Effect of Ratios of Red and White Light on the Growth and Quality of Pak Choi
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions and Treatments
2.2. Plant Growth
2.3. Photosynthetic Parameters
2.3.1. Chlorophyll Fluorescence Measurements
2.3.2. Chlorophyll (Chl) and Carotenoid Contents
2.4. Nutrients
2.4.1. Soluble Sugar Content
2.4.2. Nitrate Content
2.4.3. Soluble Protein Content
2.4.4. Vitamin C Content
2.5. Antioxidants
2.5.1. Polyphenol Content
2.5.2. Flavonoid Content
2.5.3. Ferric-Reducing Antioxidant Power
2.5.4. DPPH Radical-Scavenging Rate
2.5.5. Glucosinolate Content Determination
2.6. Statistical Analysis
3. Results
3.1. The Effect of Red and White Light Ratios on Pak Choi Growth
3.2. The Effect of Red and White Light Ratios on Pak choi Photosynthetic Capacity
3.3. The Effect of Red and White Light Ratios on Pak Choi Nutrition
3.4. The Effect of Red and White Light Ratios on Pak Choi Antioxidant Capacity
3.5. The Effect of Red and White Light Ratios on Pak Choi Glucosinolate Content
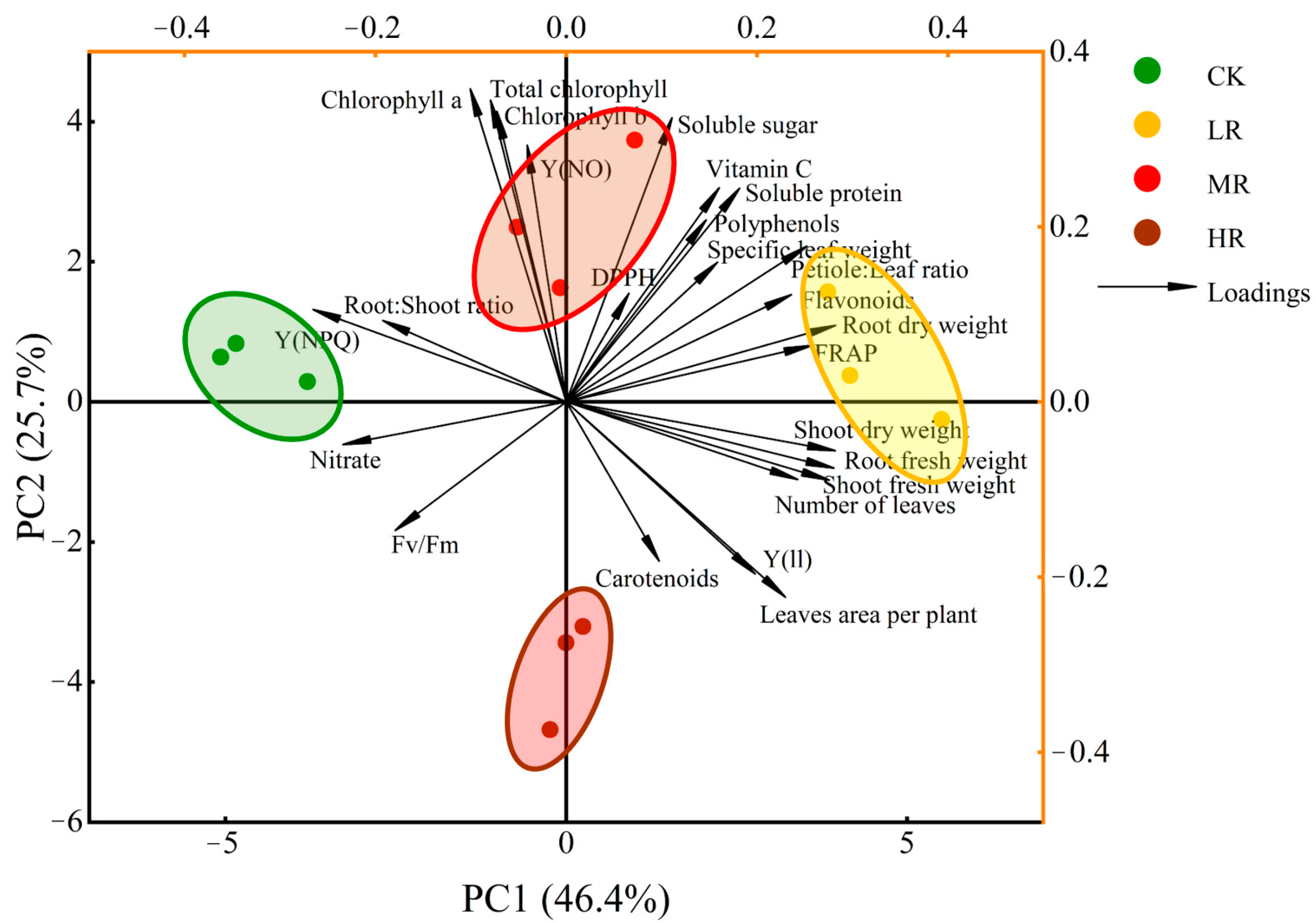
3.6. Heatmap Analysis
3.7. Multivariate Principal Component Analysis
4. Discussion
4.1. Different Red and White Light Ratios Affected Growth of Pak Choi
4.2. Different Red and White Light Ratios Affected Photosynthetic Capacity of Pak Choi
4.3. Different Red and White Light Ratios Affected Nutrition of Pak Choi
4.4. Different Red and White Light Ratios Affected Antioxidant Content and Activity of Pak Choi
4.5. Different Red and White Light Ratios Affected Glucosinolate Content of Pak Choi
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic.-Amst. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). J. Agric. Sci.-Camb. 2012, 4, 262. [Google Scholar] [CrossRef]
- Ouzounis, T.; Heuvelink, E.; Ji, Y.; Schouten, H.J.; Visser, R.G.F.; Marcelis, L.F.M. Blue and red LED lighting effects on plant biomass, stomatal conductance, and metabolite content in nine tomato genotypes. In Proceedings of the VIII International Symposium on Light in Horticulture, East Lansing, MI, USA, 22–26 May 2016; Volume 1134, pp. 251–258. [Google Scholar]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Yang, Q.; Li, T. Red/blue light ratio strongly affects steady-state photosynthesis, but hardly affects photosynthetic induction in tomato (Solanum lycopersicum). Physiol. Plant. 2019, 167, 144–158. [Google Scholar] [CrossRef]
- Son, K.-H.; Park, J.-H.; Kim, D.; Oh, M.-M. Leaf shape index, growth, and phytochemicals in two leaf lettuce cultivars grown under monochromatic light-emitting diodes. Hortic. Sci. Technol. 2012, 30, 664–672. [Google Scholar]
- Signore, A.; Bell, L.; Santamaria, P.; Wagstaff, C.; Van Labeke, M.-C. Red light is effective in reducing nitrate concentration in rocket by increasing nitrate reductase activity, and contributes to increased total glucosinolates content. Front. Plant. Sci. 2020, 11, 604. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of blue and red LED light irradiation on β-cryptoxanthin accumulation in the flavedo of citrus fruits. J. Agric. Food Chem. 2012, 60, 197–201. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Ren, J.; Guo, S.; Xu, C.; Yang, C.; Ai, W.; Tang, Y.; Qin, L. Effects of different carbon dioxide and LED lighting levels on the anti-oxidative capabilities of Gynura bicolor DC. Adv. Space Res. 2014, 53, 353–361. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant. Growth Regul. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.N.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Vitale, E.; Velikova, V.; Tsonev, T.; Costanzo, G.; Paradiso, R.; Arena, C. Manipulation of light quality is an effective tool to regulate photosynthetic capacity and fruit antioxidant properties of Solanum lycopersicum L. cv. ‘Microtom’ in a controlled environment. PeerJ 2022, 10, e13677. [Google Scholar] [CrossRef]
- Vitale, L.; Vitale, E.; Guercia, G.; Turano, M.; Arena, C.J.P. Effects of different light quality and biofertilizers on structural and physiological traits of spinach plants. Photosynthetica 2020, 58, 932–943. [Google Scholar] [CrossRef]
- Mao, P.; Duan, F.; Zheng, Y.; Yang, Q. Blue and UV-A light wavelengths positively affected accumulation profiles of healthy compounds in pak-choi. J. Sci. Food Agric. 2021, 101, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Kaviraj, M.; Rout, S.; Chakraborty, K.; Swain, P.; Nayak, P.K.; Nayak, A.K. Combined application of ascorbic acid and endophytic N-fixing Azotobacter chroococcum Avi2 modulates photosynthetic efficacy, antioxidants and growth-promotion in rice under moisture deficit stress. Microbiol. Res. 2021, 250, 126808. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L. A non-destructive method to determine chlorophyll content of leaves. Photosynthetica 1993, 26, 469–473. [Google Scholar]
- Kohyama, K.; Nishinari, K. Effect of soluble sugars on gelatinization and retrogradation of sweet potato starch. J. Agric. Food Chem. 1991, 39, 1406–1410. [Google Scholar] [CrossRef]
- Cataldo, D.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Blakesley, R.W.; Boezi, J.A. A new staining technique for proteins in polyacrylamide gels using Coomassie Brilliant Blue G250. Anal. Biochem. 1977, 82, 580–582. [Google Scholar] [CrossRef]
- Chen, G.; Mo, L.; Li, S.; Zhou, W.; Wang, H.; Liu, J.; Yang, C. Separation and determination of reduced vitamin C in polymerized hemoglobin-based oxygen carriers of the human placenta. Artif. Cell Nanomed. B 2015, 43, 152–156. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Xie, Y.; Zheng, Y.; Dai, X.; Wang, Q.; Cao, J.; Xiao, J. Seasonal dynamics of total flavonoid contents and antioxidant activity of Dryopteris erythrosora. Food Chem. 2015, 186, 113–118. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tadolini, B.; Juliano, C.; Piu, L.; Franconi, F.; Cabrini, L. Resveratrol inhibition of lipid peroxidation. Free Radic. Res. 2000, 33, 105–114. [Google Scholar] [CrossRef]
- Liu, K.; Gao, M.; Jiang, H.; Ou, S.; Li, X.; He, R.; Li, Y.; Liu, H.J.M. Light Intensity and Photoperiod Affect Growth and Nutritional Quality of Brassica Microgreens. Molecules 2022, 27, 883. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; He, Y.; Xia, R. TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv 2018, 289660. [Google Scholar] [CrossRef]
- Quian-Ulloa, R.; Stange, C. Carotenoid biosynthesis and plastid development in plants: The role of light. Int. J. Mol. Sci. 2021, 22, 1184. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zang, J.; Xu, Z.; Guo, S.; Jiao, X.; Liu, X.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of ascorbate accumulation and metabolism in lettuce by the red: Blue ratio of continuous light using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef]
- Zheng, Y.-J.; Zhang, Y.-T.; Liu, H.-C.; Li, Y.-M.; Liu, Y.-L.; Hao, Y.-W.; Lei, B.-F. Supplemental blue light increases growth and quality of greenhouse pak choi depending on cultivar and supplemental light intensity. J. Integr. Agric. 2018, 17, 2245–2256. [Google Scholar] [CrossRef]
- Amagai, Y.; Lu, N.; Hayashi, E.; Takagaki, M.; Kikuchi, M.; Ibaraki, Y.; Kozai, T. External green light as a new tool to change colors and nutritional components of inner leaves of head cabbages. J. Food Meas. Charact. 2022, 16, 269–280. [Google Scholar] [CrossRef]
- Li, Y.; Liu, N.; Ji, F.; He, D. Optimal red: Blue ratio of full spectrum LEDs for hydroponic pakchoi cultivation in plant factory. Int. J. Agric. Biol. Eng. 2022, 15, 72–77. [Google Scholar]
- Samuolienė, G.; Viršilė, A.; Haimi, P.; Miliauskienė, J. Photoresponse to different lighting strategies during red leaf lettuce growth. J. Photochem. Photobiol. B 2020, 202, 111726. [Google Scholar] [CrossRef]
- Degni, B.F.; Haba, C.T.; Dibi, W.G.; Soro, D.; Zoueu, J.T. Effect of light spectrum on growth, development, and mineral contents of okra (Abelmoschus esculentus L.). Open Agric. 2021, 6, 276–285. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kjaer, K.H.; Adnan, M.; Naznin, M.T.; Lim, J.D.; Sung, I.J.; Park, C.H.; Lim, Y.S. The evaluation of growth performance, photosynthetic capacity, and primary and secondary metabolite content of leaf lettuce grown under limited irradiation of blue and red LED light in an urban plant factory. Agriculture 2020, 10, 28. [Google Scholar] [CrossRef]
- Meng, Q.; Boldt, J.; Runkle, E.S. Blue radiation interacts with green radiation to influence growth and predominantly controls quality attributes of lettuce. J. Am. Soc. Hortic. Sci. 2020, 145, 75–87. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F. Adding blue to red supplemental light increases biomass and yield of greenhouse-grown tomatoes, but only to an optimum. Front. Plant Sci 2019, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Kowallik, W. Blue light effects on respiration. Plant Biol. 1982, 33, 51–72. [Google Scholar] [CrossRef]
- Matysiak, B.; Kowalski, A. White, blue and red LED lighting on growth, morphology and accumulation of flavonoid compounds in leafy greens. Zemdirbyste 2019, 106, 281–286. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Jiang, H.; He, R.; Song, S.; Su, W.; Liu, H. Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory. Molecules 2021, 26, 7405. [Google Scholar] [CrossRef]
- Hong, G.-J.; Hu, W.-L.; Li, J.-X.; Chen, X.-Y.; Wang, L.-J. Increased accumulation of artemisinin and anthocyanins in Artemisia annua expressing the Arabidopsis blue light receptor CRY1. Plant Mol. Biol. Rep. 2009, 27, 334–341. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of red and blue light on anthocyanin accumulation and differential gene expression in strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Klopsch, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep.-UK 2017, 7, 40807. [Google Scholar] [CrossRef] [PubMed]
- Mickens, M.A.; Torralba, M.; Robinson, S.A.; Spencer, L.E.; Romeyn, M.W.; Massa, G.D.; Wheeler, R.M. Growth of red pak choi under red and blue, supplemented white, and artificial sunlight provided by LEDs. Sci. Hortic.-Amst. 2019, 245, 200–209. [Google Scholar] [CrossRef]
- Wiesner, M.; Zrenner, R.; Krumbein, A.; Glatt, H.; Schreiner, M. Genotypic variation of the glucosinolate profile in pak choi (Brassica rapa ssp. chinensis). J. Agric. Food Chem. 2013, 61, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.A.; Sams, C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef]
- Abe, K.; Kido, S.; Maeda, T.; Kami, D.; Matsuura, H.; Shimura, H.; Suzuki, T. Glucosinolate profiles in Cardamine fauriei and effect of light quality on glucosinolate concentration. Sci. Hortic.-Amst. 2015, 189, 12–16. [Google Scholar] [CrossRef]
- Lee, G.-J.; Heo, J.-W.; Jung, C.-R.; Kim, H.-H.; Jo, J.-S.; Lee, J.-G.; Lee, G.-J.; Nam, S.-Y.; Hong, E.-Y. Effects of artificial light sources on growth and glucosinolate contents of hydroponically grown kale in plant factory. J. Bio-Environ. Control 2016, 25, 77–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, J.; Song, S.; Su, W.; Liu, H. Growth, nutritional quality and health-promoting compounds in Chinese Kale grown under different ratios of red: Blue LED lights. Agronomy 2020, 10, 1248. [Google Scholar] [CrossRef]
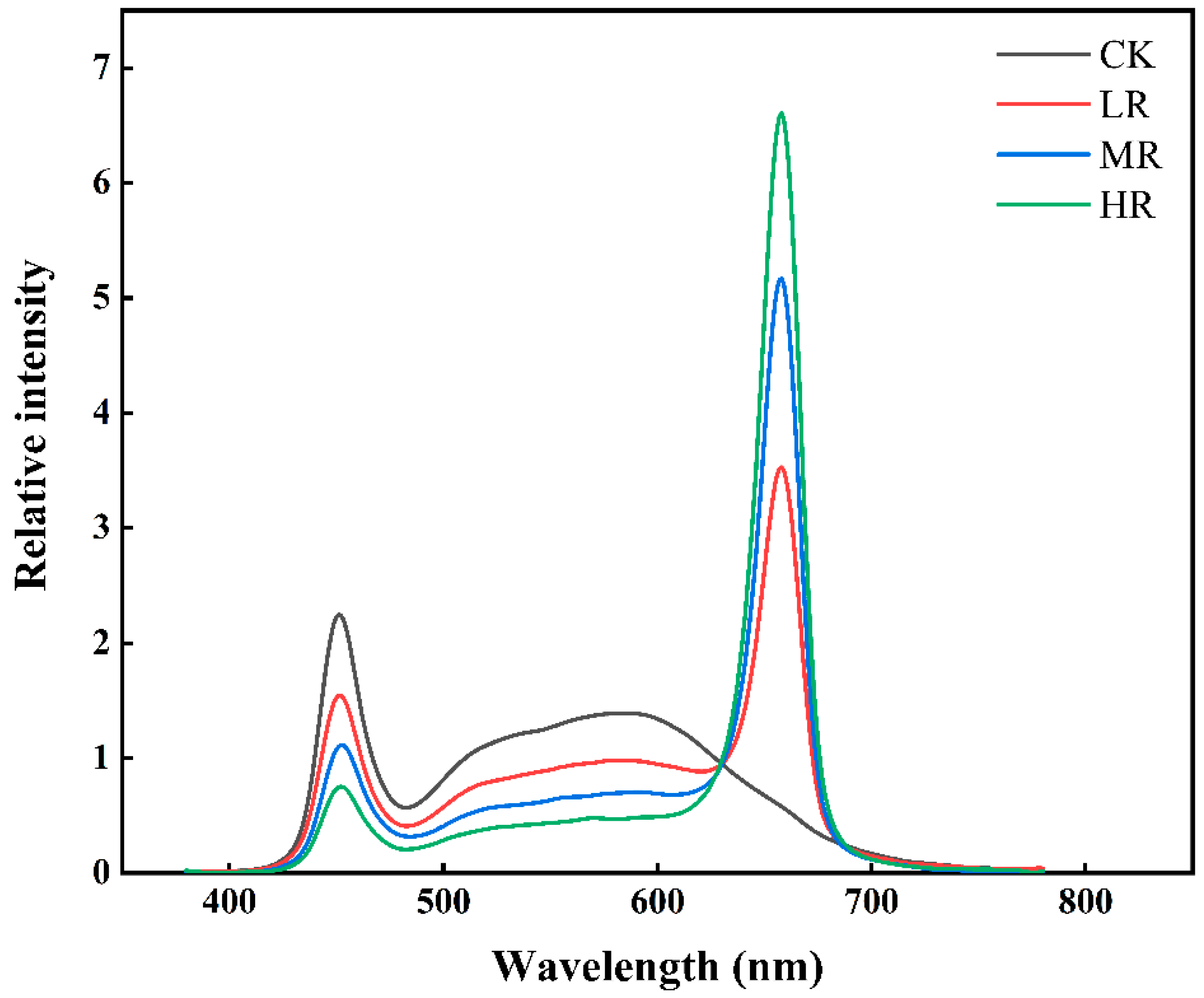
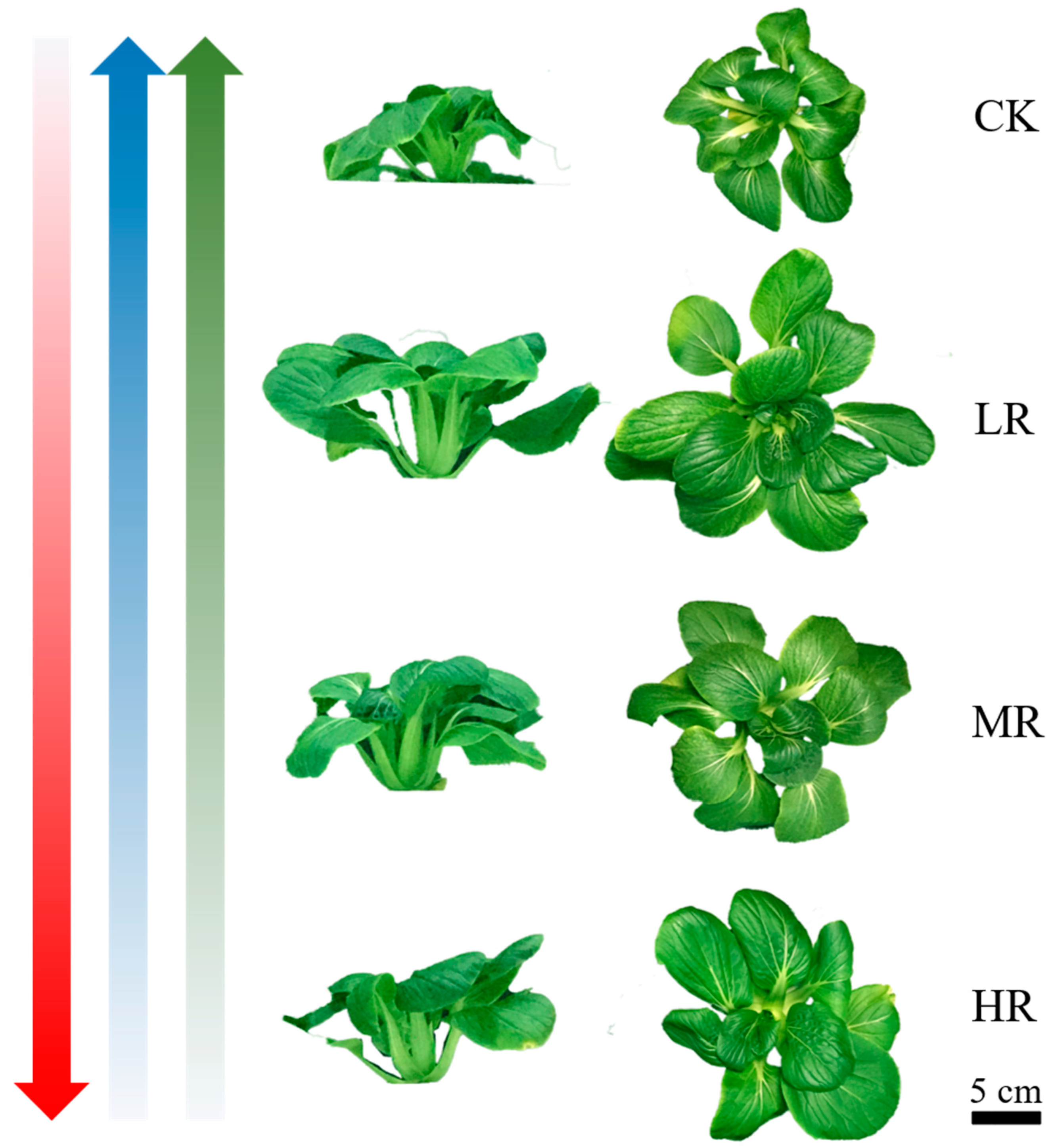

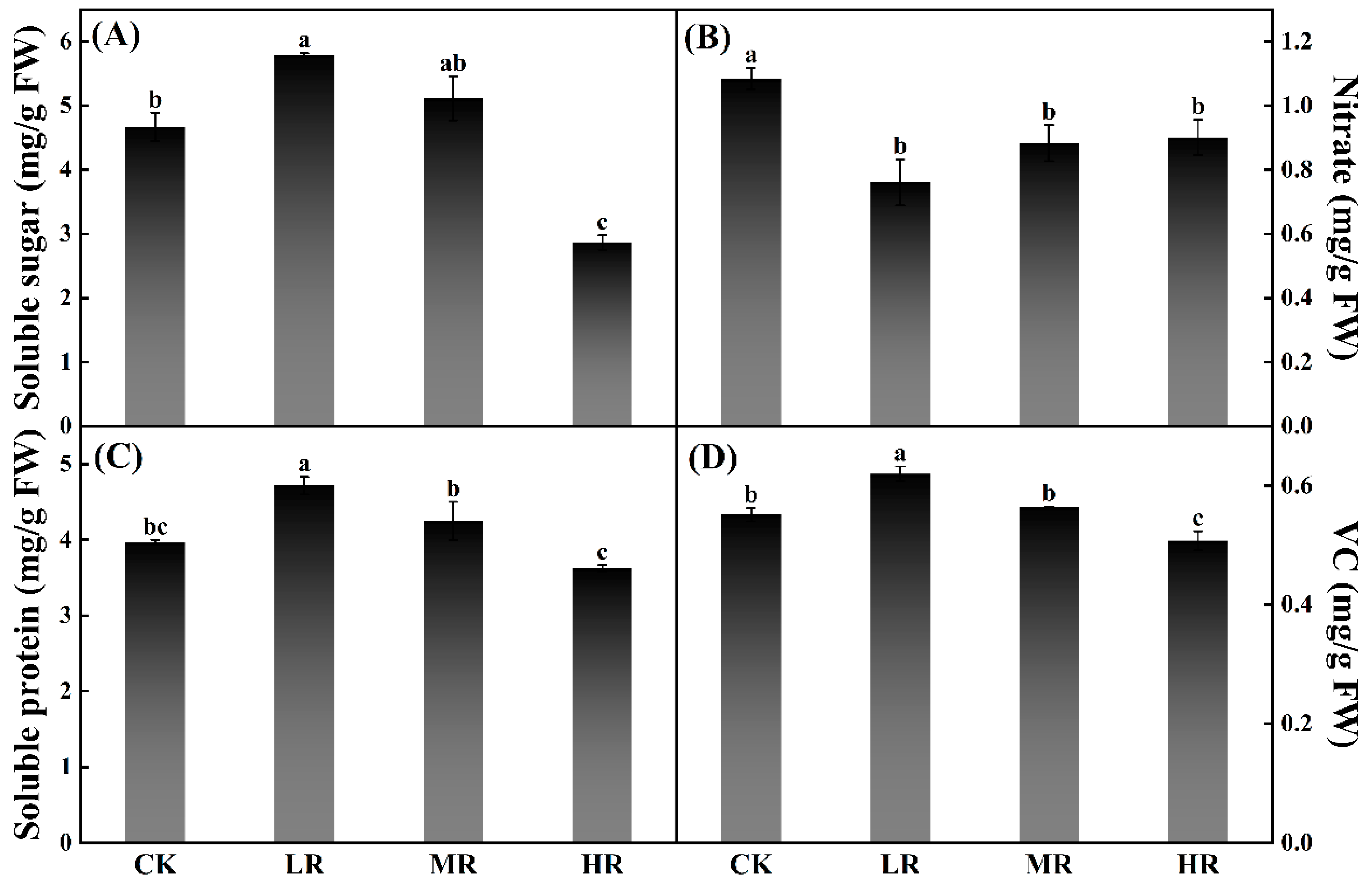


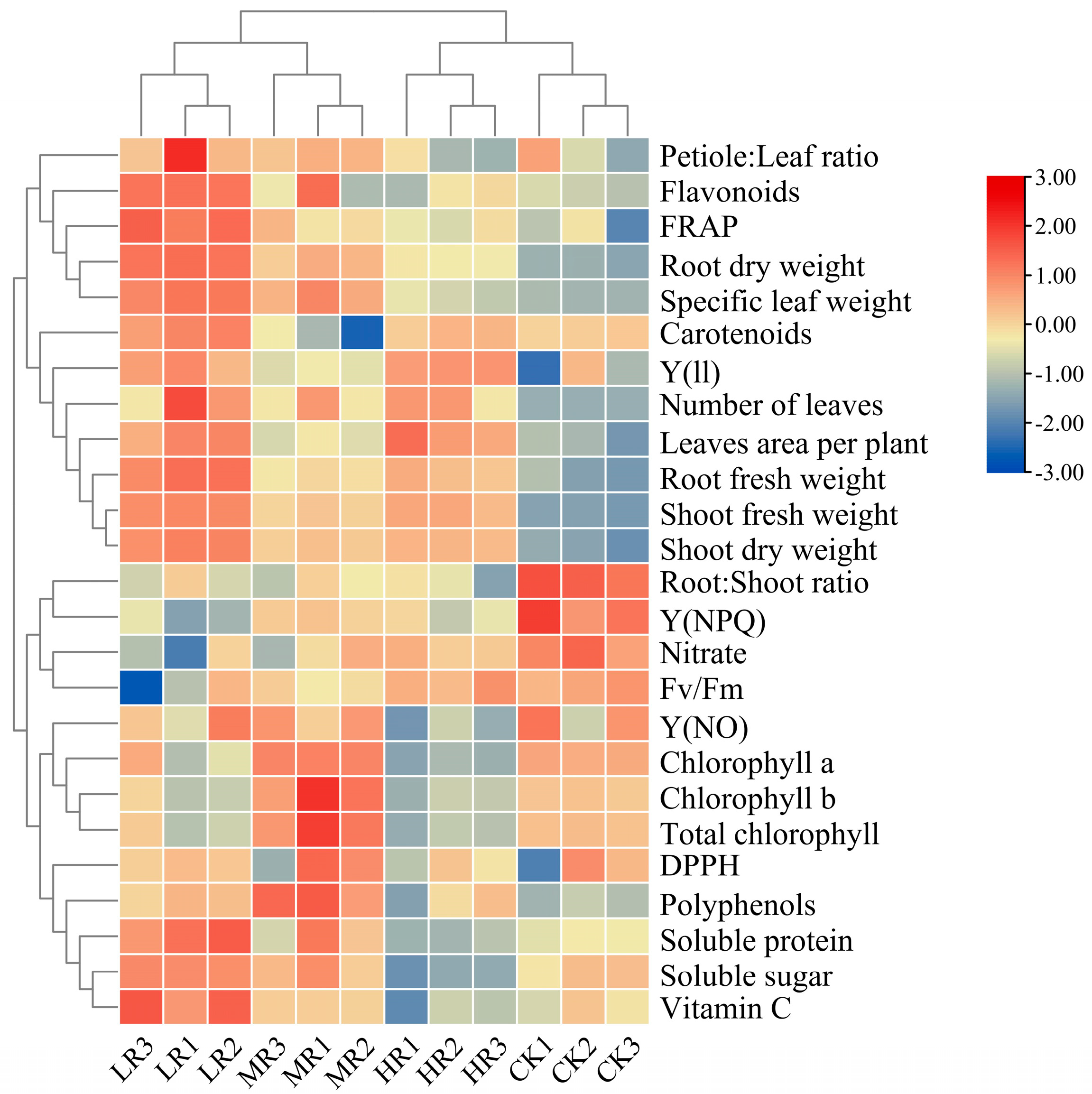
| Parameters | Lighting Treatments | |||
|---|---|---|---|---|
| CK | LR | MR | HR | |
| Single-band photon flux density (μmol·m−2·s−1) | ||||
| Blue light (400–500 nm) | 75.75 | 53.20 | 38.49 | 25.91 |
| Green light (500–600 nm) | 123.42 | 87.08 | 61.76 | 42.56 |
| Red light (600–700 nm) | 70.09 | 127.76 | 158.44 | 191.65 |
| Integrated photon flux density (μmol·m−2·s−1) | ||||
| PPFD | 269.27 | 268.05 | 258.70 | 260.12 |
| YPFD | 224.38 | 234.10 | 232.56 | 239.82 |
| Radiation ratio | ||||
| Red:Blue | 0.93 | 2.40 | 4.12 | 7.40 |
| Red:Green | 0.57 | 1.47 | 2.57 | 4.50 |
| Daily light integral (mol·m−2·d) | ||||
| 10 h | 9.70 | 9.66 | 9.32 | 9.37 |
| Treatments | CK | LR | MR | HR |
|---|---|---|---|---|
| Shoot fresh weight (g) | 60.94 ± 1.83 c | 102.12 ± 2.10 a | 87.11 ± 2.27 b | 91.71 ± 2.00 b |
| Shoot dry weight (g) | 3.06 ± 0.10 c | 5.51 ± 0.13 a | 4.61 ± 0.11 b | 4.69 ± 0.13 b |
| Root fresh weight (g) | 3.47 ± 0.14 c | 5.59 ± 0.16 a | 4.50 ± 0.13 b | 4.93 ± 0.19 b |
| Root dry weight (g) | 0.31 ± 0.01 d | 0.60 ± 0.01 a | 0.49 ± 0.02 b | 0.43 ± 0.01 c |
| Root:shoot ratio | 0.07 ± 0.01 a | 0.06 ± 0.01 b | 0.06 ± 0.01 b | 0.06 ± 0.01 b |
| Leaves area (cm2 per plant) | 767.56 ± 20.28 c | 989.95 ± 28.14 a | 872.63 ± 18.09 b | 1042.95 ± 29.61 a |
| Number of leaves | 13.00 ± 0.29 b | 14.89 ± 0.51 a | 14.33 ± 0.33 a | 14.56 ± 0.38 a |
| Petiole:leaf ratio | 0.40 ± 0.01 ab | 0.41 ± 0.01 a | 0.40 ± 0.01 ab | 0.39 ± 0.01 b |
| Specific leaf weight (mg DW/cm2) | 4.08 ± 0.03 c | 5.54 ± 0.07 a | 5.21 ± 0.15 b | 4.32 ± 0.13 c |
| Principal Components | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| Eigen Value | 11.61 | 6.43 | 2.26 | 1.49 | 1.02 |
| Variability (%) | 46.43 | 25.71 | 9.03 | 5.98 | 4.08 |
| Cumulative % | 46.43 | 72.14 | 81.17 | 87.14 | 91.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; He, R.; Li, Y.; Liu, K.; Tan, J.; Chen, Y.; Liu, X.; Liu, H. Effect of Ratios of Red and White Light on the Growth and Quality of Pak Choi. Agronomy 2022, 12, 2322. https://doi.org/10.3390/agronomy12102322
He X, He R, Li Y, Liu K, Tan J, Chen Y, Liu X, Liu H. Effect of Ratios of Red and White Light on the Growth and Quality of Pak Choi. Agronomy. 2022; 12(10):2322. https://doi.org/10.3390/agronomy12102322
Chicago/Turabian StyleHe, Xinyang, Rui He, Yamin Li, Kaizhe Liu, Jiehui Tan, Yongkang Chen, Xiaojuan Liu, and Houcheng Liu. 2022. "Effect of Ratios of Red and White Light on the Growth and Quality of Pak Choi" Agronomy 12, no. 10: 2322. https://doi.org/10.3390/agronomy12102322
APA StyleHe, X., He, R., Li, Y., Liu, K., Tan, J., Chen, Y., Liu, X., & Liu, H. (2022). Effect of Ratios of Red and White Light on the Growth and Quality of Pak Choi. Agronomy, 12(10), 2322. https://doi.org/10.3390/agronomy12102322









