Community Analysis of Culturable Sapwood Endophytes from Apulian Olive Varieties with Different Susceptibility to Xylella fastidiosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Surface Sterilization
2.2. Bacterial Endophytes Characterization
2.3. Fungal Endophytes Characterization
2.4. Molecular Identification
2.5. Statistical Analysis
3. Results
3.1. Bacterial Morphological, Biochemical, and Molecular Characterization
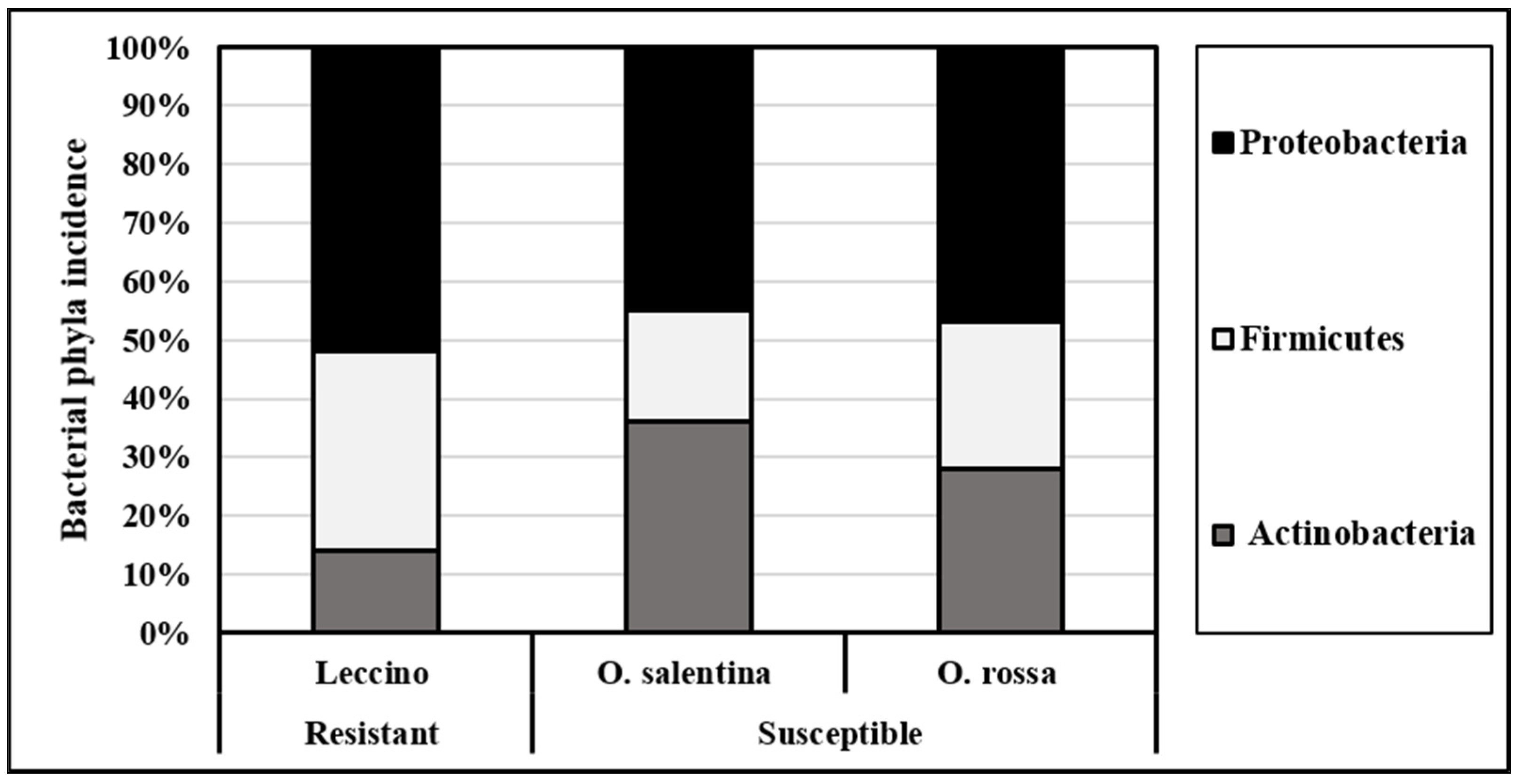
3.2. Bacterial Occurrence and Frequency Variability
3.3. Fungal Morphological and Molecular Characterization
3.4. Fungal Occurrence and Variability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar]
- Cariddi, C.; Saponari, M.; Boscia, D.; De Stradis, A.; Loconsole, G.; Nigro, F.; Porcelli, F.; Potere, O.; Martelli, G.P. Isolation of a Xylella fastidiosa strain infecting olive and oleander in Apulia, Italy. J. Plant Pathol. 2014, 96, 425–429. [Google Scholar]
- Saponari, M.; Boscia, D.; Altamura, G.; Loconsole, G.; Zicca, S.; D’Attoma, G.; Morelli, M.; Palmisano, F.; Saponari, A.; Tavano, D. Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Sci. Rep. 2017, 7, 17723. [Google Scholar] [CrossRef]
- Colella, C.; Carradore, R.; Cerroni, A. Problem setting and problem solving in the case of olive quick decline syndrome in Apulia, Italy: A sociological approach. Phytopathology 2019, 109, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Maggiore, G.; Semeraro, T.; Aretano, R.; De Bellis, L.; Luvisi, A. GIS analysis of land-use change in threatened landscapes by Xylella fastidiosa. Sustainability 2019, 11, 253. [Google Scholar] [CrossRef] [Green Version]
- Jeger, M.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Grégoire, J.C.; Miret, J.A.J.; MacLeod, A.; Navarro, M.N. Updated pest categorisation of Xylella fastidiosa. EFSA J. 2018, 16, e05357. [Google Scholar]
- Giampetruzzi, A.; Morelli, M.; Saponari, M.; Loconsole, G.; Chiumenti, M.; Boscia, D.; Savino, V.N.; Martelli, G.P.; Saldarelli, P. Transcriptome profiling of two olive cultivars in response to infection by the CoDiRO strain of Xylella fastidiosa subsp. pauca. BMC Genom. 2016, 17, 475. [Google Scholar] [CrossRef] [Green Version]
- Martelli, G.P.; Boscia, D.; Porcelli, F.; Saponari, M. The olive quick decline syndrome in south-east Italy: A threatening phytosanitary emergency. Eur. J. Plant Pathol. 2016, 144, 235–243. [Google Scholar] [CrossRef]
- Luvisi, A.; Aprile, A.; Sabella, E.; Vergine, M.; Nicoli, F.; Nutricati, E.; Miceli, A.; Negro, C.; De Bellis, L. Xylella fastidiosa subsp. pauca (CoDiRO strain) infection in four olive (Olea europaea L.) cultivars: Profile of phenolic compounds in leaves and progression of leaf scorch symptoms. Phytopathol. Mediterr. 2017, 56, 259–273. [Google Scholar]
- Sabella, E.; Aprile, A.; Genga, A.; Siciliano, T.; Nutricati, E.; Nicolì, F.; Vergine, M.; Negro, C.; De Bellis, L.; Luvisi, A. Xylem cavitation susceptibility and refilling mechanisms in olive trees infected by Xylella fastidiosa. Sci. Rep. 2019, 9, 9602. [Google Scholar] [CrossRef] [Green Version]
- Sabella, E.; Luvisi, A.; Aprile, A.; Negro, C.; Vergine, M.; Nicolì, F.; Miceli, A.; De Bellis, L. Xylella fastidiosa induces differential expression of lignification related-genes and lignin accumulation in tolerant olive trees cv. Leccino. J. Plant Physiol. 2018, 220, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Nicolì, F.; Negro, C.; Nutricati, E.; Vergine, M.; Aprile, A.; Sabella, E.; Damiano, G.; De Bellis, L.; Luvisi, A. Accumulation of azelaic acid in Xylella fastidiosa-infected olive trees: A mobile metabolite for health screening. Phytopathology 2019, 109, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Abdelfattah, A.; Malacrino, A.; Wisniewski, M.; Cacciola, S.O.; Schena, L. Metabarcoding: A powerful tool to investigate microbial communities and shape future plant protection strategies. Biol. Control 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; JJ Lugtenberg, B. Biotechnological applications of bacterial endophytes. Curr. Biotechnol. 2014, 3, 60–75. [Google Scholar] [CrossRef] [Green Version]
- Arora, N.K.; Khare, E.; Mishra, J. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Rajulu, G.; Vidal, S. Biological control through fungal endophytes: Gaps in knowledge hindering success. Curr. Biotechnol. 2018, 7, 185–198. [Google Scholar]
- Martins, F.; Pereira, J.A.; Bota, P.; Bento, A.; Baptista, P. Fungal endophyte communities in above-and belowground olive tree organs and the effect of season and geographic location on their structures. Fungal Ecol. 2016, 20, 193–201. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Sanzani, S.M.; Wisniewski, M.; Berg, G.; Cacciola, S.O.; Schena, L. Revealing cues for fungal interplay in the plant-air interface in vineyards. Front. Plant Sci. 2019, 10, 922. [Google Scholar] [CrossRef] [PubMed]
- Vergine, M.; Meyer, J.B.; Cardinale, M.; Sabella, E.; Hartmann, M.; Cherubini, P.; De Bellis, L.; Luvisi, A. The Xylella fastidiosa-Resistant Olive Cultivar “Leccino” Has Stable Endophytic Microbiota during the Olive Quick Decline Syndrome (OQDS). Pathogens 2020, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Muzzalupo, I.; Lombardo, N.; Musacchio, A.; Noce, M.E.; Pellegrino, G.; Perri, E.; Sajjad, A. DNA sequence analysis of microsatellite markers enhances their efficiency for germplasm management in an Italian olive collection. J. Am. Soc. Hortic. Sci. 2006, 131, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Oepp, B.; Bulletin, E. PM 7/24 (3) Xylella fastidiosa. EPPO Bull. 2018, 48, 175–218. [Google Scholar]
- Etminani, F.; Harighi, B. Isolation and identification of endophytic bacteria with plant growth promoting activity and biocontrol potential from wild pistachio trees. Plant Pathol. J. 2018, 34, 208. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Smith, H. Benson’s Microbiological Applications, Laboratory Manual in General Microbiology, Short Version; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Borkar, S.G. Laboratory Techniques in Plant Bacteriology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Mincuzzi, A.; Ippolito, A.; Montemurro, C.; Sanzani, S.M. Characterization of Penicillium ss and Aspergillus sect. nigri causing postharvest rots of pomegranate fruit in Southern Italy. Int. J. Food Microbiol. 2020, 314, 108389. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Hiom, S.J.; Wade, W.G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 1998, 64, 795–799. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.; Sorensen, F.C.; St Clair, J.B.; Cronn, R.C. Pacific Northwest Forest Tree Seed Zones A template for native plants? Nativ. Plants J. 2004, 5, 131–140. [Google Scholar] [CrossRef]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi; APS Press: St. Paul, MN, USA, 1999; 218p. [Google Scholar]
- Carlucci, A.; Raimondo, M.L.; Cibelli, F.; Phillips, A.J.L.; Lops, F. Pleurostomophora richardsiae, Neofusicoccum parvum and Phaeoacremonium aleophilum associated with a decline of olives in southern Italy. Phytopathol. Mediterr. 2013, 52, 517–527. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Fung, D. Rapid methods and automation in food microbiology: 25 years of development and predictions. In Global Issues in Food Science and Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 165–176. [Google Scholar]
- Fróhlich, J.; Hyde, K.D.; Petrini, O. Endophytic fungi associated with palms. Mycol. Res. 2000, 104, 1202–1212. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Guo, L.-D.; Hyde, K.D. Response of endophytic fungi of Stipa grandis to experimental plant function group removal in Inner Mongolia steppe, China. Fungal Divers. 2010, 43, 93–101. [Google Scholar] [CrossRef]
- Gibbons, J. Association analysis. In Non-Parametric Methods for Quantitative Analysis; Holt, Rinehart & Winston: New York, NY, USA, 1976; pp. 273–291. [Google Scholar]
- Sawilowsky, S.S. Nonparametric tests of interaction in experimental design. Rev. Educ. Res. 1990, 60, 91–126. [Google Scholar] [CrossRef]
- Durner, E. Effective Analysis of Interactive Effects with Non-Normal Data Using the Aligned Rank Transform, ARTool and SAS® University Edition. Horticulturae 2019, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Fausto, C.; Mininni, A.N.; Sofo, A.; Crecchio, C.; Scagliola, M.; Dichio, B.; Xiloyannis, C. Olive orchard microbiome: Characterisation of bacterial communities in soil-plant compartments and their comparison between sustainable and conventional soil management systems. Plant Ecol. Divers. 2018, 11, 597–610. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Baptista, P.; Morelli, M.; Cameirão, C.; Lino Neto, T.; Costa, D.; D’attoma, G.; Abou Kubaa, R.; Altamura, G.; Saponari, M. Differences in the endophytic microbiome of olive cultivars infected by Xylella fastidiosa across seasons. Pathogens 2020, 9, 723. [Google Scholar] [CrossRef]
- Zicca, S.; De Bellis, P.; Masiello, M.; Saponari, M.; Saldarelli, P.; Boscia, D.; Sisto, A. Antagonistic activity of olive endophytic bacteria and of Bacillus spp. strains against Xylella fastidiosa. Microbiol. Res. 2020, 236, 126467. [Google Scholar] [CrossRef]
- Compant, S.; Van Der Heijden, M.G.; Sessitsch, A. Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Mengoni, A.; Mocali, S.; Surico, G.; Tegli, S.; Fani, R. Fluctuation of endophytic bacteria and phytoplasmosis in elm trees. Microbiol. Res. 2003, 158, 363–369. [Google Scholar] [CrossRef]
- Shen, S.Y.; Fulthorpe, R. Seasonal variation of bacterial endophytes in urban trees. Front. Microbiol. 2015, 6, 427. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Valverde-Corredor, A.; Cabanás, C.G.-L.; Sesmero, R.; Mercado-Blanco, J. What lies beneath: Root-associated bacteria to improve the growth and health of olive trees. In Soil Biological Communities and Ecosystem Resilience; Springer: New York, NY, USA, 2017; pp. 107–122. [Google Scholar]
- Emmert, E.A.; Handelsman, J. Biocontrol of plant disease: A (Gram-) positive perspective. FEMS Microbiol. Lett. 1999, 171, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mina, D.; Pereira, J.A.; Lino-Neto, T.; Baptista, P. Epiphytic and endophytic bacteria on olive tree phyllosphere: Exploring tissue and cultivar effect. Microb. Ecol. 2020, 80, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Marcon, J.; Maccheroni, W.; van Elsas, J.D.; van Vuurde, J.W.; Azevedo, J.L. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl. Environ. Microbiol 2002, 68, 4906–4914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, J.L.; Araújo, W.L.; Lacava, P.T. The diversity of citrus endophytic bacteria and their interactions with Xylella fastidiosa and host plants. Genet. Mol. Biol. 2016, 39, 476–491. [Google Scholar] [CrossRef] [Green Version]
- Lacava, P.T.; Li, W.; Araújo, W.L.; Azevedo, J.L.c.; Hartung, J.S. The endophyte Curtobacterium flaccumfaciens reduces symptoms caused by Xylella fastidiosa in Catharanthus roseus. J. Microbiol. 2007, 45, 388–393. [Google Scholar]
- Lacava, P.T.; Silva-Stenico, M.E.; Araújo, W.L.; Simionato, A.V.C.; Carrilho, E.; Tsai, S.M.; Azevedo, J.L. Detection of siderophores in endophytic bacteria Methylobacterium spp. associated with Xylella fastidiosa subsp. pauca. Pesquisa Agropecuária Brasileira 2008, 43, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Godena, S.; Dminić, I.; Đermić, E. Differential susceptibility of olive varieties to olive knot disease in Istria. J. Cent. Eur. Agric. 2012, 13, 79482. [Google Scholar] [CrossRef] [Green Version]
- Gomes, T.; Pereira, J.A.; Benhadi, J.; Lino-Neto, T.; Baptista, P. Endophytic and epiphytic phyllosphere fungal communities are shaped by different environmental factors in a Mediterranean ecosystem. Microb. Ecol. 2018, 76, 668–679. [Google Scholar] [CrossRef] [Green Version]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Campos, M.D.; Rei, F.; do Rosário Félix, M. Spatial and temporal variation of fungal endophytic richness and diversity associated to the phyllosphere of olive cultivars. Fungal Biol. 2019, 123, 66–76. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Kjer, J.; Proksch, P.J.F.d. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010, 41, 1–16. [Google Scholar] [CrossRef]
- Leonhardt, S.; Hoppe, B.; Stengel, E.; Noll, L.; Moll, J.; Bässler, C.; Dahl, A.; Buscot, F.; Hofrichter, M.; Kellner, H. Molecular fungal community and its decomposition activity in sapwood and heartwood of 13 temperate European tree species. PLoS ONE 2019, 14, e0212120. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, M.; Kushveer, J.S.; Sarma, V.V. A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 2019, 10, 798–1079. [Google Scholar] [CrossRef]
- Torres, D.E.; Rojas-Martínez, R.I.; Zavaleta-Mejía, E.; Guevara-Fefer, P.; Márquez-Guzmán, G.J.; Pérez-Martínez, C. Cladosporium cladosporioides and Cladosporium pseudocladosporioides as potential new fungal antagonists of Puccinia horiana Henn., the causal agent of chrysanthemum white rust. PLoS ONE 2017, 12, e0170782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohl, J.; Scheer, C.; Holb, I.J.; Masny, S.; Molhoek, W. Toward an Integrated Use of Biological Control by Cladosporium cladosporioides H39 in Apple Scab (Venturia inaequalis) Management. Plant Dis. 2015, 99, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Paul, D.; Park, K.S. Identification of volatiles produced by Cladosporium cladosporioides CL-1, a fungal biocontrol agent that promotes plant growth. Sensors 2013, 13, 13969–13977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amatuzzi, R.F.; Cardoso, N.; Poltronieri, A.S.; Poitevin, C.G.; Dalzoto, P.; Zawadeneak, M.A.; Pimentel, I.C. Potential of endophytic fungi as biocontrol agents of Duponchelia fovealis (Zeller)(Lepidoptera: Crambidae). Braz. J. Biol. 2017, 78, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Anisha, C.; Sachidanandan, P.; Radhakrishnan, E.K. Endophytic Paraconiothyrium sp. from Zingiber officinale Rosc. displays broad-spectrum antimicrobial activity by production of danthron. Curr. Microbiol. 2018, 75, 343–352. [Google Scholar] [CrossRef]
- Tuppad, D.S.; Shishupala, S. Evaluation of endophytic fungi from Butea monosperma for antimicrobial and enzyme activity. J Med. Plants Stud. 2014, 2, 38–45. [Google Scholar]
- Chitrampalam, P.; Figuli, P.J.; Matheron, M.E.; Subbarao, K.V.; Pryor, B.M. Biocontrol of lettuce drop caused by Sclerotinia sclerotiorum and S. minor in desert agroecosystems. Plant Dis. 2008, 92, 1625–1634. [Google Scholar] [CrossRef] [Green Version]





| Group Code | No. of Isolates | Colony Morphology | Consistency | Texture | Gram | Shape | Motility | Catalase | Oxidase | IAA Test | P-Solublization |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OSB 1 | 6 | yellow, large and circular | slimy | flat | +ve | rods | + | + | + | − | + |
| OSB 2 | 10 | yellow, small and irregular | creamy | flat | +ve | rods | + | + | − | + | + |
| OSB 3 | 7 | yellow, small and irregular | slimy | flat | +ve | rods | + | + | − | + | + |
| OSB 4 | 9 | pink, large and circular | creamy | raised | −ve | rods | − | − | + | + | + |
| OSB 5 | 13 | white, small and irregular | slimy | flat | +ve | rods | − | + | + | + | − |
| OSB 6 | 14 | yellow, large and circular | mucoid | raised | −ve | rods | + | + | − | + | − |
| OSB 7 | 9 | white, large and circular | creamy | flat | +ve | rods | + | + | + | + | − |
| OSB 8 | 10 | white, small and circular | creamy | flat | −ve | rods | + | + | − | + | + |
| OSB 9 | 4 | white, small and circular | slimy | flat | −ve | rods | + | + | + | + | + |
| OSB 10 | 5 | yellow, large and circular | creamy | convex | −ve | rods | + | + | + | + | − |
| OSB 11 | 11 | brown, large and circular | mucoid | raised | +ve | rods | + | − | + | − | − |
| OSB 12 | 7 | orange, small and circular | slimy | flat | −ve | rods | + | + | + | − | + |
| OSB 13 | 5 | white, large and irregular | slimy | flat | −ve | rods | + | − | − | − | + |
| OSB 14 | 10 | red, small and circular | creamy | raised | +ve | rods | − | − | + | + | + |
| OSB 15 | 7 | yellow, large and circular | mucoid | flat | +ve | rods | + | − | − | + | − |
| OSB 16 | 3 | white, small and circular | slimy | flat | −ve | rods | + | + | + | + | − |
| Groups | Identity | Accession #N | Reference | Blast ID | Source | Total Relative Density % | ||
|---|---|---|---|---|---|---|---|---|
| Leccino | O. Salentina | O. Rossa | ||||||
| MF1 | Aspergillus sp. | MT558577-78 | MH398045.1 | 99% | L, S, and R | 9.8 | 1.77 | 4.69 |
| MF2 | Cladosporium sp. | MT558579-80 | LN834380.1 | 98% | L, S, and R | 15.7 | 11.50 | 10.94 |
| MF3 | Cytospora sp. | MT558581 | KY496629.1 | 98% | L and S | 11.8 | 2.65 | 0.00 |
| MF4 | Fusarium sp. | MT558582 | KT004553.1 | 99% | L, S, and R | 7.84 | 2.65 | 7.81 |
| MF5 | Libertasomyces platani | MT558583 | KY173416.1 | 99% | L and R | 3.92 | 0.00 | 4.69 |
| MF6 | Mycocalicium sp. | MT558584 | AJ972853.1 | 98% | S and R | 0.00 | 10.62 | 12.50 |
| MF7 | Neophaeomoniella sp. | MT558585 | NR138001.1 | 99% | L, S, and R | 5.88 | 15.05 | 14.06 |
| MF8 | Paraconiothyrium brasiliense | MT558586-87 | KR909140.1 | 99% | L | 7.84 | 0.00 | 0.00 |
| MF9 | Paraphaeosphaeria sp. | MT558588 | GU985234.1 | 99% | L, S, and R | 3.92 | 4.42 | 6.25 |
| MF10 | Penicillium sp. | MT558589-90 | MK102703.1 | 99% | L, S, and R | 9.80 | 14.16 | 10.94 |
| MF11 | Phoma sp. | MT558593 | GU183116.1 | 99% | L and S | 3.92 | 8.85 | 0.00 |
| MF12 | Pithomyces chartarum | MT558591 | MH860227.1 | 99% | L, S, and R | 7.84 | 0.88 | 4.69 |
| MF13 | Pseudophaeomoniella oleae | MT558592 | NR_137966.1 | 99% | L, S, and R | 3.92 | 14.16 | 7.81 |
| MF14 | Stigmatodiscus oculatus | MT558594 | MH756071.1 | 99% | S and R | 0.00 | 7.96 | 9.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanani, A.; Valentini, F.; Sanzani, S.M.; Santoro, F.; Minutillo, S.A.; Gallo, M.; Cavallo, G.; Mourou, M.; El Moujabber, M.; D’Onghia, A.M.; et al. Community Analysis of Culturable Sapwood Endophytes from Apulian Olive Varieties with Different Susceptibility to Xylella fastidiosa. Agronomy 2022, 12, 9. https://doi.org/10.3390/agronomy12010009
Hanani A, Valentini F, Sanzani SM, Santoro F, Minutillo SA, Gallo M, Cavallo G, Mourou M, El Moujabber M, D’Onghia AM, et al. Community Analysis of Culturable Sapwood Endophytes from Apulian Olive Varieties with Different Susceptibility to Xylella fastidiosa. Agronomy. 2022; 12(1):9. https://doi.org/10.3390/agronomy12010009
Chicago/Turabian StyleHanani, Arafat, Franco Valentini, Simona M. Sanzani, Franco Santoro, Serena A. Minutillo, Marilita Gallo, Giuseppe Cavallo, Marwa Mourou, Maroun El Moujabber, Anna M. D’Onghia, and et al. 2022. "Community Analysis of Culturable Sapwood Endophytes from Apulian Olive Varieties with Different Susceptibility to Xylella fastidiosa" Agronomy 12, no. 1: 9. https://doi.org/10.3390/agronomy12010009
APA StyleHanani, A., Valentini, F., Sanzani, S. M., Santoro, F., Minutillo, S. A., Gallo, M., Cavallo, G., Mourou, M., El Moujabber, M., D’Onghia, A. M., & Davino, S. W. (2022). Community Analysis of Culturable Sapwood Endophytes from Apulian Olive Varieties with Different Susceptibility to Xylella fastidiosa. Agronomy, 12(1), 9. https://doi.org/10.3390/agronomy12010009








