.K.; Zhou, G.S.; Guo, W.S. Urea amendment alleviated morphological and physiological damages and yield loss of winter wheat subjected to low temperature stress at jointing stage. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Liao, M.; Palta, J.A.; Fillery, I.R.P. Root characteristics of vigorous wheat improve early nitrogen uptake. Aust. J. Agric. Res. 2006, 57, 1097–1107. [Google Scholar] [CrossRef]
- Equiza, M.; Mirave, J.P.; Tognetti, J.A. Different inhibition of shoot vs. root growth at low temperature and its relationship with carbohydrate accumulation in different wheat cultivars. Ann. Bot. 1997, 5, 657–663. [Google Scholar] [CrossRef]
- Sebastian, N.; Erika, H.; Christian, K. Critically low soil temperatures for root growth and root morphology in three alpine plant species. Alpine Bot. 2016, 126, 11–22. [Google Scholar] [CrossRef]
- Buriro, M.; Oad, F.C.; Keerio, M.I.; Tunio, S.; Gandahi, A.W.; Hassan, S.W.; Oad, S.M. Wheat seed germination under the influence of temperature regimes. Sarhad J. Agric. 2011, 27, 539–543. [Google Scholar]
- Vasil′eva, G.G.; Mironova, N.V.; Glyan′ko, A.K. Peculiarities of N nutrition of spring wheat under the impact of unfavorable temperature when fertilized with urea. Agrokhimiya 2002, 9, 11–16. [Google Scholar]
- Wei, D.; Ning, S.; Lin, W. Relationship between wheat root activity and leaf senescence. Chin. J. Appl. Ecol. 2004, 9, 1565–1569. (In Chinese) [Google Scholar]
- Rinalducci, S.; Egidi, M.G.; Karimzadeh, G.; Jazii, F.R.; Zolla, L. Proteomic analysis of a spring wheat cultivar in response to prolonged cold stress. Electrophoresis 2011, 32, 1807–1818. [Google Scholar] [CrossRef]
- Janowiak, F.; Maas, B.; Dörffling, K. Importance of abscisic acid for chilling tolerance of maize seedlings. J. Plant Physiol. 2002, 159, 635–643. [Google Scholar] [CrossRef]
- Xu, H.; Wu, Z.; Xu, B.; Sun, D.; Hassan, M.A.; Cai, H.; Wu, Y.; Yu, M.; Chen, A.; Li, J.; et al. Optimized phosphorus application alleviated adverse effects of short-term low-temperature stress in winter wheat by enhancing photosynthesis and improved accumulation and partitioning of dry matter. Agronomy 2022, 12, 1700. [Google Scholar] [CrossRef]
- Kato, M.C.; Hikosaka, K.; Hirotsu, N.; Makino, A.; Hirose, T. The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. Plant Cell Physiol. 2003, 44, 318–325. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, W.; Liu, D.; Yue, S.; Cui, Z.; Chen, X.; Zou, C. Effects of nitrogen management on root morphology and zinc translocation from root to shoot of winter wheat in the field. Field Crop Res. 2014, 161, 38–45. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Khan, H.; Munsif, F.; Nie, L. Ascorbic acid priming enhances seed germination and seedling growth of winter wheat under low temperature due to late sowing in Pakistan. Agronomy 2019, 9, 757. [Google Scholar] [CrossRef]
- Effah, Z.; Li, L.; Xie, J.; Karikari, B.; Wang, J.; Zeng, M.; Wang, L.; Boamah, S.; Shanthi, J.P. Post-anthesis relationships between nitrogen isotope discrimination and yield of spring wheat under different nitrogen levels. Front Plant Sci. 2022, 13, 859655. [Google Scholar] [CrossRef]
- Azam, F.; Lodhi, A.; Farooq, S. Response of flooded rice (Oryza sativa L.) to nitrogen application at two root-zone temperature regimes in a pot experiment. Biol. Fert. Soils 2003, 38, 21–25. [Google Scholar]
- Hu, C.; Yu, J.; Sun, S.; Yan, Y.; Guo, H.; Tan, Z.; Jiang, D.; Cao, W.; Dai, T. Reduced 15N losses by winter and spring night-warming are related to root distribution of winter wheat. Front Plant Sci. 2019, 10, 771. [Google Scholar] [CrossRef]
- Gorsuch, P.A.; Pandey, S.; Atkin, O.K. Temporal heterogeneity of cold acclimation phenotypes in arabidopsis leaves. Plant Cell Environ. 2010, 33, 244–258. [Google Scholar] [CrossRef]
- Atkinson, L.J.; Sherlock, D.J.; Atkin, O.K. Source of nitrogen associated with recovery of relative growth rate in arabidopsis thaliana acclimated to sustained cold treatment. Plant Cell Environ. 2015, 38, 1023–1034. [Google Scholar] [CrossRef]
- Matić, M.; Vuković, R.; Vrandečić, K.; Čamagajevac, I.Š.; Ćosić, J.; Vulović, A.; Sabljić, K.; Sabo, N.; Dvojković, K.; Novoselović, D. Oxidative status and antioxidative response to fusarium attack and different nitrogen levels in winter wheat varieties. Plants 2021, 10, 611. [Google Scholar] [CrossRef]

| Cultivar | Treatment | Days Post Low-Temperature Stress | |||
|---|---|---|---|---|---|
| 0 d | 3 d | 8 d | 14 d | ||
| Yangmai 25 | NT | 44.80 bc | 45.11 b | 46.00 cd | 56.50 a |
| LT | 41.26 e | 43.04 c | 44.96 d | 54.17 b | |
| N-LT | 43.37 d | 45.10 b | 46.86 bcd | 54.79 ab | |
| LT-N | 41.04 e | 44.01 bc | 47.40 bc | 55.10 ab | |
| Xumai 35 | NT | 46.23 a | 46.89 a | 47.20 bc | 55.20 a |
| LT | 43.66 cd | 44.31 bc | 46.51 bcd | 50.89 b | |
| N-LT | 45.16 ab | 46.59 a | 48.31 b | 53.63 ab | |
| LT-N | 43.91 cd | 47.36 a | 50.10 a | 54.81 a | |
| Cultivar | Treatment | Nitrogen Accumulation (mg/plant) | Percentage of Total Nitrogen from 15N Urea (%) | 15N Accumulation (mg/plant) | 15N Absorption Rate (μg/d) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Root | Stem | Leaf | Root | Stem | Leaf | Root | Stem | Leaf | ||
| Yangmai 25 | N-LT | 3.7 a | 11.3 a | 26.1 a | 12.8 b | 11.2 b | 13.8 b | 0.5 a | 1.3 b | 3.6 b | 17.0 a | 45.2 b | 128.8 b |
| LT-N | 3.0 b | 10.3 b | 23.9 b | 0.6 c | 0.4 c | 0.4 c | 0.02 b | 0.1 c | 0.1 c | 0.9 b | 2.1 c | 4.6 c | |
| Xumai 35 | N-LT | 3.0 b | 9.2 c | 22.9 c | 16.7 a | 18.4 a | 21.5 a | 0.5 a | 1.7 a | 4.9 a | 17.9 a | 60.7 a | 176.1 a |
| LT-N | 2.9 b | 9.5 c | 20.6 d | 0.6 c | 0.5 c | 0.4 c | 0.02 b | 0.1 c | 0.1 c | 0.8 b | 2.4 c | 3.5 c | |
| Cultivar | Treatment | Leaf Area per Plant (cm2) | Leaf Area Change (%) | Tiller Number per Plant | Tiller Number Change (%) | Seedling Height (cm) | Seedling Height Change (%) | Above- Ground Dry Weight (g) | Above- Ground Dry Weight Change (%) | Root Dry Weight (g) | Root Dry Weight Change (%) | Ratio of Root to Shoot (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yangmai 25 | NT | 144.7 a | — | 10.2 a | — | 32.9 a | — | 1.1 a | — | 0.3 a | — | 29.6 a |
| LT | 81.6 d | −43.6 | 5.4 d | −47.1 | 27.6 c | −16.1 | 0.6 d | −40.2 | 0.1 e | −56.3 | 22.3 bc | |
| N-LT | 110.9 bc | −23.4 | 7.8 bc | −23.5 | 30.8 b | −6.3 | 0.8 b | −25.3 | 0.2 c | −40.6 | 24.4 b | |
| LT-N | 97.3 cd | −37.8 | 6.2 cd | −39.2 | 29.6 b | −10.0 | 0.7 bcd | −33.6 | 0.2 de | −50.0 | 22.7 bc | |
| Xumai 35 | NT | 140.2 a | — | 9.4 ab | — | 26.9 cd | — | 1.1 a | — | 0.3 b | — | 25.2 b |
| LT | 92.7 cd | −33.8 | 5.6 d | −40.4 | 23.1 f | −14.2 | 0.7 cd | −38.9 | 0.1 e | −48.2 | 21.1 c | |
| N-LT | 124.0 ab | −11.5 | 8.0 bc | −14.9 | 25.6 de | −4.8 | 0.8 bc | −28.7 | 0.2 cd | −33.3 | 23.3 bc | |
| LT-N | 114.6 bc | −18.2 | 7.2 cd | −23.4 | 24.3 ef | −9.7 | 0.7 bcd | −36.1 | 0.2 de | −40.7 | 22.4 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Liu, M.; Dai, C.; Zhu, Y.; Zhu, M.; Ding, J.; Zhu, X.; Zhou, G.; Guo, W. Morphology and Nitrogen Uptake and Distribution of Wheat Plants as Influenced by Applying Remedial Urea Prior to or Post Low-Temperature Stress at Seedling Stage. Agronomy 2022, 12, 2338. https://doi.org/10.3390/agronomy12102338
Li C, Liu M, Dai C, Zhu Y, Zhu M, Ding J, Zhu X, Zhou G, Guo W. Morphology and Nitrogen Uptake and Distribution of Wheat Plants as Influenced by Applying Remedial Urea Prior to or Post Low-Temperature Stress at Seedling Stage. Agronomy. 2022; 12(10):2338. https://doi.org/10.3390/agronomy12102338
Chicago/Turabian StyleLi, Chunyan, Mingmin Liu, Cunhu Dai, Yangyang Zhu, Min Zhu, Jinfeng Ding, Xinkai Zhu, Guisheng Zhou, and Wenshan Guo. 2022. "Morphology and Nitrogen Uptake and Distribution of Wheat Plants as Influenced by Applying Remedial Urea Prior to or Post Low-Temperature Stress at Seedling Stage" Agronomy 12, no. 10: 2338. https://doi.org/10.3390/agronomy12102338
APA StyleLi, C., Liu, M., Dai, C., Zhu, Y., Zhu, M., Ding, J., Zhu, X., Zhou, G., & Guo, W. (2022). Morphology and Nitrogen Uptake and Distribution of Wheat Plants as Influenced by Applying Remedial Urea Prior to or Post Low-Temperature Stress at Seedling Stage. Agronomy, 12(10), 2338. https://doi.org/10.3390/agronomy12102338










